Abstract
Long-chain ω-3 polyunsaturated fatty acids (PUFAs) are fundamental biocomponents of lipids and cell membranes. They are involved in the maintenance of cellular homeostasis and they are able to exert anti-inflammatory and cardioprotective actions. Thanks to their potential beneficial effects on the cardiovascular system, metabolic axis and body composition, we have examined their action in subjects affected by male obesity secondary hypogonadism (MOSH) syndrome. MOSH syndrome is characterized by the presence of obesity associated with the alteration of sexual and metabolic functions. Therefore, this review article aims to analyze scientific literature regarding the possible benefits of ω-3 PUFA administration in subjects affected by MOSH syndrome. We conclude that there are strong evidences supporting ω-3 PUFA administration and/or supplementation for the treatment and management of MOSH patients.
1. Introduction
It is truly fascinating to study how lifestyle modification can alter the course of a disease by modifying genetic expression and protein synthesis patterns. Thanks to modern epigenetics, researchers have found that changes in daily habits coupled with healthy nutrition can literally modulate our gene expression, in order to achieve better metabolic profiles and decrease the risk of developing an array of diseases [1]. Exploring the properties of natural compounds such as ω-3 polyunsaturated fatty acids (PUFAs) and how they can be optimally integrated in the diet is of paramount importance. Obesity represents a major public health burden and it can be defined as a pathological increase in weight and therefore in body mass index (BMI).
PUFA ω-3 would seem to exert a cardioprotective role as they improve heart rate variability, a non-invasive marker of cardiac autonomic system function, with a subsequent reduction in the risk of sudden cardiac death and arrhythmias [2]. A further beneficial effect induced by PUFAs is linked to their anti-inflammatory capacity [3] and their ability to modulate the inflammatory response. Moreover, their effects in terms of improving body composition have also been recently demonstrated [4].
Obesity is defined as a condition characterized by a pathological increase in weight and therefore in body mass index (BMI). Its interpretation is based on weight status groupings, calculated by weight in kg divided by the square of the height in meters. A BMI exceeding 30 kg/m2 is indicative of obesity, as BMI rises, its values can be further subdivided into different classes correlating with different degrees of severity and cardiovascular disease (CVD) risk (class I between 30 and 34.9 kg/m2, class II between 35 and 39.9 kg/m2 and class III ≥40kg/m2) [5]. A BMI greater than 40 kg/m2 is defined as extreme, severe or morbid, whilst having a BMI between 25 and 30 kg/m2 is described as being in a state termed pre-obesity [6,7]. A sedentary lifestyle coupled with unhealthy eating habits, characterized by the excessive consumption of high energy foods, are the root of the growing prevalence of obesity worldwide. The mechanisms which have led to such a dramatic increase in the incidence and prevalence of obesity are complex and are intertwined with environmental and societal trends [8]. It is not uncommon nowadays to see the term obesity flanked by the term epidemic or even pandemic. This is due to the sheer statistics regarding obesity, which estimate that in 2016 there were 1.9 billion overweight adults worldwide [9,10]. Obesity is the pathophysiological state determined by weight and adipose excess, which is characterized by the alteration of body composition starting from peripheral tissues such as adipose tissue, liver and muscles [11]. These alterations lead to an increased risk of the onset of arterial hypertension, CVDs and other chronic non-communicable degenerative diseases (CNCDs), such as type 2 diabetes mellitus (T2DM), male obesity secondary hypogonadism (MOSH), respiratory diseases, cancer, chronic kidney disease and psychopathological alterations that negatively impact on both quality of life and longevity [12,13,14,15].
In obese men, MOSH syndrome leads to a plethora of symptoms such as impaired fertility and sexual function, deficient bone mineralization, altered fat metabolism and body composition and the deterioration of muscle mass [16]. Epidemiological data obtained by population studies state that the prevalence of MOSH syndrome is above 45–57.5% of male obese subjects and it correlates with high-rate morbidity and mortality [17,18].
In this review article we analyzed the possible beneficial effects of ω-3 PUFA on clinical signs and symptoms of MOSH syndrome.
2. Methods
Current literature investigating the possible positive impact of ω-3 PUFA consumption on MOSH syndrome is analyzed and contextualized in this review. Specifically, research has been conducted on Medline (Pubmed) and Scopus. Such a review article analyzes studies (both in vivo and in vitro studies) published up to June 2020.
3. Structure, Metabolic Pathways and Dietary Sources of PUFA
Fatty acids (FAs) are fundamental biocomponents of lipids and cell membranes. They are made up by a hydrocarbon backbone and a carboxylic head group. FAs are classified according to the length of the hydrocarbon backbone (generally 12 to 24 carbon atoms long), and according to the presence and the number of double bonds. We can distinguish between saturated fatty acids (SFAs), which are characterized by the absence of double bonds, monounsaturated fatty acids (MUFAs), which only have one double bond and PUFAs, in which more than one double bond may be found. FAs can be further classified according to the position of the first double bond compared to carbon ω (the furthest carbon from the carboxylic group), forming two classes: ω-3 and ω-6 PUFA [19,20].
The human body can produce almost all fatty acids, except α-linolenic acid (ALA, C18:3 ω-3) and linolenic acid (LA, C18:2 ω-6) which are precursors of ω-3 and ω-6 PUFAs. These are termed “essential fatty acids” because they can only be obtained through diet [21]. Through endogenous conversion (elongation and desaturation) the organism is capable of synthesizing longer-chain counterparts such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in the ω-3 family, and γ-linolenic acid (GLA), dihomo-γ-linolenic acid (DGLA) and arachidonic acid (AA) in the ω-6 family [20,22].
Long-chain ω-3 PUFAs and long-chain ω-6 PUFAs are precursors of molecules with important biological activity called eicosanoids such as prostaglandins (PG), thromboxanes (Tx), leukotrienes (LTS), lipoxins (LXS) and resolvins. Depending on which precursor family they belong to, PUFAs can perform different biological functions. In fact, while ω-3 PUFAs carry out an anti-inflammatory function, ω-6 PUFAs elicit a proinflammatory function.
The ω-3 and ω-6 long-chain PUFAs compete to bind enzymes such as cyclooxygenase, lipoxygenase and epoxygenases, which are responsible for the release of inflammatory mediators. Thus, the equilibrium between ω-3 and ω-6 PUFA intracellular concentrations is fundamental for the maintenance of cellular homeostasis and cardiovascular (CV) protection [20,23,24]. In order for them to perform their correct biological actions, it is necessary to have a balanced PUFA intake. Recent studies suggest that an ideal ratio between ω-6/ω-3 is between 1:1 and 1:5, whilst the actual intake ratio in Western countries is of 15:1–16.7:1. Therefore, it appears necessary to maintain an adequate and balanced intake of ω-6/ω-3 in order to prevent CVD onset [25,26,27].
Regarding main food sources, PUFAs are present as precursors (ALA and LA) in plant-based products and as derivatives (EPA, DHA, AA) in meat (Table 1 and Table 2). Fish is the main source of long-chain ω-3 PUFAs, including EPA, DHA and docosapentaenoic acid (DPA), while ALA is a plant and ω-3 PUFAs are mainly found in seeds and nuts and their oils. Plant sources of ω-3 PUFAs cannot currently be considered as a replacement for seafood-derived ω-3 PUFAs. This suggests that ω-3 PUFAs, derived from different sources, have their own specific effects. Therefore, it appears necessary to have a varied and balanced diet [20,27].

Table 1.
Main dietary sources of ω-3 fatty acids.

Table 2.
Main dietary sources of ω-6 fatty acids.
ω-3 PUFAs are also known as “vitamin F”, not only are they needed for basic cellular functions such as cell signaling, membrane fluidity and structural integrity, but also for nervous system regulation [28,29]. They have a role in regulating blood pressure, clotting, glucose metabolism and inflammation [28]. Moreover, they have been related to be preventative in the occurrence of CVevents and to slow down the progression of CVDs. These concepts will be further explored in the following section [30].
4. Male Obesity Secondary Hypogonadism (MOSH) Syndrome Definition
MOSH syndrome is a clinical condition found in obese middle-aged men and epidemiological reports assert that in the last 10 years its prevalence has enhanced, even if it is currently an underestimated and underdiagnosed condition [31].
In MOSH syndrome, obesity corroborates hypogonadism to give rise to reduced levels of testosterone (T). This reduction is due to the alteration of metabolic patterns such as lipid metabolism, chronic inflammation and insulin resistance (Figure 1) [32].
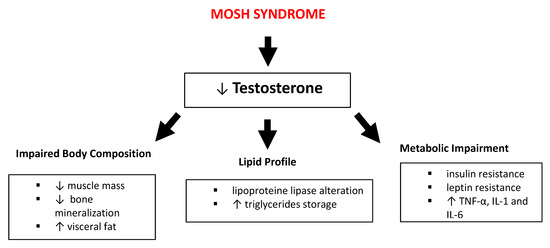
Figure 1.
Impact of male obesity secondary hypogonadism (MOSH) syndrome on body composition, lipid profile and metabolic pathways. Abbreviations: TNF-α, tumor necrosis factor-α; IL, interleukin; ↑: increase; ↓: decrease.
The pathophysiological mechanisms linking obesity with hypogonadism are complex and multifactorial [32]. Obese male subjects show a significant reduction of T levels caused by an increase of aromatase enzymes levels, released by the adipose tissue and enhanced by estrogen hormones [33], coupled with the negative feedback produced by the estrogen on the hypothalamic-pituitary axis, this is another factor decreasing the circulating T levels. Such pattern affects the lipid profile through the alteration of lipoprotein lipase presence on adipocytes and increase triglycerides (TG) storage, leading to an increase in visceral adipose deposition and total body fat. These alterations are considered particularly harmful and are highly associated with CV disease risk [33]. Moreover, these lipid profile alterations create a sort of self-perpetuating cycle between obesity and hypogonadism.
The hypertrophy of adipose tissue, characteristic of obese subjects, leads to the lowering of T levels. Metabolic impairment caused by body fat enhancement is responsible for insulin and leptin resistance, and for the increase of pro-inflammatory cytokines (such as Tumor Necrosis Factor-α - TNF-α, interleukins 1 and 6 - IL-1,IL-6) which influence hypothalamic function, in particular decreasing kisspeptin signaling [34]. Such a decrease entails the reduction of gonadotropin-releasing hormone (GnRH), which in turn decreases luteinizing hormone (LH) and follicle-stimulating hormone (FSH) secretion by anterior pituitary gonadotrophs, resulting in a T reduction and in the alteration of fertility [35].
Subjects affected by MOSH are often characterized by reduced osseous mineral density, which can be explained by the T deficiency that is strongly involved in the modulation of bone mineralization, as T is implicated in the regulation of the proliferation and differentiation of osteoblasts [16].
T induces skeletal muscle hypertrophy through numerous mechanisms including its effects in modulating pluripotent mesenchymal cell engagement. Studies have shown that elevated T levels are associated with an increase in the size of motor neurons [36]. Therefore, in subjects with MOSH, the reduction of T levels can lead to a reduction in muscle mass.
MOSH syndrome is potentially reversible. Its treatment, in addition to exogenous T administration, includes lifestyle changes such as diet therapy and physical activity aimed to reduce obesity [16,37,38].
4.1. Role of PUFA in Cardiovascular Disease
In the last few years, the role of ω-3 PUFAs has been widely debated within the scientific and medical communities in virtue of the possible role they may play in contrasting CV diseases (Table 3).

Table 3.
Studies on polyunsaturated fatty acids (PUFAs) and cardiovascular disease.
On the one hand observational studies reported an inverse association between CV diseases and dietary intake or plasma concentrations of ω-3 PUFAs (primarily EPA and DHA), suggesting that their supplementation might exert cardio protective effects, on the other hand successive clinical trials and metanalyses have speculated the absence of true benefits induced by ω-3 PUFA consumption on the CV system [39,40,41,42]. This discrepancy may be justified by the multiple variables that influence CV diseases which may lead to contrasting results. These variables render CV diseases quite heterogeneous, resulting in different responses to ω-3 PUFA treatment. We must take into consideration that this kind of treatment does not carry out the action of a “pharmaceutical” drug, but rather acts by producing a modulatory effect on the subject’s metabolism which can be more or less susceptible to a response, depending not only on the degree and type of pathological involvement but also on the subject’s genetic susceptibility. This renders the task even more articulated, particularly as an individual’s genetic susceptibility is determined by the genotype and by environmental and epigenetic changes. Even if the debate on ω-3 PUFAs is currently unresolved, it is worth underlining that their consumption has never been associated with deleterious effects on health and therefore their use can either induce positive CV effects, or in the worst case scenario, can induce a neutral effect [43]. For such reason, the following section will comment on the possible beneficial health effects induced by PUFA consumption in subjects with an elevated CV risk and in patients affected by MOSH syndrome. The cardioprotective role of ω-3 PUFAs was hypothesized for the first time in the 1950s in the Eskimo population, which presented elevated levels of plasma cholesterol but an exiguous CV mortality rate [44]. Successively, such observation was also made in the Japanese and Icelandic populations, in which there was an evidently low mortality from CV pathologies compared to Western populations [45,46]. This cardio protective effect was attributed to eating habits, in particular to elevated fish consumption. Further epidemiological studies confirmed this correlation and described the cardioprotective effects induced by ω-3 PUFA consumption [47]. In light of the data published by two large clinical randomized trials, the American Heart Association (AHA) in 2002 suggested the consumption of 1g/day EPA+DHA in patients with coronary artery disease in virtue of their cardioprotective potential [48,49,50]. Successively, the Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto (GISSI) [50,51] and Diet And Reinfarction Trial (DART) [48] studies have demonstrated a reduction in CV risk following treatment with ω-3 PUFAs, representing the milestones of clinical recommendations for ω-3 PUFA treatment in cardiopathic subjects since it was observed that the benefits outweighed any possible side effect related to their consumption [52,53]. The main cardio protective effects induced by ω-3 PUFA consumption are achieved through actions such as the reduction of plasma TG and of chronic low-grade inflammatory status, an improvement of endothelial function, cardiac functional remodeling and of cardiac contractility [51,54,55]. An in vitro study conducted in bovine aortic endothelial cells demonstrated that treatment with adiponectin is able to increase nitric oxide (NO) production by 3-fold in endothelial cells. This action is due to the phosphorylation of endothelial-nitric oxide synthase (e-NOS) by phosphatidylinositol 3-kinase-dependent pathways [56]. In 2002, the AHA affirmed that a dose between 2 and 4 g/day of ω-3 PUFA was able to treat hypertriglyceridemia [57]. In the wake of this finding, one of the principal studies, aimed at underlining an improvement in plasma TG, was conducted by Harris et al. [58] These authors observed a dose-dependent plasma TG reduction after ω-3 PUFA administration, especially in subjects who presented basal TG levels >500 mg/dL [58]. This was confirmed in subsequent clinical trials performed on subjects presenting very high triglyceride (VHT) levels (TG > 500 mg/dL) and high triglyceride (HT) levels (TG between 200 and 499 mg/dL). Results showed a 30% reduction in plasma TG in the VHT group and a reduction between 20 and 30% in the HT group following the consumption of 4 g/day of ω-3, confirming that the reduction in percentage of TG correlated with their plasma levels before treatment [59,60,61]. ω-3 PUFAs are able to contrast chronic inflammation via the reduction of macrophage-monocyte adhesion, caused by oxidized low-density lipoprotein (LDL) to the endothelial lining of the coronary vessels. This effect is coupled with the increased expression of e-NOS induced by DHA, with a consequent increase in NO release and therefore, vasodilation [62]. DHA is also able to modulate endothelial function by inducing the transcription of the gene coding for the proinflammatory cytokine TNF-α, and the inhibition of the pathway generated by nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), which causes a reduction in vascular cell adhesion molecule-1 (VCAM-1) [63]. Therefore, the actions carried out by DHA at the endothelial level suggest its vasoprotective role.
Moreover, ω-3 PUFAs induce the suppression of thromboxane A2 (a factor responsible for platelet aggregation, vasoconstriction and fibrinogen reduction) synthesis, and favor the synthesis of thromboxane A3 [64,65,66]. In this context, animal models highlighted that EPA consumption also plays a role in stabilizing the atheromatous plaque [67].
EPA and DHA inhibit a series of processes linked to inflammation, such as leukocyte chemotaxis, adhesion interactions between leukocytes and the endothelium, eicosanoid production and T cell reactivity [68]. Finally, an increase in EPA and DHA availability modifies the equilibrium between ω-3 and ω-6 PUFAs, favoring anti-inflammatory eicosanoid synthesis [69].
ω-3 PUFA consumption is associated with a better vascular function, playing a protective role in atherosclerosis, in which endothelial dysfunction is at the basis of the pathogenic process [62,70]. ω-3 PUFAs improve arterial wall rigidity [71] and it was observed that their supplementation induces a reduction in endothelial damage biomarkers such as E-selectin [72].
ω-3 PUFA supplementation was also associated with the reduction of heart rate at rest [73,74], the reduction of systolic and diastolic blood [75,76], and the increase in early and late left ventricular ejection fraction [77].
ω-3 PUFA treatment can lead to a reduction in hospitalization and CV mortality incidence [52]. Finally, the study OMEGA-REMODEL has demonstrated a reduction in cardiac remodeling and fibrosis markers in patients with acute myocardial infarction (AMI), following a supplementation of ω-3 PUFAs (4 g/day) in the diet [78]. It is hypothesized that this beneficial effect is correlated with the reduction of macrophage activation and with the inhibition of galectin-3 (Gal-3), a factor which reflects cardiac function impairment and remodeling [79]. In an elderly population in which subjects had recently undergone an AMI, there were significant inverse correlations between ω-3 PUFA content in serum phospholipids and serum levels of Gal-3, confirming the beneficial effects of ω-3 PUFAs on cardiac remodeling [79].
4.2. Impact of PUFA Consumption on Body Weight
The Mediterranean diet is known to provide a balanced supply of PUFAs [14]. In vivo studies have demonstrated that the consumption ω-3 FA is correlated with the improvement of body composition. Specifically, it is observed that there is a reduction in adipose tissue thanks to the interactions with metabolic pathways, including the glucose one [80]. A meta-analysis conducted in 2014 [4] has explored the relationship between the consumption of long-chain ω-3 PUFAs and body composition in Caucasian subjects (Table 4). The study examined 934 subjects who were getting long-chain ω-3 PUFAs from fish or from supplements. The authors have found statistically significant variations comparing results obtained between the study group and healthy subjects. The examined parameters were: body weight; BMI; fat mass (FM) %; and waist circumference (WC). Moreover, the authors have also investigated the possible gender effect tied to the consumption of long-chain ω-3 PUFAs, highlighting that in male subjects the WC diminished significantly more than in females.

Table 4.
Studies on impact of PUFA consumption on body weight.
There is considerable evidence showing that, at the cellular level, PUFAs are potent transcription regulators of genes involved in lipid metabolism. In fact, PUFAs have an important role in the inhibition of genes involved in lipogenesis, and in the promotion of genes involved in lipid oxidation [81]. Other than being prone to rapid oxidation and peroxidation, PUFAs are able to favor the synthesis of proteins involved in detoxification processes that counteract oxidative stress [82]. A study by Di Nunzio et al. [83] has shed light on the antioxidant and pro-oxidant properties of different PUFAs. The authors have demonstrated that only DHA is able to diminish susceptibility to hydrogen peroxide, which stimulates the transcription and the activation of the peroxisome proliferator-activated receptor α (PPARα). PPARα is able to favor the activity of antioxidant enzymes, such as Catalase- CAT and superoxide dismutase- SOD [83]. Therefore, the consumption of PUFAs, specifically DHA, allows an adequate antioxidant protection at the cellular level if the ω-3/ω-6 at 1:5 ratio is followed [84].
The enhancement in lipid oxidation, and the increased use of lipids as an energy source, can translate into a reduction in FM. In fact, some studies demonstrate that increased PUFA intake is associated with substantial FM loss, especially in the abdominal region [85].
Couet et al. have examined a population of lean and healthy individuals who were administered 6 g/day of visible fat for 3 weeks followed by a wash-out period lasting 10–12 weeks, followed in turn by the administration of 6 g/day of fish oil for 3 weeks. The authors have reported a statistically significant reduction in FM, whilst body weight was maintained [86].
A study by Huang et al. [87] has examined the possible genetic–dietary interactions in a population of 24,357 subjects. The authors have analyzed all known 77 single-nucleotide polymorphisms (SNPs) correlated with BMI. The data showed that consumption of fish-derived long-chain ω-3 are able to modulate gene expression related to weight gain and BMI modifications. In fact, long-chain ω-3 PUFAs were able to modify the genetic associations that determine adipose tissue accumulation in various body regions [88]. Therefore, the consumption of long-chain ω-3 PUFAs plays an important role in phenotype manifestation, modulating the expression of weight regulatory genes.
The notion that adipose tissue is simply an inert tissue that stores fat has become obsolete. On the contrary, it is now recognized as a metabolically active endocrine organ, which has the capacity to synthesize biological mediators called adipocytokines, which regulate the body’s metabolic status and influence homeostasis [89]. Adipose tissue is not solely comprised of adipocytes, but also of blood vessels and stroma, which contain the precursor cells. It is also useful to distinguish white adipose tissue (WAT) from brown adipose tissue (BAT). WAT is made up of unilocular adipocytes and is better suited for storage, while BAT adipocytes are multilocular, contain copious amounts of mitochondria and are involved in thermogenesis [90]. Diet-induced thermogenesis is a metabolic process linked with energy expenditure following the ingestion of various macronutrients (such as carbohydrates, proteins, fats and alcohol). A study by Casas-Agustench et al. has examined a population of 29 healthy males and compares the thermogenic effects induced by three isocaloric meals: the first contained high levels of PUFAs from walnuts, the second contained high levels of MUFAs from olive oil and the third contained high levels of fat from dairy products. Thermogenesis induced 5 hours after the first meal was 28% greater than the one induced by the third meal. Therefore, the quality of fats can influence the thermogenic response, even if the properties which influence lipid substrate oxidation are still not known [91].
A further feature of obese subjects is the low-grade chronic inflammatory state. The postulation that obesity is inherently linked to the latter goes hand in hand with the notion that adipose tissue in an obese individual undergoes compelling alterations in both composition and function, a process named “adipose tissue remodeling” [92]. The inflammatory status is characterized by pro-inflammatory molecules such as TNF-α, interleukin (IL)-1β, IL-6, IL-8, transforming growth factor-β, nerve growth factor and acute phase response molecules such as plasminogen activator inhibitor-1, haptoglobin; serum amyloid A, has been recognized as a driver of metabolic disease in obese subjects [93]. Therefore, a reduction of the low-grade chronic inflammatory status, consequent to a decrease of body weight, would lead to an improvement in the clinical conditions of MOSH syndrome.
A study by Lund et al. [94], other than attaining positive results regarding BMI, WC and hip circumference (HC) reduction following PUFA consumption, has highlighted an inverse correlation between ALA ω -3 consumption and levels of macrophage inflammatory protein (MIP)-1α. The latter is a chemokine which is overexpressed in obese subjects who present abdominal visceral fat accumulation. Therefore, PUFAs are able to act beneficially on MIP-1α levels, and therefore on central adiposity.
4.3. PUFA and Metabolic Axis
Long-chain ω-3 PUFAs are able to regulate numerous metabolic mechanisms apt to contrast weight gain. They enable better control of the hunger and satiety mechanism and allow better perfusion of metabolically active tissues (such as skeletal muscle) through the modulation of gene expression. They also induce fatty acid oxidation and can cause an increase in energy expenditure associated with a reduction in fat deposits [95].
Several studies suggest that long-chain ω-3 PUFAs can suppress appetite and regulate thermogenesis by inducing an increase in blood concentration of adipocyte hormones such as leptin and adiponectin [80,96,97] (Table 5). Leptin was the first hormone to be recognized for having a regulatory action at the hypothalamic level [98]. Its principal function is to control food-intake, undertaking an anorexigenic effect, however, it can also regulate energy expenditure and body weight [99]. Leptin acts upon the metabolism and food consumption, reducing appetite and increasing energy expenditure [100]. The expression and release of this hormone are positively correlated with the amount of fat mass and adipocyte dimension, and they are stimulated by hormones such as cortisol and insulin [101].

Table 5.
Studies on PUFAs and metabolic axis.
Different studies report that the reduction in leptin plasma concentration represents a short-term adaptation to the mechanism of hunger or fasting and therefore, in response to diet-induced weight loss, the levels of leptin decrease significantly [102,103]. In normal weight subjects, leptin is released into circulation and acts through hypothalamic and extra-hypothalamic brain receptors (arcuate nucleus and dorsomedial hypothalamus, respectively), inhibiting hunger and increasing thermogenesis following food intake. Moreover, in non-obese subjects, leptin acts through hypothalamic receptors, inhibiting the hunger mechanism and increasing thermogenesis during the fasting period. Decreased leptin levels provoke a reduction in central sympathetic nervous outflow and mobilize stored adipose tissue through glucocorticoid stimulation [104]. Whereas in obese subjects, even if plasma leptin concentration seems to be increased, it does not decrease food consumption and increase energy expenditure. Such a phenomenon suggests that obese subjects become leptin-resistant as reported by different authors since the 1990s [98,104]. The “leptin resistance hypothesis” was demonstrated by Enriori et al. and observed an attenuation of the phosphorylation of signal transducer and activator of transcription 3 (STAT3) in obese mice, which is a crucial factor for the action of leptin on the hypothalamic arcuate nucleus [105].
Hyperleptinemia is also associated with an increased production and release into the bloodstream of pro-inflammatory cytokines (such as TNF-α, C- reactive protein- CRP, etc.) [106,107] and to an increase of platelet aggregation and thrombosis [108]. Thus, the persistent condition of hyperleptinemia could play an unfavorable role in different organs and systems such as the CV system.
A study by Pérez-Matute et al. [109] investigated the potential anti-obesogenic and insulin-sensitizing properties associated with long-chain ω-3 PUFA consumption in an animal model, this was done by feeding the animals two different dietary regimens for the duration of 5 weeks. The control group was administered a standard laboratory diet, whilst the study group was administered a fat-rich hyperenergetic diet. These groups were further divided into two subgroups, differentiated by whether or not they were administered EPA. Results showed that EPA consumption during a fat-rich hyperenergetic diet is able to restrain weight gain and consequently leads to an increase in fat mass. This effect could be correlated with an increase in leptin levels, which causes reduced hunger. Another finding shows that the group consuming a fat-rich hyperenergetic diet and EPA supplementation showed significant weight loss, greater than the standard laboratory diet and EPA supplementation group. It can be speculated that the metabolic effects related to a fat-rich hyperenergetic diet could be correlated to its bromatological composition.
Adiponectin is a protein which regulates the endocrine functions of adipocytes, which perform autocrine and paracrine functions. Adiponectin seems to improve lipid storage, contrasting ectopic deposition of lipids [110] favoring healthy adipose tissue composition. Moreover, it can regulate energy homeostasis by modulating lipid and the glucose metabolism as well as fatty acid oxidation. A study highlighted that adiponectin is able to ameliorate insulin sensitivity in the liver and in skeletal muscles, regulating healthy adipose tissue expansion [111,112]. A study was conducted by Dimiter [113] to investigate the relationship between ω-3 PUFA consumption and circulating adiponectin levels on 35 subjects with metabolic syndrome. The subjects were subdivided into two groups: one was treated with ω-3 PUFA supplements and the control group was given a placebo for a period of three months. The results showed that the treated group demonstrated a statistically significant increase in plasma adiponectin and high-density lipoprotein (HDL) cholesterol, with a concomitant decrease in TGs, Homeostatic model assessment - insulin resistance (HOMA-IR) and CRP. These findings highlighted that supplementation with ω-3 PUFAs can contribute to a bettering of the clinical profile of metabolic syndrome patients by reducing inflammation, improving dyslipidemia and endocrine function through adiponectin-dependent mechanisms.
Long-chain ω-3 PUFAs can alter gene expression in skeletal muscle, suppressing catabolic pathways and upregulating anabolic ones. These mechanisms attenuate muscular mass loss while maintaining muscular functionality and metabolic rate [95]. The restriction of energetic intake results in efficacious fat mass reduction; however, it can often cause the loss of fat-free mass and skeletal muscle. This may negatively impact on physical performance and cause a reduction in metabolic rate by reducing lipid oxidation capacity [114]. The principal pathway responsible for muscle catabolism during energetic intake restriction is the ubiquitin-proteasome pathway [115]. EPA is able to inhibit the activity of such a pathway during periods of severe energy intake restriction. In this context, long-chain ω-3 PUFAs can augment the activation of the Protein kinase B (Akt)—Mammalian target of rapamycin (mTOR)—the Ribosomal protein S6 kinase beta-1 (S6K1) anabolic pathway in skeletal muscle-promoting anti-catabolites and anabolites [116]. In a study by Howe et al. [117], long-chain ω-3 PUFAs were able to attenuate muscle mass loss during an energy restriction diet. Moreover, an improvement of lean mass and energy balance was observed [95,118]. Successively, the same authors have observed an increase in lean mass percentage, suggesting a direct relationship between the consumption of ω-3 PUFAs and lean mass improvement [117].
5. Summary and Future Perspectives
In conclusion, there seems to be evidence that ω-3 PUFA consumption may be clinically beneficial in the treatment and clinical management of MOSH patients.
The ability of ω-3 PUFAs to act on some pathological aspects of MOSH, such as obesity, inflammation, metabolic and cardiovascular disorders, coupled with their optimal safety profile, leads us to the postulation that ω-3 PUFA assumption could be a valuable tool in ameliorating the clinical manifestations of MOSH syndrome.
In pursuance to assess this conclusion in a definitive manner and in order to define the most advantageous dosage, randomized clinical trials on a large population sample are required. Moreover, it would be interesting and useful to conduct experimental studies exploring the possible effects of ω-3 PUFA consumption on hormone profile, on the sexual sphere (T concentrations) and on body composition.
Author Contributions
Conceptualization, A.N. and N.D.D.; writing—original draft preparation, A.N., G.M., F.D.D., M.D.L., A.P.Z. and G.W.J.; writing—review and editing, N.D.D. and A.D.L.; supervision, N.D.D. and A.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviation List
| AA | Arachidonic acid |
| AHA | American Heart Association |
| Akt | Protein kinase B |
| ALA | α-linoleic acid |
| AMI | Myocardial infarction |
| BAT | Brown adipose tissue |
| BMI | Body mass index |
| CAD | Caspase-activated DNase |
| CAT | Catalase |
| CNCD | Chronic non-communicable degenerative disease |
| CRP | C-reactive protein |
| CV | Cardiovascular |
| CVD | Cardiovascular disease |
| DART | Diet And Reinfarction Trial |
| DGLA | Dihomo-γ-linoleic acid |
| DHA | Docosahexaenoic acid |
| DPA | Docosapentaenoic acid |
| eNOS | Endothelial nitric oxide synthase |
| EPA | Eicosapentaenoic acid |
| FAs | Fatty acids |
| FM | Fat mass |
| FSH | Follicle-stimulating hormone |
| Gal-3 | Galectin-3 |
| GISSI | Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto |
| GLA | γ-linoleic acid |
| GnRH | Gonadotropin-Releasing Hormone |
| HC | Hip circumference |
| HDL | High-density lipoprotein |
| HOMA-IR | Homeostatic model assessment-insulin resistance |
| HT | High triglyceride |
| IL | Interleukin |
| LA | Linoleic acid |
| LDL | Low-density lipoprotein |
| LH | Luteinizing hormone |
| LTS | Leucotriens |
| LXS | Lipoxines |
| MIP | Macrophage inflammatory protein |
| MOSH | Male obesity secondary hypogonadism |
| mTOR | Mammalian target of rapamycin |
| MUFAs | Monounsaturated fatty acids |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| PG | Prostaglandins |
| PPARα | Peroxisome proliferator- activated receptor α |
| PUFA | Polyunsaturated fatty acid |
| S6K1 | Ribosomal protein S6 kinase beta-1 |
| SFAs | Saturated fatty acids |
| SNPs | Single-nucleotide polymorphism |
| SOD | Superoxide dismutase |
| STAT3 | Signal transducer and activator of transcription 3 |
| T | Testosterone |
| T2DM | Type 2 diabetes mellitus |
| TG | Triglycerides |
| TNF-α | Tumor necrosis factor- α |
| Tx | Thromboxanes |
| VCAM-1 | Vascular cell adhesion molecule-1 |
| VHT | Very high triglyceride |
| WAT | White adipose tissue |
| WC | Waist circumference |
| WHO | World Health Organization |
References
- Barnes, S. Nutritional genomics, polyphenols, diets, and their impact on dietetics. J. Am. Diet. Assoc. 2008, 108, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.H. Omega-3 polyunsaturated Fatty acids and heart rate variability. Front. Physiol. 2011, 2, 84. [Google Scholar] [CrossRef] [PubMed]
- Raphael, W.; Sordillo, L.M. Dietary polyunsaturated fatty acids and inflammation: The role of phospholipid biosynthesis. Int. J. Mol. Sci. 2013, 14, 21167–21188. [Google Scholar] [CrossRef] [PubMed]
- Bender, N.; Portmann, M.; Heg, Z.; Hofmann, K.; Zwahlen, M.; Egger, M. Fish or n3-PUFA intake and body composition: A systematic review and meta-analysis. Obes. Rev. 2014, 15, 657–665. [Google Scholar] [CrossRef]
- Ofei, F. Obesity—A preventable disease. Ghana. Med. J. 2005, 39, 98–101. [Google Scholar]
- de Mello, A.H.; Uberti, M.F.; de Farias, B.X.; de Souza, N.A.R.; Rezin, G.T. n-3 PUFA and obesity: From peripheral tissues to the central nervous system. Br. J. Nutr. 2018, 119, 1312–1323. [Google Scholar] [CrossRef]
- Obesity, W. Obesity Classification. Available online: https://www.worldobesity.org/about/about-obesity/obesity-classification (accessed on 1 June 2020).
- Cohen, D.A. Obesity and the built environment: Changes in environmental cues cause energy imbalances. Int. J. Obes. 2008, 32 (Suppl. 7), S137–S142. [Google Scholar] [CrossRef]
- Obesity, W. Prevalence of Obesity. Available online: https://www.worldobesity.org/about/about-obesity/prevalence-of-obesity (accessed on 3 April 2020).
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef]
- Knight, J.A. Diseases and disorders associated with excess body weight. Ann. Clin. Lab. Sci. 2011, 41, 107–121. [Google Scholar]
- Kyrou, I.; Randeva, H.S.; Tsigos, C.; Kaltsas, G.; Weickert, M.O. Clinical Problems Caused by Obesity. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Grossman, A., Hershman, J.M., Kaltsas, G., Koch, C., Kopp, P., et al., Eds.; Endotext.com: South Dartmouth, MA, USA, 2000. [Google Scholar]
- World Health Organization. Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 1 June 2020).
- Di Daniele, N.; Noce, A.; Vidiri, M.F.; Moriconi, E.; Marrone, G.; Annicchiarico-Petruzzelli, M.; D’Urso, G.; Tesauro, M.; Rovella, V.; De Lorenzo, A. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget 2017, 8, 8947–8979. [Google Scholar] [CrossRef]
- Canale, M.P.; Manca di Villahermosa, S.; Martino, G.; Rovella, V.; Noce, A.; De Lorenzo, A.; Di Daniele, N. Obesity-related metabolic syndrome: Mechanisms of sympathetic overactivity. Int. J. Endocrinol. 2013, 2013, 865965. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, A.; Noce, A.; Moriconi, E.; Rampello, T.; Marrone, G.; Di Daniele, N.; Rovella, V. MOSH Syndrome (Male Obesity Secondary Hypogonadism): Clinical Assessment and Possible Therapeutic Approaches. Nutrients 2018, 10, 474. [Google Scholar] [CrossRef] [PubMed]
- Calderon, B.; Gomez-Martin, J.M.; Vega-Pinero, B.; Martin-Hidalgo, A.; Galindo, J.; Luque-Ramirez, M.; Escobar-Morreale, H.F.; Botella-Carretero, J.I. Prevalence of male secondary hypogonadism in moderate to severe obesity and its relationship with insulin resistance and excess body weight. Andrology 2016, 4, 62–67. [Google Scholar] [CrossRef]
- Anderson, J.L.; May, H.T.; Lappe, D.L.; Bair, T.; Le, V.; Carlquist, J.F.; Muhlestein, J.B. Impact of Testosterone Replacement Therapy on Myocardial Infarction, Stroke, and Death in Men With Low Testosterone Concentrations in an Integrated Health Care System. Am. J. Cardiol. 2016, 117, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Wiktorowska-Owczarek, A.; Berezinska, M.; Nowak, J.Z. PUFAs: Structures, Metabolism and Functions. Adv. Clin. Exp. Med. 2015, 24, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Das, U.N. Essential Fatty acids—A review. Curr. Pharm. Biotechnol. 2006, 7, 467–482. [Google Scholar] [CrossRef]
- Tvrzicka, E.; Kremmyda, L.S.; Stankova, B.; Zak, A. Fatty acids as biocompounds: Their role in human metabolism, health and disease--a review. Part 1: Classification, dietary sources and biological functions. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech. Repub. 2011, 155, 117–130. [Google Scholar] [CrossRef]
- Adkins, Y.; Kelley, D.S. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J. Nutr. Biochem. 2010, 21, 781–792. [Google Scholar] [CrossRef]
- Dessi, M.; Noce, A.; Bertucci, P.; Noce, G.; Rizza, S.; De Stefano, A.; Manca di Villahermosa, S.; Bernardini, S.; De Lorenzo, A.; Di Daniele, N. Plasma and erythrocyte membrane phospholipids and fatty acids in Italian general population and hemodialysis patients. Lipids Health Dis. 2014, 13, 54. [Google Scholar] [CrossRef]
- Yang, L.G.; Song, Z.X.; Yin, H.; Wang, Y.Y.; Shu, G.F.; Lu, H.X.; Wang, S.K.; Sun, G.J. Low n-6/n-3 PUFA Ratio Improves Lipid Metabolism, Inflammation, Oxidative Stress and Endothelial Function in Rats Using Plant Oils as n-3 Fatty Acid Source. Lipids 2016, 51, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, F.; Li, L.; Fan, J.; Sun, X.; Yin, Y. n-6:n-3 PUFA ratio is involved in regulating lipid metabolism and inflammation in pigs. Br. J. Nutr. 2014, 111, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed. Pharmacother. 2006, 60, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.A.; Riccioni, G.; Parrinello, G.; D’Orazio, N. Omega-3 Polyunsaturated Fatty Acids: Benefits and Endpoints in Sport. Nutrients 2018, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Merida-Ortega, A.; Rothenberg, S.J.; Torres-Sanchez, L.; Schnaas, L.; Hernandez-Alcaraz, C.; Cebrian, M.E.; Garcia-Hernandez, R.M.; Ogaz-Gonzalez, R.; Lopez-Carrillo, L. Polyunsaturated fatty acids and child neurodevelopment among a population exposed to DDT: A cohort study. Environ. Health 2019, 18, 17. [Google Scholar] [CrossRef]
- Liu, W.; Xie, X.; Liu, M.; Zhang, J.; Liang, W.; Chen, X. Serum omega-3 Polyunsaturated Fatty Acids and Potential Influence Factors in Elderly Patients with Multiple Cardiovascular Risk Factors. Sci. Rep. 2018, 8, 1102. [Google Scholar] [CrossRef]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef]
- Fernandez, C.J.; Chacko, E.C.; Pappachan, J.M. Male Obesity-related Secondary Hypogonadism—Pathophysiology, Clinical Implications and Management. Eur. Endocrinol. 2019, 15, 83–90. [Google Scholar] [CrossRef]
- Kelly, D.M.; Jones, T.H. Testosterone: A metabolic hormone in health and disease. J. Endocrinol. 2013, 217, R25–R45. [Google Scholar] [CrossRef]
- Clarke, H.; Dhillo, W.S.; Jayasena, C.N. Comprehensive Review on Kisspeptin and Its Role in Reproductive Disorders. Endocrinol. Metab. 2015, 30, 124–141. [Google Scholar] [CrossRef]
- Roseweir, A.K.; Millar, R.P. The role of kisspeptin in the control of gonadotrophin secretion. Hum. Reprod. Update 2009, 15, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Herbst, K.L.; Bhasin, S. Testosterone action on skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Giagulli, V.A.; Castellana, M.; Murro, I.; Pelusi, C.; Guastamacchia, E.; Triggiani, V.; De Pergola, G. The Role of Diet and Weight Loss in Improving Secondary Hypogonadism in Men with Obesity with or without Type 2 Diabetes Mellitus. Nutrients 2019, 11, 2975. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Gualtieri, P.; Romano, L.; Marrone, G.; Noce, A.; Pujia, A.; Perrone, M.A.; Aiello, V.; Colica, C.; De Lorenzo, A. Role of Personalized Nutrition in Chronic-Degenerative Diseases. Nutrients 2019, 11, 1707. [Google Scholar] [CrossRef] [PubMed]
- Denke, M.A. Diet, lifestyle, and nonstatin trials: Review of time to benefit. Am. J. Cardiol. 2005, 96, 3F–10F. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.S.; Martin, N.; Bridges, C.; Brainard, J.S.; Wang, X.; Brown, T.J.; Hanson, S.; Jimoh, O.F.; Ajabnoor, S.M.; Deane, K.H.; et al. Polyunsaturated fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 7, CD012345. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Warnakula, S.; Kunutsor, S.; Crowe, F.; Ward, H.A.; Johnson, L.; Franco, O.H.; Butterworth, A.S.; Forouhi, N.G.; Thompson, S.G.; et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 398–406. [Google Scholar] [CrossRef]
- Rhee, J.J.; Kim, E.; Buring, J.E.; Kurth, T. Fish Consumption, Omega-3 Fatty Acids, and Risk of Cardiovascular Disease. Am. J. Prev. Med. 2017, 52, 10–19. [Google Scholar] [CrossRef]
- Siscovick, D.S.; Barringer, T.A.; Fretts, A.M.; Wu, J.H.; Lichtenstein, A.H.; Costello, R.B.; Kris-Etherton, P.M.; Jacobson, T.A.; Engler, M.B.; Alger, H.M.; et al. Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation 2017, 135, e867–e884. [Google Scholar] [CrossRef]
- Wilber, C.G.; Levine, V.E. Fat metabolism in Alaskan eskimos. Exp. Med. Surg. 1950, 8, 422–425. [Google Scholar]
- Kagawa, Y.; Nishizawa, M.; Suzuki, M.; Miyatake, T.; Hamamoto, T.; Goto, K.; Motonaga, E.; Izumikawa, H.; Hirata, H.; Ebihara, A. Eicosapolyenoic acids of serum lipids of Japanese islanders with low incidence of cardiovascular diseases. J. Nutr. Sci. Vitaminol. 1982, 28, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Bang, H.O.; Dyerberg, J.; Sinclair, H.M. The composition of the Eskimo food in north western Greenland. Am. J. Clin. Nutr. 1980, 33, 2657–2661. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.; Chopra, S.; Jacob, J.J. A fish a day, keeps the cardiologist away!—A review of the effect of omega-3 fatty acids in the cardiovascular system. Indian. J. Endocrinol. Metab. 2013, 17, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Burr, M.L.; Fehily, A.M.; Gilbert, J.F.; Rogers, S.; Holliday, R.M.; Sweetnam, P.M.; Elwood, P.C.; Deadman, N.M. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: Diet and reinfarction trial (DART). Lancet 1989, 2, 757–761. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J.; American Heart Association; Nutrition, C. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef]
- Marchioli, R.; Schweiger, C.; Tavazzi, L.; Valagussa, F. Efficacy of n-3 polyunsaturated fatty acids after myocardial infarction: Results of GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico. Lipids 2001, 36 (Suppl. 1), S119–S126. [Google Scholar] [CrossRef]
- GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet 1999, 354, 447–455. [Google Scholar] [CrossRef]
- Tavazzi, L.; Maggioni, A.P.; Marchioli, R.; Barlera, S.; Franzosi, M.G.; Latini, R.; Lucci, D.; Nicolosi, G.L.; Porcu, M.; Tognoni, G.; et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1223–1230. [Google Scholar] [CrossRef]
- Ness, A.R.; Hughes, J.; Elwood, P.C.; Whitley, E.; Smith, G.D.; Burr, M.L. The long-term effect of dietary advice in men with coronary disease: Follow-up of the Diet and Reinfarction trial (DART). Eur. J. Clin. Nutr. 2002, 56, 512–518. [Google Scholar] [CrossRef]
- Zehr, K.R.; Walker, M.K. Omega-3 polyunsaturated fatty acids improve endothelial function in humans at risk for atherosclerosis: A review. Prostaglandins Lipid. Mediat. 2018, 134, 131–140. [Google Scholar] [CrossRef]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Polyunsaturated fatty acids and inflammation. IUBMB Life 2015, 67, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Montagnani, M.; Funahashi, T.; Shimomura, I.; Quon, M.J. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J. Biol. Chem. 2003, 278, 45021–45026. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Stone, N.J.; Ballantyne, C.; Bittner, V.; Criqui, M.H.; Ginsberg, H.N.; Goldberg, A.C.; Howard, W.J.; Jacobson, M.S.; Kris-Etherton, P.M.; et al. Triglycerides and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2011, 123, 2292–2333. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Bulchandani, D. Why do omega-3 fatty acids lower serum triglycerides? Curr. Opin. Lipidol. 2006, 17, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Ballantyne, C.M.; Kastelein, J.J.; Isaacsohn, J.L.; Braeckman, R.A.; Soni, P.N. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] trial). Am. J. Cardiol. 2011, 108, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Kastelein, J.J.; Maki, K.C.; Susekov, A.; Ezhov, M.; Nordestgaard, B.G.; Machielse, B.N.; Kling, D.; Davidson, M.H. Omega-3 free fatty acids for the treatment of severe hypertriglyceridemia: The EpanoVa fOr Lowering Very high triglyceridEs (EVOLVE) trial. J. Clin. Lipidol. 2014, 8, 94–106. [Google Scholar] [CrossRef]
- Maki, K.C.; Orloff, D.G.; Nicholls, S.J.; Dunbar, R.L.; Roth, E.M.; Curcio, D.; Johnson, J.; Kling, D.; Davidson, M.H. A highly bioavailable omega-3 free fatty acid formulation improves the cardiovascular risk profile in high-risk, statin-treated patients with residual hypertriglyceridemia (the ESPRIT trial). Clin. Ther. 2013, 35, 1400–1411.e3. [Google Scholar] [CrossRef]
- Balakumar, P.; Taneja, G. Fish oil and vascular endothelial protection: Bench to bedside. Free Radic. Biol. Med. 2012, 53, 271–279. [Google Scholar] [CrossRef]
- Wang, T.M.; Chen, C.J.; Lee, T.S.; Chao, H.Y.; Wu, W.H.; Hsieh, S.C.; Sheu, H.H.; Chiang, A.N. Docosahexaenoic acid attenuates VCAM-1 expression and NF-kappaB activation in TNF-alpha-treated human aortic endothelial cells. J. Nutr. Biochem. 2011, 22, 187–194. [Google Scholar] [CrossRef]
- Dyerberg, J.; Bang, H.O.; Stoffersen, E.; Moncada, S.; Vane, J.R. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet 1978, 2, 117–119. [Google Scholar] [CrossRef]
- Haglund, O.; Mehta, J.L.; Saldeen, T. Effects of fish oil on some parameters of fibrinolysis and lipoprotein(a) in healthy subjects. Am. J. Cardiol. 1994, 74, 189–192. [Google Scholar] [CrossRef]
- DeFilippis, A.P.; Rai, S.N.; Cambon, A.; Miles, R.J.; Jaffe, A.S.; Moser, A.B.; Jones, R.O.; Bolli, R.; Schulman, S.P. Fatty acids and TxA(2) generation, in the absence of platelet-COX-1 activity. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 428–433. [Google Scholar] [CrossRef]
- Matsumoto, M.; Sata, M.; Fukuda, D.; Tanaka, K.; Soma, M.; Hirata, Y.; Nagai, R. Orally administered eicosapentaenoic acid reduces and stabilizes atherosclerotic lesions in ApoE-deficient mice. Atherosclerosis 2008, 197, 524–533. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef]
- Egert, S.; Stehle, P. Impact of n-3 fatty acids on endothelial function: Results from human interventions studies. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.A.; Medeiros, F.; Trindade, M.; Cohen, C.; Oigman, W.; Neves, M.F. Omega-3 fatty acids supplementation improves endothelial function and arterial stiffness in hypertensive patients with hypertriglyceridemia and high cardiovascular risk. J. Am. Soc. Hypertens. 2017, 11, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Del-Rio-Navarro, B.E.; Leija-Martinez, J.; Torres-Alcantara, S.; Ruiz-Bedolla, E.; Hernandez-Cadena, L.; Barraza-Villarreal, A.; Romero-Nava, R.; Sanchez-Munoz, F.; Villafana, S.; et al. Effect of omega-3 fatty acids supplementation combined with lifestyle intervention on adipokines and biomarkers of endothelial dysfunction in obese adolescents with hypertriglyceridemia. J. Nutr. Biochem. 2019, 64, 162–169. [Google Scholar] [CrossRef]
- Rantanen, J.M.; Riahi, S.; Johansen, M.B.; Schmidt, E.B.; Christensen, J.H. Effects of Marine n-3 Polyunsaturated Fatty Acids on Heart Rate Variability and Heart Rate in Patients on Chronic Dialysis: A Randomized Controlled Trial. Nutrients 2018, 10, 1313. [Google Scholar] [CrossRef]
- Hidayat, K.; Yang, J.; Zhang, Z.; Chen, G.C.; Qin, L.Q.; Eggersdorfer, M.; Zhang, W. Effect of omega-3 long-chain polyunsaturated fatty acid supplementation on heart rate: A meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2018, 72, 805–817. [Google Scholar] [CrossRef]
- Sagara, M.; Njelekela, M.; Teramoto, T.; Taguchi, T.; Mori, M.; Armitage, L.; Birt, N.; Birt, C.; Yamori, Y. Effects of docosahexaenoic Acid supplementation on blood pressure, heart rate, and serum lipids in Scottish men with hypertension and hypercholesterolemia. Int. J. Hypertens. 2011, 2011, 809198. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Notay, K.; Klingel, S.L.; Chabowski, A.; Mutch, D.M.; Millar, P.J. Docosahexaenoic acid reduces resting blood pressure but increases muscle sympathetic outflow compared with eicosapentaenoic acid in healthy men and women. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H873–H881. [Google Scholar] [CrossRef] [PubMed]
- Ghio, S.; Scelsi, L.; Latini, R.; Masson, S.; Eleuteri, E.; Palvarini, M.; Vriz, O.; Pasotti, M.; Gorini, M.; Marchioli, R.; et al. Effects of n-3 polyunsaturated fatty acids and of rosuvastatin on left ventricular function in chronic heart failure: A substudy of GISSI-HF trial. Eur. J. Heart Fail 2010, 12, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Heydari, B.; Abdullah, S.; Pottala, J.V.; Shah, R.; Abbasi, S.; Mandry, D.; Francis, S.A.; Lumish, H.; Ghoshhajra, B.B.; Hoffmann, U.; et al. Effect of Omega-3 Acid Ethyl Esters on Left Ventricular Remodeling After Acute Myocardial Infarction: The OMEGA-REMODEL Randomized Clinical Trial. Circulation 2016, 134, 378–391. [Google Scholar] [CrossRef]
- Laake, K.; Seljeflot, I.; Schmidt, E.B.; Myhre, P.; Tveit, A.; Norseth, J.; Arnesen, H.; Solheim, S. Galectin-3, a marker of cardiac remodeling, is inversely related to serum levels of marine omega-3 fatty acids. A cross-sectional study. JRSM Cardiovasc. Dis. 2017, 6, 2048004017729984. [Google Scholar] [CrossRef]
- Takahashi, Y.; Ide, T. Dietary n-3 fatty acids affect mRNA level of brown adipose tissue uncoupling protein 1, and white adipose tissue leptin and glucose transporter 4 in the rat. Br. J. Nutr. 2000, 84, 175–184. [Google Scholar] [CrossRef]
- Clarke, S.D. Polyunsaturated fatty acid regulation of gene transcription: A mechanism to improve energy balance and insulin resistance. Br. J. Nutr. 2000, 83 (Suppl. 1), S59–S66. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Valli, V.; Bordoni, A. Pro- and anti-oxidant effects of polyunsaturated fatty acid supplementation in HepG2 cells. Prostaglandins Leukot. Essent. Fatty Acids 2011, 85, 121–127. [Google Scholar] [CrossRef]
- CREA. Linee Guida per Una Sana Alimentazione. Available online: https://www.crea.gov.it/web/alimenti-e-nutrizione/-/linee-guida-per-una-sana-alimentazione-2018 (accessed on 15 June 2020).
- Summers, L.K.; Fielding, B.A.; Bradshaw, H.A.; Ilic, V.; Beysen, C.; Clark, M.L.; Moore, N.R.; Frayn, K.N. Substituting dietary saturated fat with polyunsaturated fat changes abdominal fat distribution and improves insulin sensitivity. Diabetologia 2002, 45, 369–377. [Google Scholar] [CrossRef]
- Couet, C.; Delarue, J.; Ritz, P.; Antoine, J.M.; Lamisse, F. Effect of dietary fish oil on body fat mass and basal fat oxidation in healthy adults. Int. J. Obes. Relat. Metab. Disord 1997, 21, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Wang, T.; Heianza, Y.; Zheng, Y.; Sun, D.; Kang, J.H.; Pasquale, L.R.; Rimm, E.B.; Manson, J.E.; Hu, F.B.; et al. Habitual consumption of long-chain n-3 PUFAs and fish attenuates genetically associated long-term weight gain. Am. J. Clin. Nutr. 2019, 109, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, L.K.; Wiener, H.W.; Aslibekyan, S.; Allison, D.B.; Havel, P.J.; Stanhope, K.L.; O’Brien, D.M.; Hopkins, S.E.; Lemas, D.J.; Boyer, B.B.; et al. Linkage and association analysis of obesity traits reveals novel loci and interactions with dietary n-3 fatty acids in an Alaska Native (Yup’ik) population. Metabolism 2015, 64, 689–697. [Google Scholar] [CrossRef]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013, 9, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Glazier, B.J.; Hinkel, B.C.; Cao, J.; Liu, L.; Liang, C.; Shi, H. Neuroendocrine Regulation of Energy Metabolism Involving Different Types of Adipose Tissues. Int. J. Mol. Sci. 2019, 20, 2707. [Google Scholar] [CrossRef] [PubMed]
- Casas-Agustench, P.; Lopez-Uriarte, P.; Bullo, M.; Ros, E.; Gomez-Flores, A.; Salas-Salvado, J. Acute effects of three high-fat meals with different fat saturations on energy expenditure, substrate oxidation and satiety. Clin. Nutr. 2009, 28, 39–45. [Google Scholar] [CrossRef]
- Itoh, M.; Suganami, T.; Hachiya, R.; Ogawa, Y. Adipose tissue remodeling as homeostatic inflammation. Int. J. Inflam. 2011, 2011, 720926. [Google Scholar] [CrossRef]
- Fuentes, E.; Fuentes, F.; Vilahur, G.; Badimon, L.; Palomo, I. Mechanisms of chronic state of inflammation as mediators that link obese adipose tissue and metabolic syndrome. Mediators. Inflamm. 2013, 2013, 136584. [Google Scholar] [CrossRef]
- Lund, A.S.; Hasselbalch, A.L.; Gamborg, M.; Skogstrand, K.; Hougaard, D.M.; Heitmann, B.L.; Kyvik, K.O.; Sorensen, T.I.; Jess, T. N-3 polyunsaturated fatty acids, body fat and inflammation. Obes. Facts 2013, 6, 369–379. [Google Scholar] [CrossRef]
- Howe, P.; Buckley, J. Metabolic health benefits of long-chain omega-3 polyunsaturated fatty acids. Mil. Med. 2014, 179, 138–143. [Google Scholar] [CrossRef]
- Gray, B.; Steyn, F.; Davies, P.S.; Vitetta, L. Omega-3 fatty acids: A review of the effects on adiponectin and leptin and potential implications for obesity management. Eur. J. Clin. Nutr. 2013, 67, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Parra, D.; Ramel, A.; Bandarra, N.; Kiely, M.; Martinez, J.A.; Thorsdottir, I. A diet rich in long chain omega-3 fatty acids modulates satiety in overweight and obese volunteers during weight loss. Appetite 2008, 51, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770. [Google Scholar] [CrossRef]
- Halaas, J.L.; Gajiwala, K.S.; Maffei, M.; Cohen, S.L.; Chait, B.T.; Rabinowitz, D.; Lallone, R.L.; Burley, S.K.; Friedman, J.M. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 1995, 269, 543–546. [Google Scholar] [CrossRef]
- Auwerx, J.; Staels, B. Leptin. Lancet 1998, 351, 737–742. [Google Scholar] [CrossRef]
- Houseknecht, K.L.; Baile, C.A.; Matteri, R.L.; Spurlock, M.E. The biology of leptin: A review. J. Anim. Sci. 1998, 76, 1405–1420. [Google Scholar] [CrossRef]
- Cameron, A.J.; Welborn, T.A.; Zimmet, P.Z.; Dunstan, D.W.; Owen, N.; Salmon, J.; Dalton, M.; Jolley, D.; Shaw, J.E. Overweight and obesity in Australia: The 1999-2000 Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Med. J. Aust. 2003, 178, 427–432. [Google Scholar] [CrossRef]
- Madsen, E.L.; Bruun, J.M.; Skogstrand, K.; Hougaard, D.M.; Christiansen, T.; Richelsen, B. Long-term weight loss decreases the nontraditional cardiovascular risk factors interleukin-18 and matrix metalloproteinase-9 in obese subjects. Metabolism 2009, 58, 946–953. [Google Scholar] [CrossRef]
- Legradi, G.; Emerson, C.H.; Ahima, R.S.; Flier, J.S.; Lechan, R.M. Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology 1997, 138, 2569–2576. [Google Scholar] [CrossRef]
- Enriori, P.J.; Sinnayah, P.; Simonds, S.E.; Garcia Rudaz, C.; Cowley, M.A. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J. Neurosci. 2011, 31, 12189–12197. [Google Scholar] [CrossRef]
- Finck, B.N.; Johnson, R.W. Tumor necrosis factor-alpha regulates secretion of the adipocyte-derived cytokine, leptin. Microsc. Res. Tech. 2000, 50, 209–215. [Google Scholar] [CrossRef]
- Shamsuzzaman, A.S.; Winnicki, M.; Wolk, R.; Svatikova, A.; Phillips, B.G.; Davison, D.E.; Berger, P.B.; Somers, V.K. Independent association between plasma leptin and C-reactive protein in healthy humans. Circulation 2004, 109, 2181–2185. [Google Scholar] [CrossRef] [PubMed]
- Corsonello, A.; Perticone, F.; Malara, A.; De Domenico, D.; Loddo, S.; Buemi, M.; Ientile, R.; Corica, F. Leptin-dependent platelet aggregation in healthy, overweight and obese subjects. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Perez-Matute, P.; Perez-Echarri, N.; Martinez, J.A.; Marti, A.; Moreno-Aliaga, M.J. Eicosapentaenoic acid actions on adiposity and insulin resistance in control and high-fat-fed rats: Role of apoptosis, adiponectin and tumour necrosis factor-alpha. Br. J. Nutr. 2007, 97, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Wang, Y.; Keshaw, H.; Xu, L.Y.; Lam, K.S.; Cooper, G.J. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J. Clin. Invest. 2003, 112, 91–100. [Google Scholar] [CrossRef]
- Berg, A.H.; Combs, T.P.; Du, X.; Brownlee, M.; Scherer, P.E. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 2001, 7, 947–953. [Google Scholar] [CrossRef]
- Dadson, K.; Liu, Y.; Sweeney, G. Adiponectin action: A combination of endocrine and autocrine/paracrine effects. Front. Endocrinol. 2011, 2, 62. [Google Scholar] [CrossRef]
- Dimiter, D. Effect of omega-3 fatty acids on plasma adiponectin levels in Metabolic syndrome subjects. Inter. J. Obes. 2007, 31. [Google Scholar]
- Whitehouse, A.S.; Tisdale, M.J. Downregulation of ubiquitin-dependent proteolysis by eicosapentaenoic acid in acute starvation. Biochem. Biophys. Res. Commun. 2001, 285, 598–602. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Rovella, V.; Cusijmano, A.; Di Daniele, N. Beneficial effects of physical activity on uremic sarcopenia. Med. Dello Sport 2018. [Google Scholar] [CrossRef]
- Andrade-Vieira, R.; Han, J.H.; Marignani, P.A. Omega-3 polyunsaturated fatty acid promotes the inhibition of glycolytic enzymes and mTOR signaling by regulating the tumor suppressor LKB1. Cancer Biol. Ther. 2013, 14, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Howe, P.; Coates, A.; Murphy, K.; Pettman, T.; Milte, C.; Buckley, J. Higher erythrocyte LCn-3 PUFA content is associated with a healthier body composition. Australas. Med. J. 2014, 1, 60. [Google Scholar]
- Wing, S.S.; Goldberg, A.L. Glucocorticoids activate the ATP-ubiquitin-dependent proteolytic system in skeletal muscle during fasting. Am. J. Physiol. 1993, 264, E668–E676. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).










