Diet, Stress and Mental Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Effects of Diet on Health
2.2. The Influence of Diet on Mood and Psychiatric Disorders
2.3. Diet Interventions for Depression
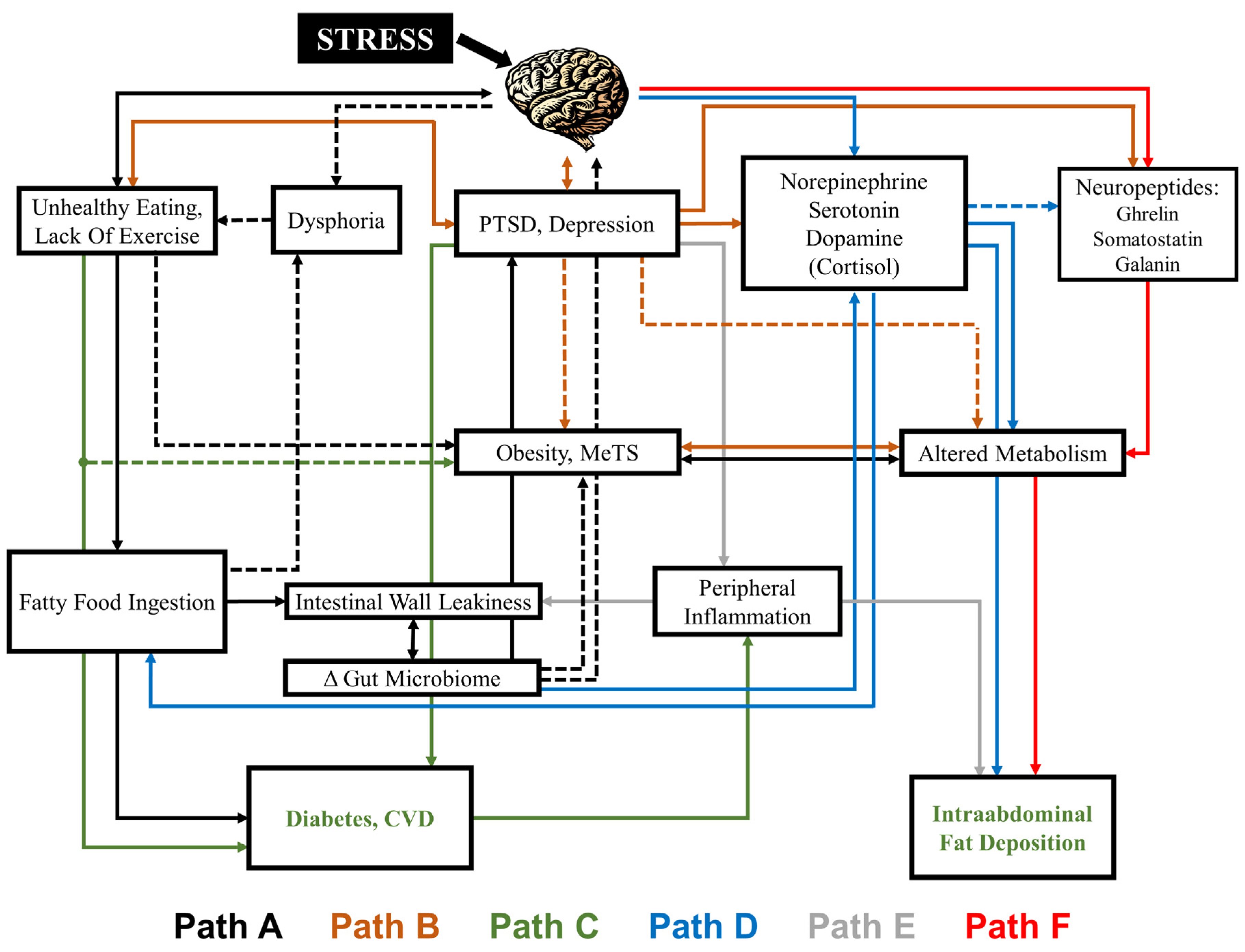
2.4. Neurotransmitters and Neuropeptides Affected by Diet, Obesity and Psychiatric Disorders Related to Stress
2.5. The Gut-Brain Connection
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean diet and health status: A meta-analysis. BMJ 2008, 337, a1344. [Google Scholar] [CrossRef] [PubMed]
- Perk, J.; De Backer, G.; Gohlke, H.; Graham, I.; Reiner, Z.; Verschuren, M.; Albus, C.; Benlian, P.; Boysen, G.; Cifkova, R.; et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2012, 33, 1635–1701. [Google Scholar] [CrossRef] [PubMed]
- Vaccarino, V.; Bremner, J.D. Behavioral, emotional and neurobiological determinants of coronary heart disease risk in women. Neurosci. Biobehav. Rev. 2017, 74, 297–309. [Google Scholar] [CrossRef]
- Vaccarino, V.; Goldberg, J.; Rooks, C.; Shah, A.J.; Veledar, E.; Faber, T.L.; Votaw, J.R.; Forsberg, C.W.; Bremner, J.D. Post-traumatic stress disorder and incidence of coronary heart disease: A twin study. J. Am. Coll. Cardiol. 2013, 62, 97–978. [Google Scholar] [CrossRef] [PubMed]
- Vaccarino, V.; Votaw, J.; Faber, T.; Veledar, E.; Murrah, N.V.; Jones, L.R.; Zhao, J.; Su, S.; Goldberg, J.; Raggi, J.P.; et al. Major depression and coronary flow reserve detected by positron emission tomography. Arch. Intern. Med. 2009, 169, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- Carney, R.M.; Freedland, K.E. Depression and coronary heart disease. Nat. Rev. Cardiol. 2017, 14, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Penninx, B.W. Depression and cardiovascular disease: Epidemiological evidence on their linking mechanisms. Neurosci. Biobehav. Rev. 2017, 74, 277–286. [Google Scholar] [CrossRef]
- Freedland, K.E.; Carney, R.M. Depression as a risk factor for adverse outcomes in coronary heart disease. BMC Med. 2013, 11, 131. [Google Scholar] [CrossRef]
- Vaccarino, V.; Mayer, E.; Bremner, J.D. Stress and Health. In Posttraumatic Stress Disorder: From Neurobiology to Treatment; Bremner, J.D., Ed.; Wiley-Blackwell Press: Hoboken, NJ, USA, 2016. [Google Scholar]
- Vaccarino, V.; Bremner, J.D. Psychiatric and behavioral aspects of cardiovascular disease. In Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine; Zipes, D.P., Libby, P., Bonow, R.O., Mann, D.L., Tomaselli, G.F., Eds.; Elsevier-Saunders: Philadelphia, PA, USA, 2018; pp. 1880–1889. [Google Scholar]
- Freeman, M.P.; Rapaport, M.H. Omega-3 fatty acids and depression: From cellular mechanisms to clinical care. J. Clin. Psychiatry 2011, 72, 258–259. [Google Scholar] [CrossRef]
- Williamson, D.F.; Thompson, T.J.; Anda, R.F.; Dietz, W.H.; Felitti, V. Body weight and obesity in adulthood and self-reported abuse in childhood. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 1075–1082. [Google Scholar] [CrossRef]
- Fowler, N.; Vo, P.T.; Sisk, C.L.; Klump, K.L. Stress as a potential moderator of ovarian hormone influences on binge eating in women. F1000Research 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Moazzami, K.; Lima, B.B.; Sullivan, S.; Shah, A.; Bremner, J.D.; Vaccarino, V. Independent and joint association of obesity and metabolic syndrome with depression and inflammation. Health Psychol. 2019, 38, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.D. (Ed.) Posttraumatic Stress Disorder: From Neurobiology to Treatment, 1st ed.; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Valassi, E.; Schacchi, M.; Cavagnini, F. Neuroendocrine control of food intake. Nutr. Metab. Cardiovasc. Dis. 2007, 18, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.D. Obesity linked to smaller cerebral volume: What should we make of this? Psychosom. Med. 2009, 71, 483–484. [Google Scholar] [CrossRef]
- Mayer, E. The Mind-Gut Connection: How the Hidden Conversation Within Our Bodies Affects Our Mood, Our Choices, and Our Overall Health; HarperCollins: New York, NY, USA, 2016. [Google Scholar]
- American Psychiatric Association. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Ladabaum, U.; Mannalithara, A.; Myer, P.A.; Singh, G. Obesity, abdominal obesity, physical activity, and caloric intake in US adults: 1988 to 2010. Am. J. Med. 2014, 127, 717–727.e12. [Google Scholar] [CrossRef]
- Flegal, K.M.; Carroll, M.D.; Ogden, C.L.; Johnson, C.L. Prevalence and trends in obesity among US adults, 1999–2000. J. Am. Med. Assoc. 2002, 288, 1723–1727. [Google Scholar] [CrossRef]
- Hogue, C.W., Jr.; Stearns, J.D.; Colantuoni, E.; Robinson, K.A.; Stierer, T.; Mitter, N.; Pronovost, P.J.; Needham, D.M. The impact of obesity on outcomes after critical illness: A meta-analysis. Intensive Care Med. 2009, 35, 1152–1170. [Google Scholar] [CrossRef]
- Bremner, J.D. Before You Take That Pill: Why the Drug Industry May Be Bad for Your Health: Risks and Side Effects You Won’t Find on the Label of Commonly Prescribed Drugs, Vitamins, and Supplements; Penguin/Avery: New York, NY, USA, 2008. [Google Scholar]
- Pollan, M. The Omnivore’s Dilemma: A Natural History of Four Meals; Penguin Press: New York, NY, USA, 2006. [Google Scholar]
- Mathes, W.F.; Brownley, K.A.; Mo, X.; Bulik, C.M. The biology of binge eating. Appetite 2009, 52, 545–553. [Google Scholar] [CrossRef]
- Olshansky, S.J.; Passaro, D.J.; Hershow, R.C.; Layden, J.; Carnes, B.A.; Brody, J.; Hayflick, L.; Butler, R.N.; Allison, D.B.; Ludwig, D.S. A potential decline in life expectancy in the United States in the 21st century. N. Engl. J. Med. 2005, 352, 1138–1145. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Anand, S.S.; Islam, S.; Rosengren, A.; Franzosi, M.G.; Steyn, K.; Yusufali, A.H.; Keltai, M.; Diaz, R.; Rangarajan, S.; Yusuf, S. Risk factors for myocardial infarction in women and men: Insights from the INTERHEART study. Eur. Heart J. 2008, 29, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Alberti, K.G.M.M.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 1 April 2019).
- Ko, G.T.; Chan, J.C.; Tsang, L.W.; Yeung, V.T.; Chow, C.C.; Cockram, C.S. Outcomes of screening for diabetes in high-risk Hong Kong Chinese subjects. Diabetes Care 2000, 23, 1290–1294. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, Y. Duration of obesity and risk of non-insulin-dependent diabetes mellitus. Biomed. Pharmacother. 2000, 54, 80–84. [Google Scholar] [CrossRef]
- Sakurai, Y.; Teruya, K.; Shimada, N.; Umeda, T.; Tanaka, H.; Muto, T.; Kondo, T.; Nakamura, K.; Yoshizawa, N. Association between duration of obesity and risk of non-insulin-dependent diabetes mellitus. The Sotetsu Study. Am. J. Epidemiol. 1999, 149, 256–260. [Google Scholar] [CrossRef] [PubMed]
- García-Toro, M.; Vicens-Pons, E.; Gili, M.; Roca, M.; Serrano-Ripoll, M.J.; Vives, M.; Leivad, A.; Yáñez, A.M.; Bennasar-Veny, M.; Oliván-Blázquez, B. Obesity, metabolic syndrome and Mediterranean diet: Impact on depression outcome. J. Affect. Disord. 2016, 194, 105–108. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Lindstrom, J.; Eriksson, J.G.; Valle, T.T.; Halalainen, H.; Ilanne-Parikka, P.; Keinanen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef]
- Halton, T.L.; Willett, W.C.; Liu, S.; Manson, J.E.; Albert, C.M.; Rexrode, K.; Hu, F.B. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N. Engl. J. Med. 2006, 355, 1991–2002. [Google Scholar] [CrossRef]
- de Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: Final report of the Lyon Diet Heart Study. Circulation 1999, 99, 779–785. [Google Scholar] [CrossRef]
- Dai, J.; Lampert, R.; Wilson, P.W.; Goldberg, J.; Ziegler, T.R.; Vaccarino, V. Mediterranean dietary pattern is associated with improved cardiac autonomic function among middle-aged men: A twin study. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 366–373. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Orfanos, P.; Norat, T.; Bueno-de-Mesquita, B.; Ocke, M.C.; Peeters, P.H.M.; van der Schouw, Y.T.; Boeing, H.; Hoffmann, K.; Boffetta, P.; et al. Modified Mediterranean diet and survival: EPIC-elderly prospective cohort study. Br. Med. J. 2005, 330, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Rexrode, K.M.; Mantzoros, C.S.; Manson, J.E.; Willett, W.C.; Hu, F.B. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009, 119, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean Diet supplemented with extra-virgin olive oil or nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, S.; Friedman, M.A.; Arent, S.M. Understanding the relation between obesity and depression: Causal mechanisms and implications for treatment. Clin. Psychol. Sci. Pr. 2008, 15, 1–20. [Google Scholar] [CrossRef]
- Fernanda Fernandes, M.; Mutch, D.M.; Leri, F. The relationship between fatty acids and different depression-related brain regions, and their potential role as biomarkers of response to antidepressants. Nutrients 2017, 9, 298. [Google Scholar] [CrossRef]
- Weltens, N.; Zhao, D.; Van Oudenhove, L. Where is the comfort in comfort foods? Mechanisms linking fat signaling, reward, and emotion. Neurogastroenterol. Motil. 2014, 26, 303–315. [Google Scholar] [CrossRef]
- Van Oudenhove, L.; McKie, S.; Lassman, D.; Uddin, B.; Paine, P.; Coen, S.; Gregory, L.; Tack, J.; Aziz, Q. Fatty acid–induced gut-brain signaling attenuates neural and behavioral effects of sad emotion in humans. J. Clin. InvestIG. 2011, 121, 3094–3099. [Google Scholar] [CrossRef]
- Tomiyama, A.J.; Dallman, M.F.; Epel, E.S. Comfort food is comforting to those most stressed: Evidence of the chronic stress response network in high stress women. Psychoneuroendocrinology 2011, 36, 1513–1519. [Google Scholar] [CrossRef]
- Shively, C.A.; Register, T.C.; Clarkson, T.B. Social stress, visceral obesity, and coronary artery atherosclerosis: Product of a primate adaptation. Am. J. Primatol. 2009, 71, 742–751. [Google Scholar] [CrossRef]
- Dong, M.; Giles, W.H.; Felitti, V.J.; Dube, S.R.; Williams, J.E.; Chapman, D.P.; Anda, R.F. Insights into causal pathways for ischemic heart disease: Adverse childhood experiences study. Circulation 2004, 110, 1761–1766. [Google Scholar] [CrossRef]
- Rich-Edwards, J.W.; Spiegelman, D.; Lividoti Hibert, E.N.; Jun, H.J.; Todd, T.J.; Kawachi, I.; Wright, R.J. Abuse in childhood and adolescence as a predictor of type 2 diabetes in adult women. Am. J. Prev. Med. 2010, 39, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Jimenez, M.P.; Roberts, C.T.; Loucks, E.B. The role of adverse childhood experiences in cardiovascular disease risk: A review with emphasis on plausible mechanisms. Curr. Cardiol. Rep. 2015, 17, 88. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.K.; Boudry, G.; Lemay, D.G.; Raybould, H.E. Changes in intestinal barrier function and gut microbiota in high fat diet-fed rats are dynamic and region dependent. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G840–G851. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Anda, R.F.; Felitti, V.J.; Walker, J.; Whitfield, C.; Bremner, J.D.; Perry, B.D.; Dube, S.R.; Giles, W.H. The enduring effects of childhood abuse and related experiences in childhood: A convergence of evidence from neurobiology and epidemiology. Eur. Arch. Psychiatry Clin. Neurosci. 2006, 256, 174–186. [Google Scholar] [CrossRef]
- Lin, P.-Y.; Su, K.-P. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J. Clin. Psychiatry 2007, 68, 1056–1061. [Google Scholar] [CrossRef]
- Lacasse, J.R.; Leo, J. Serotonin and depression: A disconnect between the advertisements and the scientific literature. PLoS Med. 2005, 2, e392. [Google Scholar] [CrossRef]
- Wells, A.S.; Read, N.W.; Uvnas-Moberg, K.; Alster, P. Influences of fat and carbohydrate on postprandial sleepiness, mood, and hormones. Physiol. Behav. 1997, 61, 679–686. [Google Scholar] [CrossRef]
- Wells, A.S.; Read, N.W.; Macdonald, I.A. Effects of carbohydrate and lipid on resting energy expenditure, heart rate, sleepiness, and mood. Physiol. Behav. 1998, 63, 621–628. [Google Scholar] [CrossRef]
- Pischke, C.R.; Frenda, S.; Ornish, D.; Weidner, G. Lifestyle changes are related to reductions in depression in persons with elevated coronary risk factors. Psychol. Health 2010, 29, 1–24. [Google Scholar] [CrossRef]
- Pellegrin, K.L.; O’Neil, P.M.; Stellefson, E.J.; Fossey, M.D.; Ballenger, J.C.; cochrane, C.E.; Currey, H.S. Average daily nutrient intake and mood among obese women. Nutr. Res. 1998, 18, 1103–1112. [Google Scholar] [CrossRef]
- Sánchez-Villegas, A.; Delgado-Rodríguez, M.; Alonso, A.; Schlatter, J.; Lahortiga, F.; Serra Majem, L.; Martínez-González, M.A. Association of the Mediterranean dietary pattern with the incidence of depression: The Seguimiento Universidad de Navarra/University of Navarra follow-up (SUN) cohort. Arch. Gen. Psychiatry 2009, 66, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Marx, W.; Dash, S.; Carney, R.; Teasdale, S.B.; Solmi, M.; Stubbs, B.; Schuch, F.B.; Carvalho, A.F.; Jacka, F.; et al. The effects of dietary improvement on symptoms of depression and anxiety: A meta-analysis of randomized controlled trials. Psychosom. Med. 2019, 81, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, U.; Mishra, S.; Xu, J.; Levin, S.; Gonzales, J.; Barnard, N.D. A multicenter randomized controlled trial of a nutrition intervention program in a multiethnic adult population in the corporate setting reduces depression and anxiety and improves quality of life: The GEICO study. Am. J. Health Promot. 2015, 29, 245–254. [Google Scholar] [CrossRef] [PubMed]
- ndevelt, R.; Lemberger, J.; Bregman, J.; Kowen, G.; Berger-Fecht, I.; Lander, H.; Karpati, T.; Shahar, D. Intensive dietary intervention by a dietitian as a case manager among community dwelling older adults: The EDIT study. J. Nutr. Health Aging 2011, 15, 624–630. [Google Scholar] [CrossRef]
- Forster, S.E.; Powers, H.J.; Foulds, G.A.; Flower, D.J.; Hopkinson, K.; Parker, S.G.; Young, T.A.; Saxton, J.; Pockley, A.G.; Williams, E.A. Improvement in nutritional status reduces the clinical impact of infections in older adults. J. Am. Geriatr. Soc. 2012, 60, 1645–1654. [Google Scholar] [CrossRef]
- Scheier, M.F.; Helgeson, V.S.; Schulz, R.; Colvin, S.; Berga, S.; Bridges, M.W.; Knapp, J.; Gerszten, K.; Pappert, W.S. Interventions to enhance physical and psychological functioning among younger women who are ending nonhormonal adjuvant treatment for early-stage breast cancer. J. Clin. Oncol. 2005, 23, 4298. [Google Scholar] [CrossRef]
- Halyburton, A.K.; Brinkworth, G.D.; Wilson, C.J.; Noakes, M.; Buckley, J.D.; Keogh, J.B.; Clifton, P.M. Low- and high-carbohydrate weight-loss diets have similar effects on mood but not cognitive performance. Am. J. Clin. Nutr. 2007, 86, 580–587. [Google Scholar] [CrossRef]
- Assaf, A.R.; Beresford, S.A.; Risica, P.M.; Aragaki, A.; Brunner, R.L.; Bowen, D.J.; Naughton, M.; Rosal, M.C.; Snetselaar, L.; Wenger, N. Low-fat dietary pattern intervention and health-related quality of life: The Women’s Health Initiative randomized controlled dietary modification trial. J. Acad. Nutr. Diet. 2016, 116, 259–271. [Google Scholar] [CrossRef]
- Nieman, D.C.; Custer, W.F.; Butterworth, D.E.; Utter, A.C.; Henson, D.A. Psychological response to exercise training and/or energy restriction in obese women. J. Psychosom. Res. 2000, 48, 23–29. [Google Scholar] [CrossRef]
- Kiernan, M.; King, A.C.; Stefanick, M.L.; Killen, J.D. Men gain additional psychological benefits by adding exercise to a weight-loss program. Obesity 2001, 9, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, C.M.; Doherty, M.; Avery, A.J.; Read, A.; Taylor, M.A.; Sach, T.H.; Silcocks, P.; Muir, K.R. Effects of dietary intervention and quadriceps strengthening exercises on pain and function in overweight people with knee pain: Randomised controlled trial. Br. Med. J. 2009, 339, b3170. [Google Scholar] [CrossRef] [PubMed]
- Serrano Ripoll, M.J.; Oliván-Blázquez, B.; Vicens-Pons, E.; Roca, M.; Gili, M.; Leiva, A.; García-Campayo, J.; Demarzo, M.P.; García-Toro, M. Lifestyle change recommendations in major depression: Do they work? J. Affect. Disord. 2015, 183, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Wardle, J.; Rogers, P.; Judd, P.; Taylor, M.A.; Rapoport, L.; Green, M.; Nicholson Perry, K. Randomized trial of the effects of cholesterol-lowering dietary treatment on psychological function. Am. J. Med. 2000, 108, 547–553. [Google Scholar] [CrossRef]
- Hyyppä, M.T.; Kronholm, E.; Virtanen, A.; Leino, A.; Jula, A. Does simvastatin affect mood and steroid hormone levels in hypercholesterolemic men? A randomized double-blind trial. Psychoneuroendocrinology 2003, 28, 181–194. [Google Scholar] [CrossRef]
- McMillan, L.; Owen, L.; Kras, M.; Scholey, A. Behavioural effects of a 10-day Mediterranean diet. Results from a pilot study evaluating mood and cognitive performance. Appetite 2011, 56, 143–147. [Google Scholar] [CrossRef]
- Garcia-Toro, M.; Ibarra, O.; Gili, M.; Salva, J.; Monzón, S.; Vives, M.; Serrano, M.J.; Garcia-Campayo, J.; Roca, M. Effectiveness of hygienic-dietary recommendations as enhancers of antidepressant treatment in patients with Depression: Study protocol of a randomized controlled trial. BMC Public Health 2010, 10, 404. [Google Scholar] [CrossRef]
- Toobert, D.J.; Glasgow, R.E.; Strycker, L.A.; Barrera, M.; Ritzwoller, D.P.; Weidner, G. Long-term effects of the Mediterranean lifestyle program: A randomized clinical trial for postmenopausal women with type 2 diabetes. Int. J. Behav. Nutr. Phys. Act. 2007, 4, 1–7. [Google Scholar] [CrossRef]
- Garcia-Toro, M.; Gili, M.; Ibarra, O.; Monzón, S.; Vives, M.; Garcia-Campayo, J.; Gomez-Juanes, R.; Roca, M. Metabolic syndrome improvement in depression six months after prescribing simple hygienic-dietary recommendations. BMC Res. Notes 2014, 7, 339. [Google Scholar] [CrossRef]
- Kasckow, J.; Klaus, J.; Morse, J.; Oslin, D.; Luther, J.; Fox, L.; Reynolds, C.; Haas, G.L. Using problem solving therapy to treat veterans with subsyndromal depression: A pilot study. Int. J. Geriatr. Psychiatry 2014, 29, 1255–1261. [Google Scholar] [CrossRef]
- Kasckow, J.; Morse, J.; Begley, A.; Anderson, S.; Bensasi, S.; Thomas, S.; Quinn, S.C.; Reynolds, C.F.r. Treatment of post-traumatic stress disorder symptoms in emotionally distressed individuals. Psychiatry Res. 2014, 220, 370–375. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jacka, F.N.; O’Neil, A.; Opie, R.; Itsiopoulos, C.; Cotton, S.; Mohebbi, M.; Castle, D.; Dash, S.; Mihalopoulos, C.; Chatterton, M.L.; et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. 2017, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Castro-Diehl, C.; Wood, A.C.; Redline, S.; Reid, M.; Johnson, D.A.; Maras, J.E.; Jacobs, D.R., Jr.; Shea, S.; Crawford, A.; St-Onge, M.P. Mediterranean diet pattern and sleep duration and insomnia symptoms in the Multi-Ethnic Study of Atherosclerosis. Sleep 2018, 41. [Google Scholar] [CrossRef] [PubMed]
- Campanini, M.Z.; Guallar-Castillon, P.; Rodriguez-Artalejo, F.; Lopez-Garcia, E. Mediterranean Diet and Changes in Sleep Duration and Indicators of Sleep Quality in Older Adults. Sleep 2017, 40. [Google Scholar] [CrossRef] [PubMed]
- Mamalaki, E.; Anastasiou, C.A.; Ntanasi, E.; Tsapanou, A.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.M.; Sakka, P.; Scarmeas, N.; Yannakoulia, M. Associations between the mediterranean diet and sleep in older adults: Results from the hellenic longitudinal investigation of aging and diet study. Geriatr. Gerontol. Int. 2018, 18, 1543–1548. [Google Scholar] [CrossRef]
- Jaussent, I.; Dauvilliers, Y.; Ancelin, M.L.; Dartigues, J.F.; Tavernier, B.; Touchon, J.; Ritchie, K.; Besset, A. Insomnia symptoms in older adults: Associated factors and gender differences. Am. J. Geriatr. Psychiatry 2011, 19, 88–97. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, D.; Ni, N.; Ren, H.; Luo, C.; He, C.; Kang, J.-X.; Wan, J.-B.; Su, H. Omega-3 polyunsaturated fatty acids protect neural progenitor cells against oxidative injury. Mar. Drugs 2014, 12, 2341–2356. [Google Scholar] [CrossRef]
- Russell, F.D.; Bürgin-Maunder, C.S. Distinguishing health benefits of eicosapentaenoic and docosahexaenoic acids. Mar. Drugs 2012, 10, 2535–2559. [Google Scholar] [CrossRef]
- Brouwer, I.A.; Zock, P.L.; Camm, A.J.; Bocker, D.; Hauer, R.N.W.; Wever, E.F.D.; Dullemeijer, C.; Ronden, J.E.; Katan, M.B.; Lubinski, A.; et al. Effect of fish oil on ventricular tachyarrhythmia and death in patients with implantable cardioverter defibrillators: The study on Omega-3 fatty acids and ventricular arrhthmia (SOFA) randomized trial. J. Am. Med. Assoc. 2006, 295, 2613–2619. [Google Scholar] [CrossRef]
- Burr, M.L.; Fehily, A.M.; Gilbert, J.F.; Rogers, S.; Holliday, R.M.; Sweetnam, P.M. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction. Lancet 1989, 2, 757–761. [Google Scholar] [CrossRef]
- Hooper, L.; Thompson, R.L.; Harrison, R.A.; Summerbell, C.D.; Ness, A.R.; Moore, H.J.; Worthington, H.V.; Durrington, P.N.; Higgins, J.P.; Capps, N.E.; et al. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: Systematic review. BMJ 2006, 332, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.D.; McCaffery, P. The neurobiology of retinoic acid in affective disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Danese, A.; Moffitt, T.E.; Pariante, C.M.; Ambler, A.; Poulton, R.; Caspi, A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch. Gen. Psychiatry 2008, 65, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and its discontents: The role of cytokines in the pathphysiology of depression. Biol. Psychiatry 2009, 65, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Rapaport, M.H.; Nierenberg, A.A.; Schettler, P.J.; Kinkead, B.; Cardoos, A.; Walker, R.; Mischoulon, D. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: A proof-of-concept study. Mol. Psychiatry 2016, 21, 71–79. [Google Scholar] [CrossRef]
- Allam-Ndoul, B.; Guénard, F.; Barbier, O.; Vohl, M.-C. Effect of n-3 fatty acids on the expression of inflammatory genes in THP-1 macrophages. Lipids Health Dis. 2016, 15, 1–7. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P.-Y. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Lima, B.B.; Hammadah, M.; Wilmot, K.; Pearce, B.D.; Shah, A.; Levantsevych, O.; Kaseer, B.; Obideen, M.; Gafeer, M.M.; Kim, J.H.; et al. Posttraumatic Stress Disorder is associated with enhanced interleukin-6 response to mental stress in subjects with a recent myocardial infarction. Brain. Behav. Immun. 2019, 75, 26–33. [Google Scholar] [CrossRef]
- Serhan, C.N. Novel pro-resolving lipid mediators in inflammation are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Chiang, N.; Serhan, C.N. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol. Asp. Med. 2017, 58, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.F.; Dona, M.; Fredman, G.; Krishnamoorthy, S.; Irimia, D.; Serhan, C.N. Resolvin E2 formation and impact in inflammation-resolution. J. Immunol. 2012, 188, 4527–4534. [Google Scholar] [CrossRef] [PubMed]
- Deyama, S.; Ishikawa, Y.; Yoshikawa, K.; Shimoda, K.; Ide, S.; Satoh, M.; Minami, M. Resolvin D1 and D2 reverse lipopolysaccharide-induced depression-like behaviors through the mTORC1 signaling pathway. Int. J. Neuropsychopharmacol. 2017, 20, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, K.; Bernier, J.; Godbout, R.; Rousseau, G. Resolvin D1, a metabolite of omega-3 polyunsaturated fatty acid, decreases post-myocardial infarct depression. Mar. Drugs 2014, 12, 5306–5407. [Google Scholar] [CrossRef] [PubMed]
- Deyama, S.; Shimoda, K.; Suzuki, H.; Ishikawa, Y.; Ishimura, K.; Fukuda, H.; Hitora-Imamura, N.; Ide, S.; Satoh, M.; Kaneda, K.; et al. Resolvin E1/E2 ameliorate lipopolysaccharide-induced depression-like behaviors via ChemR23. Psychopharmacology 2018, 235, 329–336. [Google Scholar] [CrossRef]
- Ishikawaa, Y.; Deyamaa, S.; Shimodaa, K.; Yoshikawaa, K.; Idea, S.; Satohd, M.; Minamia, M. Rapid and sustained antidepressant effects of resolvin D1 and D2 in a chronic unpredictable stress model. Behav. Brain Res. 2017, 332, 233–236. [Google Scholar] [CrossRef]
- Alcocer-Gómez, E.; de Miguel, M.; Casas-Barquero, N.; Núñez-Vasco, J.; Sánchez-Alcazar, J.A.; Fernández-Rodríguez, A.; Cordero, M.D. NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain. Behav. Immun. 2014, 36, 111–117. [Google Scholar] [CrossRef]
- Akosile, W.; Voisey, J.; Lawford, B.; Colquhounc, D.; Young, R.M.; Mehta, D. The inflammasome NLRP12 is associated with both depression and coronary artery disease in Vietnam veterans. Psychiatry Res. 2018, 270, 775–779. [Google Scholar] [CrossRef]
- Vaccarino, V.; Brennan, M.L.; Miller, A.H.; Bremner, J.D.; Ritchie, J.C.; Lindau, F.; Veledar, E.; Su, S.; Murrah, N.V.; Jones, L.; et al. Association of major depressive disorder with serum myeloperoxidase and other markers of inflammation: A twin study. Biol. Psychiatry 2008, 64, 476–483. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Belury, M.A.; Porter, K.; Beversdorf, D.Q.; Lemeshow, S.; Glaser, R. Depressive symptoms, omega-6 fatty acids, and inflammation in older adults. Psychosom. Med. 2007, 69, 217–224. [Google Scholar] [CrossRef]
- Sublette, M.E.; Hibbeln, J.R.; Galfalvy, H.; Oquendo, M.A.; Mann, J.J. Omega-3 polyunsaturated essential fatty acid status as a predictor of future suicide risk. Am. J. Psychiatry 2006, 163, 1100–1102. [Google Scholar] [CrossRef] [PubMed]
- Capuron, L.; Su, S.; Miller, A.H.; Bremner, J.D.; Goldberg, J.; Vogt, G.J.; Maisano, C.; Jones, L.; Murrah, N.V.; Vaccarino, V. Depressive symptoms and metabolic syndrome: Is inflammation the underlying link? Biol. Psychiatry 2008, 64, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Gharekhani, A.; Khatami, M.-R.; Dashti-Khavidaki, S.; Razeghi, E.; Noorbala, A.-A.; Hashemi-Nazari, S.-S.; Mansournia, M.-A. The effect of omega-3 fatty acids on depressive symptoms and inflammatory markers in maintenance hemodialysis patients: A randomized, placebo-controlled clinical trial. Eur. J. Clin. Pharmacol. 2014, 70, 655–665. [Google Scholar] [CrossRef]
- Su, K.-P.; Lai, H.-C.; Yang, H.-T.; Su, W.-P.; Peng, C.-Y.; Chang, J.P.-C.; Chang, H.-C.; Pariante, C.M. Omega-3 fatty acids in the prevention of interferon-alpha-induced depression: Results from a randomized, controlled trial. Biol. Psychiatry 2014, 76, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Poppitt, S.D.; Howe, C.A.; Lithander, F.E.; Silvers, K.M.; Lin, R.B.; Croft, J.; Ratnasabapathy, Y.; Gibson, R.A.; Anderson, C.S. Effects of moderate-dose omega-3 fish oil on cardiovascular risk factors and mood after ischemic stroke: A randomized, controlled trial. Stroke 2009, 40, 3485–3492. [Google Scholar] [CrossRef]
- Sinn, N.; Milte, C.M.; Street, S.J.; Buckley, J.D.; Coates, A.M.; Petkov, J.; Howe, P.R. Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: A 6-month randomised controlled trial. Br. J. Nutr. 2012, 107, 1682–1693. [Google Scholar] [CrossRef]
- Einvik, G.; Ekeberg, O.; Lavik, J.G.; Ellingsen, I.; Klemsdal, T.O.; Hjerkinn, E. The influence of long-term awareness of hyperlipidemia and of 3 years of dietary counseling on depression, anxiety, and quality of life. J. Psychosom. Res. 2010, 68, 567–572. [Google Scholar] [CrossRef]
- Andreeva, V.A.; Galan, P.; Torres, M.; Julia, C.; Hercberg, S.; Kesse-Guyot, E. Supplementation with B vitamins or n-3 fatty acids and depressive symptoms in cardiovascular disease survivors: Ancillary findings from the SUpplementation with FOLate, vitamins B-6 and B-12 and/or OMega-3 fatty acids (SU.FOL.OM3) randomised trial. Am. J. Clin. Nutr. 2012, 96, 208–214. [Google Scholar] [CrossRef]
- Giltay, E.J.; Geleijnse, J.M.; Kromhout, D. Effects of n-3 fatty acids on depressive symptoms and dispositional optimism after myocardial infarction. Am. J. Clin. Nutr. 2011, 94, 1442–1450. [Google Scholar] [CrossRef]
- Doornbos, B.; van Goor, S.A.; Dijck-Brouwer, D.A.; Schaafsma, A.; Korf, J.; Muskiet, F.A. Supplementation of a low dose of DHA or DHA+AA does not prevent peripartum depressive symptoms in a small population based sample. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 49–52. [Google Scholar] [CrossRef]
- Llorente, A.M.; Jensen, C.L.; Voigt, R.G.; Fraley, J.K.; Berretta, M.C.; Heird, W.C. Effect of maternal docosahexaenoic acid supplementation on postpartum depression and information processing. Am. J. Obstet. Gynecol. 2003, 188, 1348–1353. [Google Scholar] [CrossRef] [PubMed]
- Makrides, M.; Gibson, R.A.; McPhee, A.J.; Yelland, L.; Quinlivan, J.; Ryan, P.; the DOMInO Investigative Team. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: A randomized controlled trial. JAMA 2010, 304, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Mozurkewich, E.L.; Clinton, C.M.; Chilimigras, J.L.; Hamilton, S.E.; Allbaugh, L.J.; Berman, D.R.; Marcus, S.M.; Romero, V.C.; Treadwell, M.C.; Keeton, K.L.; et al. The Mothers, Omega-3, and Mental Health Study: A double-blind, randomized controlled trial. Am. J. Obstet. Gynecol. 2013, 208, e1-9. [Google Scholar] [CrossRef]
- Stoll, A.L.; Severus, E.; Freeman, M.P.; Rueter, S.; Zboyan, H.A.; Diamond, E.; Cress, K.K.; Marangell, L.B. Omega 3 fatty acids in bipolar disorder: A preliminary double-blind, placebo-controlled trial. Arch. Gen. Psychiatry 1999, 56, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.P.; Hibbeln, J.R.; Wisner, K.L.; Davis, J.M.; Mischoulon, D.; Peet, M.; Keck, P.E.; Marangell, L.B.; Richardson, A.J.; Lake, J.; et al. Omega-3 fatty acids: Evidence basis of treatment and future research in psychiatry. J. Clin. Psychiatry 2006, 67, 1954–1967. [Google Scholar] [CrossRef]
- Peet, M.; Horrobin, D.F. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch. Gen. Psychiatry 2002, 59, 913–919. [Google Scholar] [CrossRef]
- Su, K.P.; Huang, S.Y.; Chiu, T.-H.; Huang, K.-C.; Huang, C.-L.; Chang, H.-C.; Pariante, C.M. Omega-3 fatty acids for major depressive disorder during pregnancy: Results from a randomized, double-blind, placebo-controlled trial. J. Clin. Psychiatry 2008, 69, 644–651. [Google Scholar] [CrossRef]
- Nemets, H.; Nemets, B.; Apter, A.; Bracha, Z.; Belmaker, R.H. Omega-3 treatment of childhood depression: A controlled, double-blind pilot study. Am. J. Psychiatry 2006, 163, 1098–1100. [Google Scholar] [CrossRef]
- Freeman, M.P.; Davis, M.; Sinha, P.; Wisner, K.L.; Hibbeln, J.R.; Gelenberg, A.J. Omega-3 fatty acids and supportive psychotherapy for perinatal depression: A randomized placebo-controlled study. J. Affect. Disord. 2008, 110, 142–148. [Google Scholar] [CrossRef]
- Parker, G.; Gibson, N.A.; Brotchie, H.; Heruc, G.; Rees, A.M.; Hadzi-Pavlovic, D. Omega-3 fatty acids and mood disorders. Am. J. Psychiatry 2006, 163, 969–978. [Google Scholar] [CrossRef]
- Grosso, G.; Pajak, A.; Marventano, S.; Castellano, S.; Galvano, F.; Bucolo, C.; Drago, F.; Caraci, F. Role of omega-3 fatty acids in the treatment of depressive disorders: A comprehensive meta-analysis of randomized clinical trials. PLoS ONE 2014, 9, e96905. [Google Scholar] [CrossRef] [PubMed]
- Hallahan, B.; Ryan, T.; Hibbeln, J.R.; Murray, I.T.; Glynn, S.; Ramsden, C.E.; SanGiovanni, J.P.; Davis, J.M. Efficacy of omega-3 highly unsaturated fatty acids in the treatment of depression. Br. J. Psychiatry 2016, 209, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.G.; Bentsen, H.; Puri, B.K. Eicosapentaenoic acid appears to be the key omega-3 fatty acid component associated with efficacy in major depressive disorder: A critique of Bloch and Hannestad and updated metaanalysis. Mol. Psychiatry 2012, 17, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Mocking, R.J.; Harmsen, I.; Assies, J.; Koeter, M.W.; Ruhé, H.G.; Schene, A.H. Metaanalysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl. Psychiatry 2016, 6, e756. [Google Scholar] [CrossRef] [PubMed]
- Sublette, M.E.; Ellis, S.P.; Geant, A.L.; Mann, J.J. Metaanalysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J. Clin. Psychiatry 2011, 72, 1577–1584. [Google Scholar] [CrossRef]
- Appleton, K.M.; Rogers, P.J.; Ness, A.R. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am. J. Clin. Nutr. 2010, 91, 757–770. [Google Scholar] [CrossRef]
- Gertsik, L.; Poland, R.E.; Bresee, C.; Rapaport, M.H. Omega-3 fatty acid augmentation of citalopram treatment for patients with major depressive disorder. J. Clin. Psychopharmacol. 2012, 32, 61–64. [Google Scholar] [CrossRef]
- Lesperance, F.; Frasure-Smith, N.; St-Andre, E.; Turecki, G.; Lesperance, P.; Wisniewski, S.R. The efficacy of omega-3 supplementation for major depression: A randomized controlled trial. J. Clin. Psychiatry 2011, 72, 1054–1062. [Google Scholar] [CrossRef]
- Felger, J.C.; Li, Z.; Haroon, E.; Woolwine, B.J.; Jung, M.Y.; Hu, X.; Miller, A.H. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatry 2016, 21, 1358–1365. [Google Scholar] [CrossRef]
- Bot, M.; Pouwer, F.; Assies, J.; Jansen, E.H.; Diamant, M.; Snoek, F.J.; Beekman, A.T.; de Jonge, P. Eicosapentaenoic acid as an add-on to antidepressant medication for co-morbid major depression in patients with diabetes mellitus: A randomized, double-blind placebo controlled study. J. Affect. Disord. 2010, 126, 282–286. [Google Scholar] [CrossRef]
- da Silva, T.M.; Munhoz, R.P.; Alvarez, C.; Naliwaiko, K.; Kiss, A.; Andreatini, R.; Ferraz, A.C. Depression in Parkinson’s disease: A double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. J. Affect. Disord. 2008, 111, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Grenyer, B.F.S.; Crowe, T.; Meyer, B.; Owen, A.J.; Grigonis-Deane, E.M.; Caputi, P.; Howe, P.R.C. Fish oil supplementation in the treatment of major depression: A randomised double-blind placebo-controlled trial. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 1393–1396. [Google Scholar] [CrossRef] [PubMed]
- Silvers, K.M.; Woolley, C.C.; Hamilton, F.C.; Watts, P.M.; Watson, R.A. Randomised double-blind placebo-controlled trial of fish oil in the treatment of depression. Prostaglandins Leukot. Essent. Fat. Acids 2005, 72, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Tayama, J.; Ogawa, S.; Nakaya, N.; Sone, T.; Hamaguchi, T.; Takeoka, A.; Hamazaki, K.; Okamura, H.; Yajima, J.; Kobayashi, M.; et al. Omega-3 polyunsaturated fatty acids and psychological intervention for workers with mild to moderate depression: A double-blind randomized controlled trial. J. Affect. Disord. 2019, 245, 364–370. [Google Scholar] [CrossRef]
- Marangell, L.B.; Martinez, J.M.; Zboyan, H.A.; Kertz, B.; Seung Kim, H.F.; Puryear, L.J. A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am. J. Psychiatry 2003, 160, 996–998. [Google Scholar] [CrossRef]
- Mischoulon, D.; Papakostas, G.I.; Dording, C.M.; Farabaugh, A.H.; Sonawalla, S.B.; Agoston, A.M.; Smith, J.; Beaumont, E.C.; Dahan, L.E.; Alpert, J.E.; et al. A double-blind, randomized controlled trial of ethyl-eicosapentaenoate for major depressive disorder. J. Clin. Psychiatry 2009, 70, 1636–1644. [Google Scholar] [CrossRef]
- Rees, A.-M.; Austin, M.-P.; Parker, G.B. Omega-3 fatty acids as a treatment for perinatal depression: Randomized double-blind placebo-controlled trial. Aust. N. Z. J. Psychiatry 2008, 42, 199–205. [Google Scholar] [CrossRef]
- Carney, R.M.; Freedland, K.E.; Rubin, E.H.; Rich, M.W.; Steinmeyer, B.C.; Harris, W.S. Omega-3 augmentation of sertraline in treatment of depression in patients with coronary heart disease: A randomized controlled trial. J. Am. Med. Assoc. 2009, 302, 1651–1657. [Google Scholar] [CrossRef]
- Carney, R.M.; Freedland, K.E.; Rubin, E.H.; Rich, M.W.; Steinmeyer, B.C.; Harris, W.S. A randomized placebo-controlled trial of omega-3 and sertraline in depressed patients with or at risk for coronary heart disease. J. Clin. Psychiatry 2019, 80, 19m12742. [Google Scholar] [CrossRef]
- Almeida, O.P.; Flicker, L.; Lautenshlager, N.T.; Leedman, P.; Vasikaran, S.; van Bockxmeer, F.M. Contribution of the MTHFR gene to the causal pathway for depression, anxiety and cognitive impairment in later life. Neurobiol. Aging 2005, 26, 251–257. [Google Scholar] [CrossRef]
- Bressa, G.M. S-adenosyl-l-methionine (SAMe) as antidepressant: Meta-analysis of clinical studies. Acta Neurol. Scand. 1994, 89, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Bottiglieri, T. Homocysteine and folate metabolism in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Tolmunen, T.; Hintikka, J.; Voutilainene, S.; Ruusunen, A.; Alfthan, G.; Nyyssonen, K.; Viinamaki, H.; Kaplan, G.A.; Salonen, J.T. Association between depressive symptoms and serum concentrations of homocysteine in men: A population study. Am. J. Clin. Nutr. 2004, 80, 1574–1578. [Google Scholar] [CrossRef] [PubMed]
- Tiemeier, T.; van Tuijl, H.R.; Hofman, A.; Meijer, J.; Kiliaan, A.J.; Breteler, M.M. Vitamin B12, folate, and homocysteine in depression: The Rotterdam Study. Am. J. Psychiatry 2002, 159, 2099–2101. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.H.; Carney, M.W.P.; Toone, B.K. Methylation and Mood. Lancet 1984, 324, 196–198. [Google Scholar] [CrossRef]
- Dedoussis, G.V.; Panagiotakos, D.B.; Chrysohoou, C.; Pitsavos, C.; Zampelas, A.; Choumerianou, D.; Stefanadis, C. Effect of interaction between adherence to a Mediterranean diet and the methylenetetrahydrofolate reductase 677C-->T mutation on homocysteine concentrations in healthy adults: The ATTICA Study. Am. J. Clin. Nutr. 2004, 80, 849–854. [Google Scholar] [CrossRef][Green Version]
- Kim, J.M.; Stewart, R.; Kim, S.W.; Yang, S.J.; Shin, I.S.; Yoon, J.S. Predictive value of folate, vitamin B12 and homocysteine levels in late-life depression. Br. J. Psychiatry 2008, 192, 268–274. [Google Scholar] [CrossRef]
- Forti, P.; Rietti, E.; Pisacane, N.; Olivelli, V.; Dalmonte, E.; Mecocci, P.; Ravaglia, G. Blood homocysteine and risk of depression in the elderly. Arch. Gerontol. Geriatr. 2010, 51, 21–25. [Google Scholar] [CrossRef]
- Nabi, H.; Bochud, M.; Glaus, J.; Lasserre, A.M.; Waeber, G.; Vollenweider, P.; Preisig, M. Association of serum homocysteine with major depressive disorder: Results from a large population-based study. Psychoneuroendocrinology 2013, 38, 2309–2318. [Google Scholar] [CrossRef]
- Papakostas, G.I.; Petersen, T.; Lebowitz, B.D.; Mischoulon, D.; Ryan, J.L.; Nierenberg, A.A.; Bottiglieri, T.; Alpert, J.E.; Rosenbaum, J.F.; Fava, M. The relationship between serum folate, vitamin B12, and homocysteine levels in major depressive disorder and the timing of improvement with fluoxetine. Int. J. Neuropsychopharmacol. 2005, 8, 523–528. [Google Scholar] [CrossRef]
- Papakostas, G.I.; Petersen, T.; Mischoulon, D.; Green, C.H.; Nierenberg, A.A.; Bottiglieri, T.; Rosenbaum, J.F.; Alpert, J.E.; Fava, M. Serum folate, vitamin B12, and homocysteine in major depressive disorder, Part 2: Predictors of relapse during the continuation phase of pharmacotherapy. J. Clin. Psychiatry 2004, 65, 1096–1098. [Google Scholar] [CrossRef] [PubMed]
- Papakostas, G.I.; Petersen, T.; Mischoulon, D.; Ryan, J.L.; Nierenberg, A.A.; Bottiglieri, T.; Rosenbaum, J.F.; Alpert, J.E.; Fava, M. Serum folate, vitamin B12, and homocysteine in major depressive disorder, Part 1: Predictors of clinical response in fluoxetine-resistant depression. J. Clin. Psychiatry 2004, 65, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Papakostas, G.I.; Iosifescu, D.V.; Renshaw, P.F.; Lyoo, I.K.; Lee, H.K.; Alpert, J.E.; Nierenberg, A.A.; Fava, M. Brain MRI white matter hyperintensities and one-carbon cycle metabolism in non-geriatric outpatients with major depressive disorder (Part II). Psychiatry Res. 2005, 140, 301–307. [Google Scholar] [CrossRef] [PubMed]
- McMahon, J.A.; Green, T.J.; Skeaff, C.M.; Knight, R.G.; Mann, J.I.; Williams, S.M. A controlled trial of homocysteine lowering and cognitive performance. N. Engl. J. Med. 2006, 354, 2764–2772. [Google Scholar] [CrossRef]
- De Koning, E.J.; Zwaluw, N.L.; Wijngaarden, J.P.; Sohl, E.; Brouwer-Brolsma, E.M.; van Marwijk, H.W.; Enneman, A.W.; Swart, K.M.; van Dijk, S.C.; Ham, A.C.; et al. Effects of two-year vitamin B(12) and folic acid supplementation on depressive symptoms and quality of life in older adults with elevated homocysteine concentrations: Additional results from the B-PROOF Study, an RCT. Nutrients 2016, 8, 748. [Google Scholar] [CrossRef]
- Schefft, C.; Kilarski, L.L.; Bschor, T.B.; Köhler, S. Efficacy of adding nutritional supplements in unipolar depression: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. 2017, 27, 1090–1109. [Google Scholar] [CrossRef]
- Okereke, O.I.; Cook, N.R.; Albert, C.M.; Van Denburgh, M.; Buring, J.E.; Manson, J.E. Effect of long-term supplementation with folic acid and B vitamins on risk of depression in older women. Br. J. Psychiatry 2015, 206, 324–331. [Google Scholar] [CrossRef]
- Kwok, T.; Wu, Y.; Lee, J.; Lee, R.; Yung, C.Y.; Choi, G.; Lee, V.; Harrison, J.; Lam, L.; Mok, V. A randomized placebo-controlled trial of using B vitamins to prevent cognitive decline in older mild cognitive impairment patients. Clin. Nutr. 2019, S0261-5614, 33132. [Google Scholar] [CrossRef]
- Mech, A.W.; Farah, A. Correlation of clinical response with homocystein reduction during therapy with reduced B vitamins in patients with MDD who are positive for MTHFR C677T or A1298C polymorphism: A randomized, double-blind, placebo-controlled study. J. Clin. Psychiatry 2016, 77, 668–671. [Google Scholar] [CrossRef]
- Coppen, A.; Bailey, J. Enhancement of the antidepressant action of fluoxetine by folic acid: A randomised, placebo controlled trial. J. Affect. Disord. 2000, 60, 121–130. [Google Scholar] [CrossRef]
- Papakostas, G.I.; Shelton, R.C.; Zajecka, J.M.; Etemad, B.; Rickels, K.; Clain, A.; Baer, L.; Dalton, E.D.; Sacco, G.R.; Schoenfeld, D.; et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: Results of two randomized, double-blind, parallel-sequential trials. Am. J. Psychiatry 2012, 169, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Resler, G.; Lavie, R.; Campos, J.; Mata, S.; Urbina, M.; García, A.; Apitz, R.; Lima, L. Effect of folic acid combined with fluoxetine in patients with major depression on plasma homocysteine and vitamin B12, and serotonin levels in lymphocytes. Neuroimmunomodulation 2008, 15, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Bedson, E.; Bell, D.; Carr, D.; Carter, B.; Hughes, D.; Jorgensen, A.; Lewis, H.; Lloyd, K.; Mccaddon, A.; Moat, S.; et al. Folate Augmentation of Treatment--Evaluation for Depression (FolATED): Randomised trial and economic evaluation. Health Technol. Assess. 2014, 18, 1–159. [Google Scholar] [CrossRef] [PubMed]
- Almeida, O.P.; Ford, A.H.; Hirani, V.; Singh, V.; vanBockxmeer, F.M.; McCaul, K.; Flicker, L. B vitamins to enhance treatment response to antidepressants in middle-aged and older adults: Results from the B-VITAGE randomised, double-blind, placebo-controlled trial. Br. J. Psychiatry 2014, 205, 450–457. [Google Scholar] [CrossRef]
- Papakostas, G.I.; Shelton, R.C.; Zajecka, J.M.; Bottiglieri, T.; Roffman, J.; Cassiello, C.; Stahl, S.M.; Fava, M. Effect of adjunctive L-methylfolate 15 mg among inadequate responders to SSRIs in depressed patients who were stratified by biomarker levels and genotype: Results from a randomized clinical trial. J. Clin. Psychiatry 2014, 75, 855–863. [Google Scholar] [CrossRef]
- Khosravi, M.; Sotoudeh, G.; Amini, M.; Raisi, F.; Mansoori, A.; Hosseinzadeh, M. The relationship between dietary patterns and depression mediated by serum levels of Folate and vitamin B12. BMC Psychiatry 2020, 20, 63. [Google Scholar] [CrossRef]
- Assies, J.; Mocking, R.J.T.; Lok, A.; Koeter, M.W.J.; Bockting, C.L.H.; Visser, I.; Pouwer, F.; Ruhé, H.G.; Schene, A.H. Erythrocyte fatty acid profiles and plasma homocysteine, folate and vitamin B6 and B12 in recurrent depression: Implications for co-morbidity with cardiovascular disease. Psychiatry Res. 2015, 229, 992–998. [Google Scholar] [CrossRef]
- Moorthy, D.; Peter, I.; Scott, T.M.; Parnell, L.D.; Lai, C.-Q.; Crott, J.W.; Ordovás, J.M.; Selhub, J.; Griffith, J.; Rosenberg, I.H.; et al. Status of vitamins B-12 and B-6 but not of folate, homocysteine, and the methylenetetrahydrofolate reductase C677T polymorphism are associated with impaired cognition and depression in adults. J. Nutr. 2012, 143, 1554–1560. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Shroff, M.R.; Beydoun, H.A.; Zonderman, A.B. Serum folate, vitamin B-12, and homocysteine and their association with depressive symptoms among U.S. adults. Psychosom. Med. 2010, 72, 862–873. [Google Scholar] [CrossRef]
- Penninx, B.W.; Guralnik, J.M.; Ferrucci, L.; Fried, L.P.; Allen, R.H.; Stabler, S.P. Vitamin B(12) deficiency and depression in physically disabled older women: Epidemiological evidence from the Women’s Health and Aging Study. Am. J. Psychiatry 2000, 157, 715–721. [Google Scholar] [CrossRef]
- Elstgeest, L.E.; Brouwer, I.A.; Penninx, B.W.; van Schoor, N.M.; Visser, M. Vitamin B(12), homocysteine and depressive symptoms: A longitudinal study among older adults. Eur. J. Clin. Nutr. 2017, 71, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Schoenaker, D.A.; Hebert, J.R.; Mishra, G.D. Association between inflammatory potential of diet and risk of depression in middle-aged women: The Australian Longitudinal Study on Women’s Health. Br. J. Nutr. 2016, 116, 1077–1086. [Google Scholar] [CrossRef]
- Akbaraly, T.; Kerlau, C.; Wyart, M.; Chevallier, N.; Ndiaye, L.; Shivappa, N.; Hebert, J.R.; Kivimaki, M. Dietary inflammatory index and recurrence of depressive symptoms: Results from the Whitehall II Study. Clin. Psychol. Sci. 2016, 4, 1125–1134. [Google Scholar] [CrossRef]
- Sanchez-Villegas, A.; Ruiz-Canela, M.; de la Fuente-Arrillaga, C.; Gea, A.; Shivappa, N.; Hebert, J.R.; Martinez-Gonzalez, M.A. Dietary inflammatory index, cardiometabolic conditions and depression in the Seguimiento Universidad de Navarra cohort study. Br. J. Nutr. 2015, 114, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Haghighatdoost, F.; Feizi, A.; Esmaillzadeh, A.; Feinle-Bisset, C.; Keshteli, A.H.; Afshar, H.; Adibi, P. Association between the dietary inflammatory index and common mental health disorders profile scores. Clin. Nutr. 2019, 38, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Imayama, I.; Alfano, C.M.; Kong, A.; Foster-Schubert, K.E.; Bain, C.E.; Xiao, L.; Duggan, C.; Wang, C.-Y.; Campbell, K.L.; Blackburn, G.L.; et al. Dietary weight loss and exercise interventions effects on quality of life in overweight/obese postmenopausal women: A randomized controlled trial. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 118. [Google Scholar] [CrossRef]
- Molendijk, M.L.; Fried, E.I.; Van der Does, W. The SMILES trial: Do undisclosed recruitment practices explain the remarkably large effect? BMC Med. 2018, 16, 243. [Google Scholar] [CrossRef]
- Horton, R. Expression of concern: Indo-Mediterranean Diet Heart Study. Lancet 2005, 366, 354–356. [Google Scholar] [CrossRef]
- Bloch, M.H.; Hannestad, J. Omega-3 fatty acids for the treatment of depression: Systematic review and meta-analysis. Mol. Psychiatry 2012, 17, 1272–1282. [Google Scholar] [CrossRef]
- Vermetten, E.; Bremner, J.D. Circuits and systems in stress. I. Preclinical studies. Depress. Anxiety 2002, 15, 126–147. [Google Scholar] [CrossRef]
- Charney, D.S.; Bremner, J.D. The neurobiology of anxiety disorders. In Neurobiology of Mental Illness; Charney, D.S., Nestler, E.J., Bunney, S.S., Eds.; Oxford University Press: Oxford, UK, 1999; pp. 494–517. [Google Scholar]
- Inoue, T.; Tsuchiya, K.; Koyama, T. Regional changes in dopamine and serotonin activation with various intensity of physical and psychological stress in the rat brain. Pharmacol. Biochem. Behav. 1994, 49, 911–920. [Google Scholar] [CrossRef]
- Petty, F.; Kramer, G.L.; Wu, J. Serotonergic modulation of learned helplessness. Ann. N. Y. Acad. Sci. 1997, 821, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Vermetten, E.; Bremner, J.D. Circuits and systems in stress. II. Applications to neurobiology and treatment of PTSD. Depress. Anxiety 2002, 16, 14–38. [Google Scholar] [CrossRef] [PubMed]
- Southwick, S.M.; Krystal, J.H.; Bremner, J.D.; Morgan, C.A.; Nicolaou, A.; Nagy, L.M.; Johnson, D.R.; Heninger, G.R.; Charney, D.S. Noradrenergic and serotonergic function in posttraumatic stress disorder. Arch. Gen. Psychiatry 1997, 54, 749–758. [Google Scholar] [CrossRef]
- Yehuda, R. Post-traumatic stress disorder. N. Engl. J. Med. 2002, 346, 108–114. [Google Scholar] [CrossRef]
- Bremner, J.D.; Krystal, J.H.; Southwick, S.M.; Charney, D.S. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse 1996, 23, 28–38. [Google Scholar] [CrossRef]
- Bremner, J.D.; Krystal, J.H.; Southwick, S.M.; Charney, D.S. Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse 1996, 23, 39–51. [Google Scholar] [CrossRef]
- Abercrombie, E.D.; Jacobs, B.L. Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. I. Acutely presented stressful and non-stressful stimuli. J. Neurosci. 1987, 7, 2837–2847. [Google Scholar] [CrossRef]
- Abercrombie, E.D.; Jacobs, B.L. Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. II. Adaptation to chronically presented stressful stimuli. J. Neurosci. 1987, 7, 2844–2848. [Google Scholar] [CrossRef]
- Foote, S.L.; Bloom, F.E.; Aston-Jones, G. Nucleus locus coeruleus: New evidence of anatomical and physiological specificity. Physiol. Behav. 1983, 63, 844–914. [Google Scholar]
- Levine, E.S.; Litto, W.J.; Jacobs, B.L. Activity of cat locus coeruleus noradrenergic neurons during the defense reaction. Brain Res. 1990, 531, 189–195. [Google Scholar] [CrossRef]
- Redmond, D.; Huang, Y. New evidence for a locus coeruleus-norepinephrine connection with anxiety. Life Sci. 1979, 25, 2149–2162. [Google Scholar] [CrossRef]
- Jedema, H.P.; Finlay, J.M.; Sved, A.F.; Grace, A.A. Chronic cold exposure potentiates CRH-evoked increases in electrophysiologic activity of locus coeruleus neurons. Biol. Psychiatry 2001, 49, 351–359. [Google Scholar] [CrossRef]
- Nisenbaum, L.K.; Abercrombie, E.D. Presynaptic alterations associated with enhancement of evoked release and synthesis of NE in hippocampus of chemically cold stressed rats. Brain Res. 1993, 608, 280–287. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Shipley, M.T.; Chouvet, G.; Ennis, M.; VanBockstaele, E.J.; Pieribone, V.; Shiekhattar, R. Afferent regulation of locus coeruleus neurons: Anatomy, physiology and pharmacology. Prog. Brain Res. 1991, 88, 47–75. [Google Scholar]
- Southwick, S.M.; Krystal, J.H.; Morgan, C.A.; Johnson, D.; Nagy, L.M.; Nicolaou, A.; Heninger, G.R.; Charney, D.S. Abnormal noradrenergic function in posttraumatic stress disorder. Arch. Gen. Psychiatry 1993, 50, 266–274. [Google Scholar] [CrossRef]
- Bremner, J.D.; Innis, R.B.; Ng, C.K.; Staib, L.; Duncan, J.; Bronen, R.; Zubal, G.; Rich, D.; Krystal, J.H.; Dey, H.; et al. PET measurement of cerebral metabolic correlates of yohimbine administration in posttraumatic stress disorder. Arch. Gen. Psychiatry 1997, 54, 246–256. [Google Scholar] [CrossRef]
- Rossi, J.; Zolovick, A.J.; Davies, R.F.; Panksepp, J. The role of norepinephrine in feeding behavior. Neurosci. Biobehav. Rev. 1982, 6, 195–204. [Google Scholar] [CrossRef]
- Wise, R.A. Role of brain dopamine in food reward and reinforcement. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2006, 361, 1149–1158. [Google Scholar] [CrossRef]
- Bremner, J.D.; Pearce, B. Neurotransmitter, neurohormonal, and neuropeptidal function in stress and PTSD. In Posttraumatic Stress Disorder: From Neurobiology to Treatment; Bremner, J.D., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2016; pp. 181–232. [Google Scholar]
- Kalivas, P.W.; Abhold, R. Enkephalin release in to the ventral tegmental area in response to stress: Modulation of mesocortical dopamine. Biol. Psychiatry 1987, 414, 339–348. [Google Scholar] [CrossRef]
- Krisch, B. Somatostatin-immunoreactive fiber projections into the brain stem and the spinal cord of the rat. Cell Tissue Res. 1981, 217, 531–552. [Google Scholar] [CrossRef] [PubMed]
- Vecsei, L.; Kiraly, C.; Bollok, I.; Nagy, A.; Varga, J.; Penke, B.; Telegdy, G. Comparative studies with somatostatin and cysteamine in different behavioral tests on rats. Pharmacol. Biochem. Behav. 1984, 21, 833–837. [Google Scholar] [CrossRef]
- Benyassi, A.; Gavalda, A.; Armario, A.; Arancibia, S. Role of somatostatin in the acute immobilization stress-induced GH decrease in rat. Life Sci. 1993, 52, 361–370. [Google Scholar] [CrossRef]
- Bremner, J.D.; Licinio, J.; Darnell, A.; Krystal, J.H.; Owens, M.; Southwick, S.M.; Nemeroff, C.B.; Charney, D.S. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am. J. Psychiatry 1997, 154, 624–629. [Google Scholar]
- Weiss, J.M.; Bonsall, R.W.; Demetrikopoulos, M.K.; Emery, M.S.; West, C.H.K. Galanin: A significant role in depression? Ann. N. Y. Acad. Sci. 1998, 863, 364–384. [Google Scholar] [CrossRef]
- Karlsson, R.M.; Holmes, A. Galanin as a modulator of anxiety and depression and a therapeutic target for affective disease. Amino Acids 2006, 31, 231–239. [Google Scholar] [CrossRef]
- Yildiz, B.O.; Suchard, M.A.; Wong, M.L.; McCann, S.M.; Licinio, J. Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc. Natl. Acad. Sci. USA 2004, 101, 10434–10439. [Google Scholar] [CrossRef]
- Stengel, A.; Wang, L.; Tache, Y. Stress-related alterations of acyl and desacyl ghrelin circulating levels: Mechanisms and functional implications. Peptides 2011, 32, 2208–2217. [Google Scholar] [CrossRef][Green Version]
- Spencer, S.J.; Xu, L.; Clarke, M.A.; Lemus, M.; Reichenbach, A.; Geenen, B.; Kozicz, T.; Andrews, Z.B. Ghrelin regulates the hypothalamic-pituitary-adrenal axis and restricts anxiety after acute stress. Biol. Psychiatry 2012, 72, 457–465. [Google Scholar] [CrossRef]
- Chuang, J.C.; Perello, M.; Sakata, I.; Osborne-Lawrence, S.; Savitt, J.M.; Lutter, M.; Zigman, J.M. Ghrelin mediates stress-induced food-reward behavior in mice. J. Clin. Investig. 2011, 121, 2684–2692. [Google Scholar] [CrossRef]
- Meyer, R.M.; Burgos-Robles, A.; Liu, E.; Correia, S.S.; Goosens, K.A. A ghrelin-growth hormone axis drives stress-induced vulnerability to enhanced fear. Mol. Psychiatry 2014, 19, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.T.; Dowd, S.E.; Galley, J.D.; Hufnagle, A.R.; Allen, R.G.; Lyte, M. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain. Behav. Immun. 2011, 25, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011, 141, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Cani, P.D.; Everard, A. Talking microbes: When gut bacteria interact with diet and host organs. Mol. Nutr. Food Res. 2016, 60, 58–66. [Google Scholar] [CrossRef]
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbes and the brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef]
- De Lartigue, G.; Barbier de La Serre, C.; Raybould, H.E. Vagal afferents in high gat diet-induced obesity: Intestinal microflora, gut inflammation and cholecystokinin. Physiol. Behav. 2011, 105, 100–105. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Melancholic microbes: A link between gut microbiota and depression? Neurogastroenterol. Motil. 2013, 25, 713–719. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef]
- Tillisch, K.; Labus, J.; Kilpatrick, L.; Jiang, Z.; Stains, J.; Ebrat, B.; Guyonnet, D.; Legrain-Raspaud, S.; Trotin, B.; Naliboff, B.; et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013, 144, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, J.A.; Bayak, M.A.; Moore, K.A. Effects of exercise training on older patients with major depression. Arch. Intern. Med. 1999, 159, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
| Author-Year | Study | Population | Intervention | Sample | Outcome measure | Result | |
|---|---|---|---|---|---|---|---|
| Agarwal 2015 | GEICO | Healthy employees | Vegan diet v no intervention | Randomized (n = 292) | SF-36 |  | depression, anxiety, QOL |
| Almeida 2014 | B-VITAGE Adjunctive to antidepressants | Major depression >age 50 | VB6/VB12/Fol v Pla | Randomized (n = 153) | MADRS |  | relapse, MADRS NS |
| Andreevna 2012 | SU.FOLOM3 | CAD patients | EPA/DHA v placebo | Randomized (n = 2501) | GDS | NS depression | |
| Assaf 2016 | WHI Study | Women | Low fat diet v no intervention | Randomized (48,835) | CES-D | NS depression | |
| Bedson 2014 | FolATED Adunctive to antidepressants | Patients with depression | Fol v pla | Randomized (n = 475) | BDI, MADRS | NS depression | |
| Bot 2010 | Patients with MDD & Diabetes | EPA v placebo | Randomized (n = 25) | MADRAS | NS depression | ||
| Carney 2009 | Adjunctive to antidepressants | Patients with CAD & depression | DHA/EPA v placebo | Randomized (n = 122) | HDRS, BDI | NS depression | |
| Carney 2019 | Adjunctive to antidepressants | Patients with CAD or hi risk & depression | EPA v placebo | Randomized (n = 144) | HDRS, BDI | NS depression | |
| Coppen 2000 | Adjunctive to antidepressants | Major depression | Fol v pla | Randomized (n = 127) | HDRS |  | depression |
| Da Silva 2008 | Adjunct ±antidepressants | Patients with PD & depression | Fish oil v placebo | Randomized (n = 31) | MADRAS, BDI |  | MADRS depression, BDI NS |
| De Koning 2016 | B-PROOF | Community sample, older adults | Fol/VB12 v pla | Randomized (n = 2,919) | GDS | NS depression | |
| Doornbos 2009 | Pregnant women | EPA/DHA v placebo | Randomized (n = 119) | EPDS | NS depression | ||
| Endevelt 2011 | Israel | Elderly at risk for malnutrition | 5 visits with dietician & MD v booklet v nothing | Randomized (n = 127) | GDS |  | Depression with dietician & MD |
| Einvik 2010 | Oslo Diet & Antismoking Study | Patients with hyperlipidemia | n-3 PUFAs v placebo v dietary counseling | Randomized (n =563) | HADS | NS depression | |
| Forster 2012 | UK | Elderly in community | Diet intervention v supplement v placebo | Randomized (n = 217) | GDS | NS depression | |
| Freeman 2008 | Adjunctive to therapy | Perinatal MDD | DHA/EPA v placebo | Randomized (n = 51) | HDRS | NS depression | |
| Garcia Toro 2016 | Adjunctive to antidepressants | Patients with depression | Med Diet adherenec | Observational (n = 273) | BDI | NS depression | |
| Gharekhani 2014 | Hemodialysis patients with depressive symptoms | DHA/EPA v placebo | Randomized (n = 54) | BDI |  | Depression | |
| Gertsik 2012 | Adjunctive to antidepressants | Patients with depression | Citalopram + EPA/DHA/O3FA v Cit + pla | Randomized (n = 46) | HDRS |  | depression |
| Grenyer 2007 | ±Adjunctive to antidepressants | Patients with depression | DHA/EPA (fish oil) v placebo | Randomized (n = 83) | HDRS, BDI | NS depression | |
| Halyburton 2007 | Overweight or Obese patients | Low carb high fat v high carb low fat diet | Randomized (n = 93) | POMS, BDI |  | Weight in both groups, cognition low fat, NS depression | |
| Hyypa 2003 | Men with hyper- cholesterolemia | Simvistatin v Med Diet v placebo | Randomized (n = 120) | BSI |  | NS depression | |
| Imayama 2011 | Obese and overweight post-menopausal women | Weight loss diet, exercise v no intervention | Randomized (n = 439) | BSI |  | Depression diet/exercise, NS depression diet alone | |
| Jacka 2017 | SMILES study | Patients with clinical depression | Med. Diet v Social Support | Randomized (n = 67) | MADRAS |  | Depression |
| Jazayeri 2008 | Patients with clinical depression | Flu + EPA v flu + pla v EPA + pla | Randomized (n = 60) | HDRS |  | Depression | |
| Jenkinson 2009 | Obese patients with knee pain | Diet v exercise v no intervention | Randomized (n = 389) | HADS |  | Depression | |
| Kasckow 2014 | Veterans with symptoms of depression | Diet Education Group v PST | Randomized (n = 45) | BDI, SF-36 |  | NS depression, GMH | |
| Kasckow 2014 | Veterans with symptoms of depression | Diet Education Group v PST | Randomized (n = 60) | BDI | NS depression | ||
| Kwok 2019 | Older MCI+ H-Hcy | VB12/fol v pla | Randomized (n = 279) | CDR, HDRS |  | Depression | |
| Llorente 2003 | Obese and overweight post-menopausal women | DHA v placebo | Randomized (n = 439) | BSI | NS depression | ||
| Lesperance 2011 | Patients with depression | EPA/DHA v placebo | Randomized (n = 432) | IDS | NS depression | ||
| Llorente 2003 | Pregnant women | DHA v pla | Randomized (n = 99) | BDI | |||
| Lucas 2009 | Middle aged women with symptoms of depression | EPA/DHA v pla | Randomized (n = 120) | HDRS | NS depression | ||
| Makrides 2010 | DOMInO study | Pregnant women | DHA v pla | Randomized (n = 2399) | EPDS | NS depression | |
| Marangell 2003 | Patients with depression | DHA v placebo | Randomized (n = 36) | MADRAS | NS depression | ||
| McMillan 2011 | Young healthy women | Med Diet v no intervention | Randomized (n = 25) | POMS |  | NS depression, cognition . vigor | |
| Mech 2016 | MDD+ MTHFR | VB6/VB12 v pla | Randomized (n = 330) | MADRS |  | depression | |
| Mischoulon 2009 | Patients with depression | EPA v placebo | Randomized (n = 35) | HDRS | NS depression | ||
| Mischoulon 2015 | Patients with depression | EPA v DHA v placebo | Randomized (n = 196) | HDRS | NS depression | ||
| Mozaffari-Khosravi 2013 | Patients with depression | EPA v DHA v placebo | Randomized (n = 81) | HDRS |  | Depression EPA, DHA NS depression | |
| Mozurkewich 2013 | The Mothers, Omega-3 and Mental Health Study | Pregnant with symptoms of depression | EPA Fish oil v DHA fish oil v placebo | Randomized (n = 118) | BDI | NS depression | |
| Nemets 2002 | Adjunctive to antidepressants | Patients with MDD | EPA v placebo | Randomized (n = 20) | HDRS |  | Depression |
| Nemets 2006 | Children with depression | EPA/DHA v placebo | Randomized (n = 28) | CDI, CDRS |  | Depression | |
| Nieman 2000 | Obese women | Exercise and/or weight loss diet v wait list | Randomized (n = 91) | POMS | NS depression | ||
| Okereke 2015 | WAFACS | Normal women | VB6/VB12/Fol v pla | Randomized (n = 4331) | Clin dx | NS depression | |
| Peet 2002 | Adjunct to TAU | Patients with MDD | EPA v placebo | Randomized (n = 70) | HDRS, MADRAS |  | Depression |
| Poppitt 2009 | Patients with Ischemic Stroke | Fish Oil with O3FA v pla | Randomized (n = 102) | GHQ | NS depression | ||
| Rees 2008 | Perinatal MDD | DHA/EPA (fish oil) v placebo | Randomized (n = 26) | HDRS, MADRAS | NS depression | ||
| Rondanelli 2010 | Elderly women with depression | DHA/EPA v placebo | Randomized (n = 46) | GDS |  | depression | |
| Scheier 2005 | Young Women with Breast Cancer | Nutrion group v Health Ed v TAU | Randomized (n = 252) | CES-D |  | Depression with nutrition group | |
| Serrano Ripoll 2015 | Primary Care Patients | Diet & exercise instructions v control | Randomized (n = 273) | BDI | NS depression | ||
| Silvers 2005 | Patients with MD in treatment | EPA/DHA (Fish oil) v placebo | Randomized (n = 77) | HDRS | NS depression | ||
| Sinn 2012 | Patients with MCI | DHA v EPA v linoleic acid | Randomized (n = 50) | GDS |  | Depression | |
| Stoll 1999 | Patients with Bipolar Disorder | EPA/DHA (Fish oil) v placebo | Randomized (n = 30) | Time to relapse (clinical) | Delayed relapse | ||
| Su 2003 | Adjunct to TAU | MDD | EPA/DHA v placebo | Randomized (n = 28) | HDRS |  | depression |
| Su 2008 | Pregnant women with MDD | EPA/DHA v placebo | Randomized (n = 36) | HDRS |  | depression | |
| Su 2014 | Interferon patients | DHA or EPA v placebo | Randomized (n = 162) | Mini |  | Depression onset | |
| Tajalizadekhoob 2011 | Adjunct to TAU | Elderly with mild to mod depression | EPA/DHA (Fish oil) v placebo | Randomized (n = 66) | GDS |  | depression |
| Tayama 2019 | Patients with mild to mod depression | EPA/DHA v placebo | Randomized (n = 90) | BDI | NS depression | ||
| Toobert 2007 | Med Lifestyle Program | Postmenopausal women with DM2 | Med Diet & exercise v control | Randomized (n = 279) | CES-D | NS depression or QOL | |
| Wardle 2000 | Patients with hyper- cholesterolemia | Low fat v Med Diet v wait list | Randomized (n = 176) | BDI, POMS | NS depression | ||
 = decrease;
= decrease;  = increase; VB6 = Vitamin B-6WHI = Women’s Health Initiative.
= increase; VB6 = Vitamin B-6WHI = Women’s Health Initiative.© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bremner, J.D.; Moazzami, K.; Wittbrodt, M.T.; Nye, J.A.; Lima, B.B.; Gillespie, C.F.; Rapaport, M.H.; Pearce, B.D.; Shah, A.J.; Vaccarino, V. Diet, Stress and Mental Health. Nutrients 2020, 12, 2428. https://doi.org/10.3390/nu12082428
Bremner JD, Moazzami K, Wittbrodt MT, Nye JA, Lima BB, Gillespie CF, Rapaport MH, Pearce BD, Shah AJ, Vaccarino V. Diet, Stress and Mental Health. Nutrients. 2020; 12(8):2428. https://doi.org/10.3390/nu12082428
Chicago/Turabian StyleBremner, J. Douglas, Kasra Moazzami, Matthew T. Wittbrodt, Jonathon A. Nye, Bruno B. Lima, Charles F. Gillespie, Mark H. Rapaport, Bradley D. Pearce, Amit J. Shah, and Viola Vaccarino. 2020. "Diet, Stress and Mental Health" Nutrients 12, no. 8: 2428. https://doi.org/10.3390/nu12082428
APA StyleBremner, J. D., Moazzami, K., Wittbrodt, M. T., Nye, J. A., Lima, B. B., Gillespie, C. F., Rapaport, M. H., Pearce, B. D., Shah, A. J., & Vaccarino, V. (2020). Diet, Stress and Mental Health. Nutrients, 12(8), 2428. https://doi.org/10.3390/nu12082428





