Plasma Vitamin C Concentrations Were Negatively Associated with Tingling, Prickling or Pins and Needles Sensation in Patients with Postherpetic Neuralgia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Subjects
2.2. Measurement of Plasma Vitamin C Concentrations
2.3. Evaluation of Spontaneous Pain and Items in the LANSS Questionnaire
2.4. Statistical Analysis
3. Results
3.1. Primary Outcomes: Correlations between Spontaneous Pain/Items in the LANSS and Plasma Vitamin C in PHN Patients
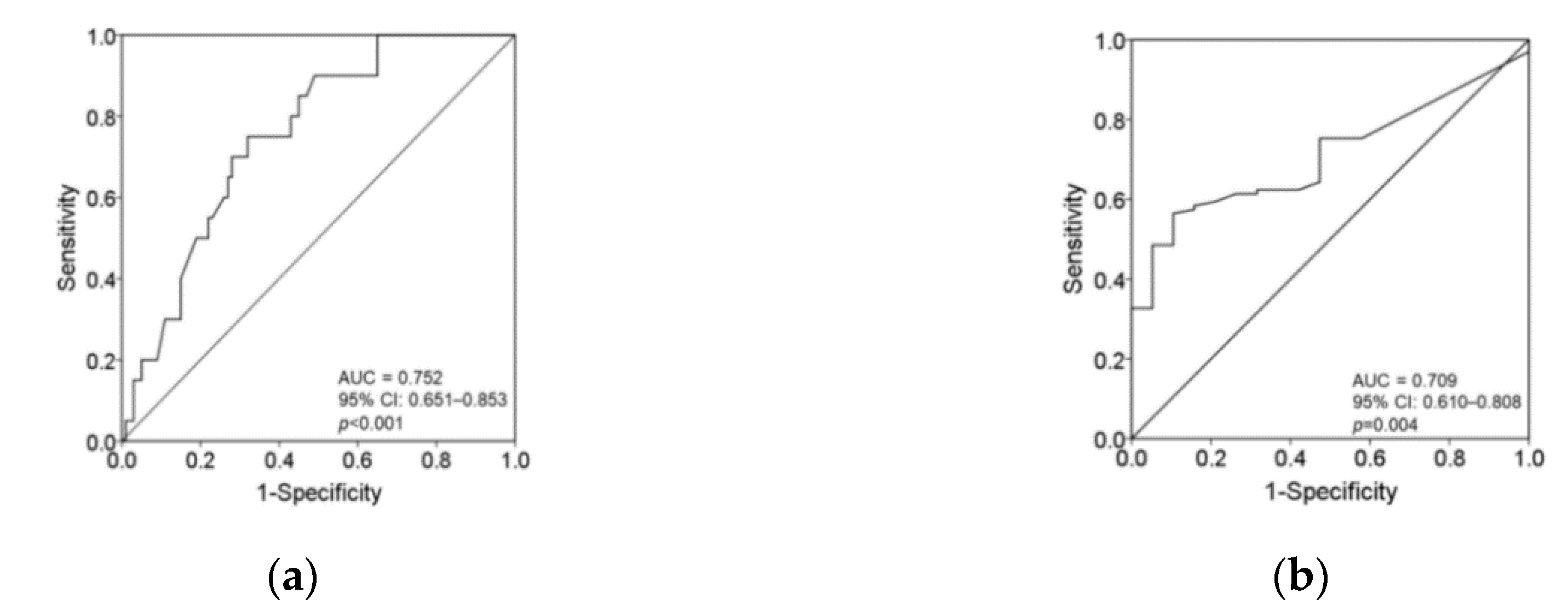
3.2. Secondary Outcomes: The Cutoff for Plasma Vitamin C Concentrations to Predict Tingling, Prickling or Pins and Needles Sensation
3.3. The Proportions of Items in the LANSS Questionnaire in Patients with Different Plasma Vitamin C
3.4. Factors Associated with Plasma Vitamin C deficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
Abbreviations
| AUC | area under the ROC curve |
| H. pylori | Helicobacter pylori |
| HZ | herpes zoster |
| LANSS | Leeds assessment of neuropathic symptoms and signs Pain Scale |
| NMDA | N-methyl-D-aspartate |
| NRS | numeric rating pain scale |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| PHN | postherpetic neuralgia |
| PUD | peptic ulcer disease |
| ROS | reactive oxygen species |
| VZV | varicella-zoster virus |
Appendix A
| Chi Mei Medical Center Pain Clinic |
| First Visit Questionnaire |
| 1. Does the pain produce unpleasant sensations such as tingling, prickling or pins and needles? | Yes (5) No (0) |
| 2. Is there a different skin aspect in the painful areas, i.e., skin redder than usual or appearing mottled? | Yes (5) No (0) |
| 3. Does stroking the skin in the painful area or wearing tight clothing items produce unpleasant sensations? | Yes (3) No (0) |
| 4. Do you experience any sensations like electric shocks, bursting or jumping corresponding to painful episodes, i.e., unexplained bursts of pain? | Yes (2) No (0) |
| 5. Do you experience burning sensations in the painful areas or a sudden temperature change? | Yes (1) No (0) |
| 6. Result from stroking the nonpainful area and the described painful area with cotton wool: | Allodynia in painful area (5) Normal sensations in both areas (0) |
| 7. Result to touching (pinprick) both areas with a 23-gauge needle: | Altered PPT in the painful area (3) Equal sensations in both areas (0) |
References
- Yawn, B.P.; Saddier, P.; Wollan, P.C.; St Sauver, J.L.; Kurland, M.J.; Sy, L.S. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin. Proc. 2007, 82, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Gebremeskel, B.G.; Acosta, C.J. Systematic review of incidence and complications of herpes zoster: Towards a global perspective. BMJ Open 2014, 4, e004833. [Google Scholar] [CrossRef] [PubMed]
- Pappagallo, M.; Oaklander, A.L.; Quatrano-Piacentini, A.L.; Clark, M.R.; Raja, S.N. Heterogenous patterns of sensory dysfunction in postherpetic neuralgia suggest multiple pathophysiologic mechanisms. Anesthesiology 2000, 92, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.H.; Lv, M.M.; Wang, S.; Chen, L.; Qian, N.S.; Tang, Y.; Zhang, X.D.; Ren, P.C.; Gao, C.J.; Sun, X.D.; et al. Spinal astrocytic activation is involved in a virally-induced rat model of neuropathic pain. PLoS ONE 2011, 6, e23059. [Google Scholar] [CrossRef]
- Jagodic, M.M.; Pathirathna, S.; Joksovic, P.M.; Lee, W.; Nelson, M.T.; Naik, A.K.; Su, P.; Jevtovic-Todorovic, V.; Todorovic, S.M. Upregulation of the T-type calcium current in small rat sensory neurons after chronic constrictive injury of the sciatic nerve. J. Neurophysiol. 2008, 99, 3151–3156. [Google Scholar] [CrossRef]
- Nelson, M.T.; Joksovic, P.M.; Su, P.; Kang, H.W.; Van Deusen, A.; Baumgart, J.P.; David, L.S.; Snutch, T.P.; Barrett, P.Q.; Lee, J.H.; et al. Molecular mechanisms of subtype-specific inhibition of neuronal T-type calcium channels by ascorbate. J. Neurosci. 2007, 27, 12577–12583. [Google Scholar] [CrossRef]
- Gilden, D.H.; Cohrs, R.J.; Hayward, A.R.; Wellish, M.; Mahalingam, R. Chronic varicella-zoster virus ganglionitis—A possible cause of postherpetic neuralgia. J. Neurovirol. 2003, 9, 404–407. [Google Scholar] [CrossRef]
- Munoz-Quiles, C.; Lopez-Lacort, M.; Orrico-Sanchez, A.; Diez-Domingo, J. Impact of postherpetic neuralgia: A six year population-based analysis on people aged 50 years or older. J. Infect. 2018, 77, 131–136. [Google Scholar] [CrossRef]
- Carr, A.C.; McCall, C. The role of vitamin C in the treatment of pain: New insights. J. Transl. Med. 2017, 15, 77. [Google Scholar] [CrossRef]
- Yang, Z.; Copolov, D.L.; Lim, A.T. Ascorbic acid augments the adenylyl cyclase-cAMP system mediated POMC mRNA expression and beta-endorphin secretion from hypothalamic neurons in culture. Brain Res. 1996, 706, 243–248. [Google Scholar] [CrossRef]
- Wu, H.Y.; Mao, X.F.; Tang, X.Q.; Ali, U.; Apryani, E.; Liu, H.; Li, X.Y.; Wang, Y.X. Spinal interleukin-10 produces antinociception in neuropathy through microglial beta-endorphin expression, separated from antineuroinflammation. Brain. Behav. Immun. 2018, 73, 504–519. [Google Scholar] [CrossRef] [PubMed]
- Przewlocka, B.; Mika, J.; Labuz, D.; Toth, G.; Przewlocki, R. Spinal analgesic action of endomorphins in acute, inflammatory and neuropathic pain in rats. Eur. J. Pharmacol. 1999, 367, 189–196. [Google Scholar] [CrossRef]
- Wang, C.L.; Yang, D.J.; Yuan, B.Y.; Qiu, T.T. Antiallodynic effects of endomorphin-1 and endomorphin-2 in the spared nerve injury model of neuropathic pain in mice. Anesth. Analg. 2017, 125, 2123–2133. [Google Scholar] [CrossRef]
- Orestes, P.; Bojadzic, D.; Lee, J.; Leach, E.; Salajegheh, R.; Digruccio, M.R.; Nelson, M.T.; Todorovic, S.M. Free radical signalling underlies inhibition of CaV3.2 T-type calcium channels by nitrous oxide in the pain pathway. J. Physiol. 2011, 589, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.J.; Chi, Y.N.; Chen, W.; Liu, F.Y.; Cui, S.; Liao, F.F.; Cai, J.; Wan, Y. Increased expression of CaV3.2 T-type calcium channels in damaged DRG neurons contributes to neuropathic pain in rats with spared nerve injury. Mol. Pain 2018, 14, 1744806918765808. [Google Scholar] [CrossRef]
- Chen, J.Y.; Chu, C.C.; So, E.C.; Hsing, C.H.; Hu, M.L. Treatment of postherpetic neuralgia with intravenous administration of vitamin C. Anesth. Analg. 2006, 103, 1616–1617. [Google Scholar] [CrossRef]
- Thomas, S.L.; Wheeler, J.G.; Hall, A.J. Micronutrient intake and the risk of herpes zoster: A case-control study. Int. J. Epidemiol. 2006, 35, 307–314. [Google Scholar] [CrossRef][Green Version]
- Chen, J.Y.; Chang, C.Y.; Feng, P.H.; Chu, C.C.; So, E.C.; Hu, M.L. Plasma vitamin C is lower in postherpetic neuralgia patients and administration of vitamin C reduces spontaneous pain but not brush-evoked pain. Clin. J. Pain 2009, 25, 562–569. [Google Scholar] [CrossRef]
- Chen, J.Y.; Chu, C.C.; Lin, Y.S.; So, E.C.; Shieh, J.P.; Hu, M.L. Nutrient deficiencies as a risk factor in Taiwanese patients with postherpetic neuralgia. Br. J. Nutr. 2011, 106, 700–707. [Google Scholar] [CrossRef]
- Chen, J.Y.; Chang, C.Y.; Lin, Y.S.; Hu, M.L. Nutritional factors in herpes zoster, postherpetic neuralgia, and zoster vaccination. Popul. Health Manag. 2012, 15, 391–397. [Google Scholar] [CrossRef]
- Schencking, M.; Vollbracht, C.; Weiss, G.; Lebert, J.; Biller, A.; Goyvaerts, B.; Kraft, K. Intravenous vitamin C in the treatment of shingles: Results of a multicenter prospective cohort study. Med. Sci. Monit. 2012, 18, CR215–CR224. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, S. Vitamin C for attenuating postherpetic neuralgia pain: An emerging treatment alternative. J. Headache Pain 2012, 13, 591. [Google Scholar] [CrossRef] [PubMed]
- Beissner, F.; Brandau, A.; Henke, C.; Felden, L.; Baumgartner, U.; Treede, R.D.; Oertel, B.G.; Lotsch, J. Quick discrimination of A(delta) and C fiber mediated pain based on three verbal descriptors. PLoS ONE 2010, 5, e12944. [Google Scholar] [CrossRef] [PubMed]
- Ballaz, S.; Morales, I.; Rodríguez, M.; Obeso, J.A. Ascorbate prevents cell death from prolonged exposure to glutamate in an in vitro model of human dopaminergic neurons. J. Neurosci. Res. 2013, 91, 1609–1617. [Google Scholar] [CrossRef]
- May, J.M. Vitamin C transport and its role in the central nervous system. Subcell. Biochem. 2012, 56, 85–103. [Google Scholar] [CrossRef]
- Domith, I.; Socodato, R.; Portugal, C.C.; Munis, A.F.; Duarte-Silva, A.T.; Paes-de-Carvalho, R. Vitamin C modulates glutamate transport and NMDA receptor function in the retina. J. Neurochem. 2018, 144, 408–420. [Google Scholar] [CrossRef]
- Bennett, M. The LANSS Pain Scale: The Leeds assessment of neuropathic symptoms and signs. Pain 2001, 92, 147–157. [Google Scholar] [CrossRef]
- Frei, B.; Forte, T.M.; Ames, B.N.; Cross, C.E. Gas phase oxidants of cigarette smoke induce lipid peroxidation and changes in lipoprotein properties in human blood plasma. Protective effects of ascorbic acid. Biochem. J. 1991, 277 Pt 1, 133–138. [Google Scholar] [CrossRef]
- Tawfeek, H.I.; Muhyaddin, O.M.; al-Sanwi, H.I.; al-Baety, N. Effect of maternal dietary vitamin C intake on the level of vitamin C in breastmilk among nursing mothers in Baghdad, Iraq. Food Nutr. Bull. 2002, 23, 244–247. [Google Scholar] [CrossRef]
- Nair, S.; Norkus, E.P.; Hertan, H.; Pitchumoni, C.S. Micronutrient antioxidants in gastric mucosa and serum in patients with gastritis and gastric ulcer: Does Helicobacter pylori infection affect the mucosal levels? J. Clin. Gastroenterol. 2000, 30, 381–385. [Google Scholar] [CrossRef]
- Aditi, A.; Graham, D.Y. Vitamin C, gastritis, and gastric disease: A historical review and update. Dig. Dis. Sci. 2012, 57, 2504–2515. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.; Loughrey, C.M.; Lightbody, J.H.; McNamee, P.T.; Young, I.S. Effect of hemodialysis on total antioxidant capacity and serum antioxidants in patients with chronic renal failure. Clin. Chem. 1995, 41, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Pincemail, J.; Vanbelle, S.; Degrune, F.; Cheramy-Bien, J.P.; Charlier, C.; Chapelle, J.P.; Giet, D.; Collette, G.; Albert, A.; Defraigne, J.O. Lifestyle behaviours and plasma vitamin C and beta-carotene levels from the ELAN population (Liege, Belgium). J. Nutr. Metab. 2011, 2011, 494370. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; Gnann, J.W., Jr.; Oaklander, A.L.; Raja, S.N.; Schmader, K.E.; Whitley, R.J. Diagnosis and assessment of pain associated with herpes zoster and postherpetic neuralgia. J. Pain 2008, 9, S37–S44. [Google Scholar] [CrossRef]
- Joesoef, R.M.; Harpaz, R.; Leung, J.; Bialek, S.R. Chronic medical conditions as risk factors for herpes zoster. Mayo Clin. Proc. 2012, 87, 961–967. [Google Scholar] [CrossRef]
- Chen, J.Y.; Cheng, T.J.; Chang, C.Y.; Lan, K.M.; Weng, S.F.; Sheu, M.J.; Tseng, S.F.; Hu, M.L. Increased incidence of herpes zoster in adult patients with peptic ulcer disease: A population-based cohort study. Int. J. Epidemiol. 2013, 42, 1873–1881. [Google Scholar] [CrossRef]
- Chen, J.Y.; Lan, K.M.; Sheu, M.J.; Tseng, S.F.; Weng, S.F.; Hu, M.L. Peptic ulcer as a risk factor for postherpetic neuralgia in adult patients with herpes zoster. J. Med. Virol. 2015, 87, 222–229. [Google Scholar] [CrossRef]
- Yang, Y.W.; Chen, Y.H.; Wang, K.H.; Wang, C.Y.; Lin, H.W. Risk of herpes zoster among patients with chronic obstructive pulmonary disease: A population-based study. CMAJ 2011, 183, E275–E280. [Google Scholar] [CrossRef]
- Forbes, H.J.; Bhaskaran, K.; Thomas, S.L.; Smeeth, L.; Clayton, T.; Langan, S.M. Quantification of risk factors for herpes zoster: Population based case-control study. BMJ 2014, 348, g2911. [Google Scholar] [CrossRef]
- Chen, J.Y.; Chang, C.Y.; Lan, K.M.; Sheu, M.J.; Lu, C.L.; Hu, M.L. Is peptic ulcer disease a risk factor of postherpetic neuralgia in patients with herpes zoster? Med. Hypotheses 2013, 81, 834–838. [Google Scholar] [CrossRef]
- Del Pozo, J.L.; van de Beek, D.; Mandrekar, J.N.; Daly, R.C.; McGregor, C.G.; Azanza, J.R.; Patel, R. High serum cholesterol levels are associated with herpes zoster infection after heart transplantation. Clin. Infect. Dis. 2010, 50, 121–122. [Google Scholar] [CrossRef] [PubMed]
- Littlefield, L.G.; Joiner, E.E. Analysis of chromosome aberrations in lymphocytes of long-term heavy smokers. Mutat. Res. 1986, 170, 145–150. [Google Scholar] [CrossRef]
- Freeman, R.; Baron, R.; Bouhassira, D.; Cabrera, J.; Emir, B. Sensory profiles of patients with neuropathic pain based on the neuropathic pain symptoms and signs. Pain 2014, 155, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; Jensen, M.P.; Gammaitoni, A.R.; Olaleye, D.O.; Galer, B.S. Symptom profiles differ in patients with neuropathic versus non-neuropathic pain. J. Pain 2007, 8, 118–126. [Google Scholar] [CrossRef]
- Baron, R.; Binder, A.; Wasner, G. Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010, 9, 807–819. [Google Scholar] [CrossRef]
- Shah, S.A.; Yoon, G.H.; Kim, H.O.; Kim, M.O. Vitamin C neuroprotection against dose-dependent glutamate-induced neurodegeneration in the postnatal brain. Neurochem. Res. 2015, 40, 875–884. [Google Scholar] [CrossRef]
- Chen, J.Y.; Lin, Y.T.; Wang, L.K.; Hung, K.C.; Lan, K.M.; Ho, C.H.; Chang, C.Y. Hypovitaminosis D in postherpetic neuralgia—High prevalence and inverse association with pain: A retrospective study. Nutrients 2019, 11, 2787. [Google Scholar] [CrossRef]
- Arundine, M.; Tymianski, M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium 2003, 34, 325–337. [Google Scholar] [CrossRef]
- Lu, R.; Kallenborn-Gerhardt, W.; Geisslinger, G.; Schmidtko, A. Additive antinociceptive effects of a combination of vitamin C and vitamin E after peripheral nerve injury. PLoS ONE 2011, 6, e29240. [Google Scholar] [CrossRef]
- Wang, L.K.; Chuang, C.C.; Chen, J.Y. Relief of acute herpetic pain by intravenous vitamin C: The dosage may make a difference. Ann. Dermatol. 2018, 30, 262–263. [Google Scholar] [CrossRef]
- Hemila, H. Vitamin C and infections. Nutrients 2017, 9, 339. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, D.J.; Na, C.H.; Shin, B.S. A study of intravenous administration of vitamin C in the treatment of acute herpetic pain and postherpetic neuralgia. Ann. Dermatol. 2016, 28, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Vadillo, M.A.; Konstantinidis, E.; Shanks, D.R. Underpowered samples, false negatives, and unconscious learning. Psychon. Bull. Rev. 2016, 23, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, A.; Luna, J.D.; Del Pozo, E.; Galvez, R. Diagnostic accuracy of two questionnaires for the detection of neuropathic pain in the Spanish population. Eur. J. Pain 2014, 18, 101–109. [Google Scholar] [CrossRef]
- McCorry, L.K. Physiology of the autonomic nervous system. Am. J. Pharm. Educ. 2007, 71, 78. [Google Scholar] [CrossRef]
- May, J.M.; Qu, Z.C.; Meredith, M.E. Mechanisms of ascorbic acid stimulation of norepinephrine synthesis in neuronal cells. Biochem. Biophys. Res. Commun. 2012, 426, 148–152. [Google Scholar] [CrossRef]
- May, J.M.; Qu, Z.C.; Nazarewicz, R.; Dikalov, S. Ascorbic acid efficiently enhances neuronal synthesis of norepinephrine from dopamine. Brain Res. Bull. 2013, 90, 35–42. [Google Scholar] [CrossRef]
- Nandi, A.; Mukhopadhyay, C.K.; Ghosh, M.K.; Chattopadhyay, D.J.; Chatterjee, I.B. Evolutionary significance of vitamin C biosynthesis in terrestrial vertebrates. Free Radic. Biol. Med. 1997, 22, 1047–1054. [Google Scholar] [CrossRef]
- Woodward, M.; Tunstall-Pedoe, H.; McColl, K. Helicobacter pylori infection reduces systemic availability of dietary vitamin C. Eur. J. Gastroenterol. Hepatol. 2001, 13, 233–237. [Google Scholar] [CrossRef]
- Biondi, C.; Pavan, B.; Dalpiaz, A.; Medici, S.; Lunghi, L.; Vesce, F. Expression and characterization of vitamin C transporter in the human trophoblast cell line HTR-8/SVneo: Effect of steroids, flavonoids and NSAIDs. Mol. Hum. Reprod. 2007, 13, 77–83. [Google Scholar] [CrossRef]
- Henry, E.B.; Carswell, A.; Wirz, A.; Fyffe, V.; McColl, K.E. Proton pump inhibitors reduce the bioavailability of dietary vitamin C. Aliment. Pharmacol. Ther. 2005, 22, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Parruti, G.; Tontodonati, M.; Rebuzzi, C.; Polilli, E.; Sozio, F.; Consorte, A.; Agostinone, A.; Di Masi, F.; Congedo, G.; D’Antonio, D.; et al. Predictors of pain intensity and persistence in a prospective Italian cohort of patients with herpes zoster: Relevance of smoking, trauma and antiviral therapy. BMC Med. 2010, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Viscovich, M.; Poulsen, H.E. Ascorbic acid recycling in human erythrocytes is induced by smoking in vivo. Free Radic. Biol. Med. 2003, 35, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Harbecke, R.; Oxman, M.N.; Arnold, B.A.; Ip, C.; Johnson, G.R.; Levin, M.J.; Gelb, L.D.; Schmader, K.E.; Straus, S.E.; Wang, H.; et al. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. J. Med. Virol. 2009, 81, 1310–1322. [Google Scholar] [CrossRef] [PubMed]
- Gariballa, S. Poor vitamin C status is associated with increased depression symptoms following acute illness in older people. Int. J. Vitam. Nutr. Res. 2014, 84, 12–17. [Google Scholar] [CrossRef]
- Irwin, M.R.; Levin, M.J.; Carrillo, C.; Olmstead, R.; Lucko, A.; Lang, N.; Caulfield, M.J.; Weinberg, A.; Chan, I.S.; Clair, J.; et al. Major depressive disorder and immunity to varicella-zoster virus in the elderly. Brain. Behav. Immun. 2011, 25, 759–766. [Google Scholar] [CrossRef]
- Schmader, K.; George, L.K.; Burchett, B.M.; Pieper, C.F.; Hamilton, J.D. Racial differences in the occurrence of herpes zoster. J. Infect. Dis. 1995, 171, 701–704. [Google Scholar] [CrossRef]
- Julian, T.; Syeed, R.; Glascow, N.; Angelopoulou, E.; Zis, P. B12 as a Treatment for Peripheral Neuropathic Pain: A Systematic Review. Nutrients 2020, 12, 2221. [Google Scholar] [CrossRef]

| Parameters | Mean (SD) |
|---|---|
| Age, mean (SD) (years) | 66.45 (11.34) |
| Body height, mean (SD) (cm) | 158.67 (11.42) |
| Body weight, mean (SD) (kg) | 55.99 (11.86) |
| Male, n (%) | 63 (52.5%) |
| Duration of pain, mean (SD) (months) | 8.55 (7.50) |
| Plasma concentrations of vitamin C (6–15 mg/L) | |
| mean (SD) (mg/L) | 6.34 (3.80) |
| Well-nourished (≥10 mg/L; 56.8 μmol/L), n (%) | 19 (15.8%) |
| Adequate (6–10 mg/L; 34.1–56.8 μmol/L), n (%) | 38 (31.7%) |
| Deficiency (<6 mg/L; 34.1 μmol/L), n (%) | 63 (52.5%) |
| Comorbidities/habits (n, %) | |
| Hypertension | 47 (39.2%) |
| Diabetes mellitus | 31 (25.8%) |
| Peptic ulcer disease | 37 (30.8%) |
| Cancers | 12 (10.0%) |
| COPD | 15 (12.5%) |
| Chronic kidney disease with dialysis | 0 (0%) a |
| Hypercholesterolemia | 13 (10.8%) |
| Smoking (Male: 31; Female: 0; heavy smokers b: 21) | 31 (25.8%) |
| Alcohol intake | 5 (4.2%) |
| Low intake of fruits and vegetables c | 46 (38.3%) |
| Spearman Correlation Coefficient | p | |
|---|---|---|
| Plasma vitamin C concentrations vs. Spontaneous pain (NRS 0–10) | −0.420 * | <0.001 |
| vs. Items in the LANSS questionnaire | ||
| Tingling, prickling or pins and needles sensation (NRS 0–10) | −0.449 * | <0.001 |
| A different skin aspect (Yes: 1; No: 0) | −0.250 | 0.007 |
| Abnormally sensitive to touch (Yes: 1; No: 0) | −0.231 | 0.011 |
| Sudden electric shocks, bursting, jumping pain (NRS 0–10) | −0.104 | 0.265 |
| Burning pain (NRS 0–10) | −0.173 | 0.058 |
| Allodynia (NRS 0–10) | −0.139 | 0.131 |
| Altered pin-prick threshold (Yes: 1; No: 0) | −0.113 | 0.218 |
| Cutoff for Plasma Vitamin C Concentrations | ≥7.05 mg/L (n = 47) | <7.05 mg/L (n = 73) | p | ≥5.68 mg/L (n = 57) | <5.68 mg/L (n = 63) | p |
|---|---|---|---|---|---|---|
| Tingling, prickling or pins and needles sensations, n (%) | 29 (61.7) | 71 (97.3) | <0.001 | 44 (74.6) | 59 (96.7) | <0.001 |
| A different skin aspect in the painful areas, n (%) | 26 (55.3) | 51 (69.9) | 0.105 | 34 (57.6) | 43 (70.5) | 0.142 |
| Abnormally sensitive to touch in the painful area, n (%) | 18 (38.3) | 36 (49.3) | 0.236 | 23 (39.0) | 31 (50.8) | 0.193 |
| Sudden electric shocks, bursting or jumping pain, n (%) | 24 (51.1) | 39 (48.9) | 0.800 | 32 (54.2) | 31 (50.8) | 0.708 |
| Burning pain, n (%) | 9 (19.1) | 22 (30.1) | 0.180 | 13 (22.0) | 18 (29.5) | 0.350 |
| Allodynia in painful area, n (%) | 23 (48.9) | 45 (61.6) | 0.170 | 29 (49.2) | 39 (63.9) | 0.102 |
| Altered pin-prick threshold, n (%) | 20 (42.6) | 21 (28.8) | 0.120 | 24 (40.7) | 17 (27.9) | 0.139 |
| Well-Nourished | Deficient | |||||
|---|---|---|---|---|---|---|
| Cutoff for Plasma Vitamin C Concentrations | ≥10 mg/L (n = 19) | <10 mg/L (n = 101) | p | ≥6.0 mg/L (n = 57) | <6.0 mg/L (n = 63) | |
| Tingling, prickling or pins and needles sensations, n (%) | 11 (57.9) | 89 (88.1) | <0.001 | 39 (68.4) | 61 (96.8) | <0.001 |
| A different skin aspect in the painful areas, n (%) | 8 (42.1) | 69 (68.3) | 0.029 | 33 (57.9) | 44 (69.8) | 0.173 |
| Abnormally sensitive to touch in the painful area, n (%) | 6 (31.6) | 48 (47.5) | 0.200 | 22 (38.6) | 32 (50.8) | 0.180 |
| Sudden electric shocks, bursting or jumping pain, n (%) | 6 (31.6) | 57 (56.4) | 0.047 | 31 (54.4) | 32 (50.8) | 0.694 |
| Burning pain, n (%) | 2 (10.5) | 29 (28.7) | 0.097 | 12 (21.1) | 19 (30.2) | 0.255 |
| Allodynia in painful area, n (%) | 7 (36.8) | 61 (60.4) | 0.057 | 28 (49.1) | 40 (63.5) | 0.113 |
| Altered pin-prick threshold, n (%) | 10 (52.6) | 31 (30.7) | 0.064 | 24 (42.1) | 17 (27.0) | 0.081 |
| Vitamin C Deficiency (<6 mg/L) (n = 63, 52.5%) | ||||
|---|---|---|---|---|
| Variables | Crude Odds Ratio (95% CI) | pb | Adjusted Odds Ratio (95% CI) | pb |
| Gender (male vs. female) | 2.23 (1.08, 4.64) | 0.030 * | ||
| Age (≥70 vs. <70 years old) | 0.89 (0.43, 1.84) | 0.761 | ||
| Hypertension | 2.48 (1.16, 5.31) | 0.018 * | ||
| Diabetes mellitus | 1.95 (0.84, 4.53) | 0.120 | ||
| Peptic ulcer disease | 5.95 (2.60, 13.61) | <0.001 * | 3.25 (1.28–8.28) | 0.014 * |
| Cancer | 3.0 (0.77, 11.69) | 0.100 | ||
| COPD | 1.42 (0.47, 4.26) | 0.534 | ||
| Hypercholesterolemia | 1.06 (0.33, 3.37) | 0.918 | ||
| Smoking | 10.65 (4.30, 26.37) | <0.001 * | 3.60 (1.33–9.77) | 0.010 * |
| Alcohol intake | 3.80 (0.41, 35.01) | 0.208 | ||
| Low intake of fruits and vegetables before outbreaks of herpes zoster a | 11.61 (4.53, 29.72) | <0.001 * | 2.66 (1.09–6.48) | 0.032 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-K.; Lin, Y.-T.; Hung, K.-C.; Chang, C.-Y.; Wu, Z.-F.; Hu, M.-L.; Chen, J.-Y. Plasma Vitamin C Concentrations Were Negatively Associated with Tingling, Prickling or Pins and Needles Sensation in Patients with Postherpetic Neuralgia. Nutrients 2020, 12, 2384. https://doi.org/10.3390/nu12082384
Wang L-K, Lin Y-T, Hung K-C, Chang C-Y, Wu Z-F, Hu M-L, Chen J-Y. Plasma Vitamin C Concentrations Were Negatively Associated with Tingling, Prickling or Pins and Needles Sensation in Patients with Postherpetic Neuralgia. Nutrients. 2020; 12(8):2384. https://doi.org/10.3390/nu12082384
Chicago/Turabian StyleWang, Li-Kai, Yao-Tsung Lin, Kuo-Chuan Hung, Chia-Yu Chang, Zhi-Fu Wu, Miao-Lin Hu, and Jen-Yin Chen. 2020. "Plasma Vitamin C Concentrations Were Negatively Associated with Tingling, Prickling or Pins and Needles Sensation in Patients with Postherpetic Neuralgia" Nutrients 12, no. 8: 2384. https://doi.org/10.3390/nu12082384
APA StyleWang, L.-K., Lin, Y.-T., Hung, K.-C., Chang, C.-Y., Wu, Z.-F., Hu, M.-L., & Chen, J.-Y. (2020). Plasma Vitamin C Concentrations Were Negatively Associated with Tingling, Prickling or Pins and Needles Sensation in Patients with Postherpetic Neuralgia. Nutrients, 12(8), 2384. https://doi.org/10.3390/nu12082384