The Impact of Protein Supplementation Targeted at Improving Muscle Mass on Strength in Cancer Patients: A Scoping Review
Abstract
1. Introduction
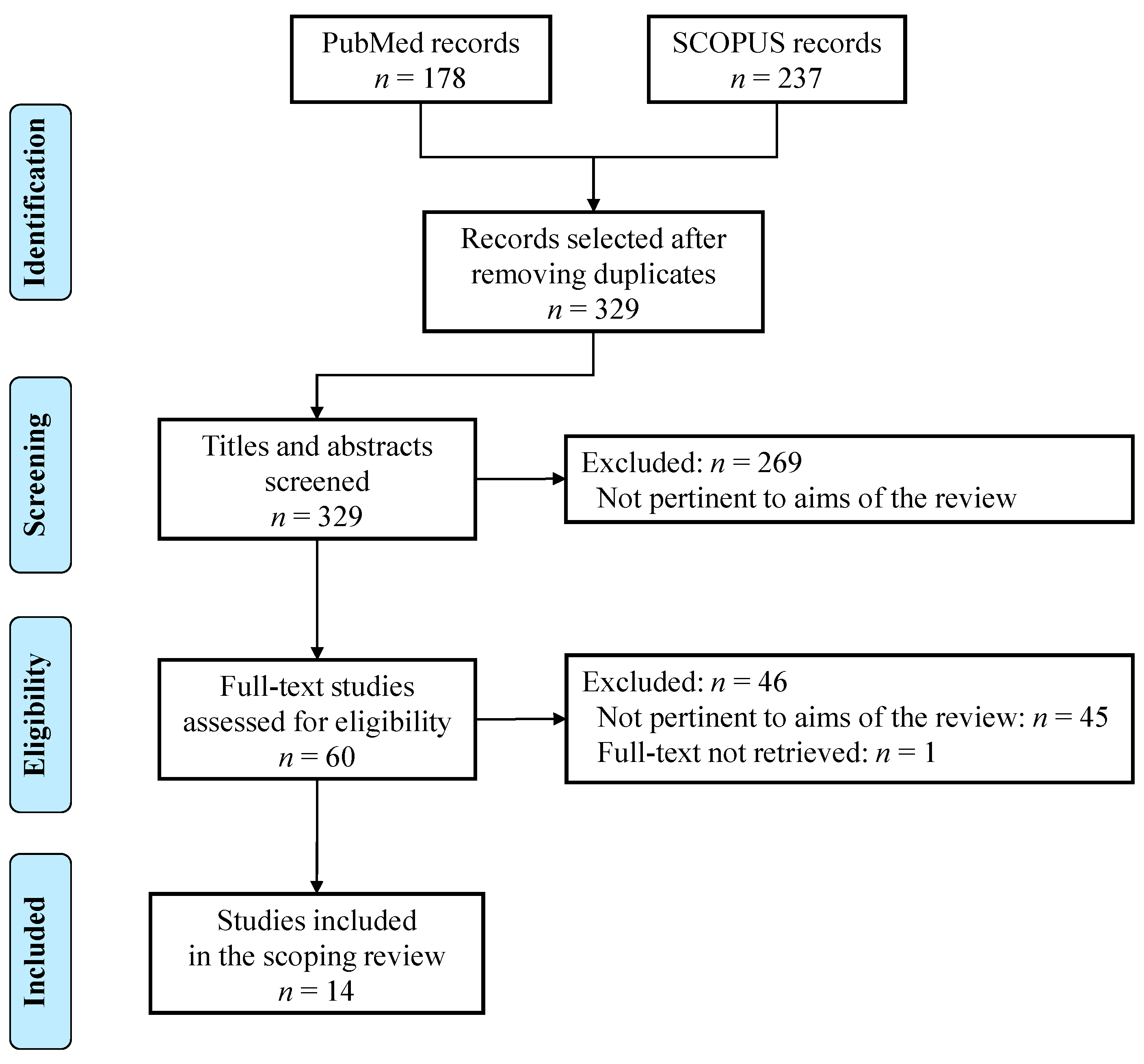
2. Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Study Selection
2.4. Synthesis of Results
3. Results
3.1. Trials with Amino Acids, Proteins, and Protein-Derived Dietary Supplements
3.2. Trials with Nutritional Supplementation Including Proteins and Other Macronutrients
4. Discussion
Limitations
5. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lieffers, J.R.; Bathe, O.F.; Fassbender, K.; Winget, M.; Baracos, V.E. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br. J. Cancer 2012, 107, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Baracos, V.E.; Arribas, L. Sarcopenic obesity: Hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, ii1–ii9. [Google Scholar] [CrossRef] [PubMed]
- Bozzetti, F. Forcing the vicious circle: Sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 2107–2118. [Google Scholar] [CrossRef] [PubMed]
- Mir, O.; Coriat, R.; Blanchet, B.; Durand, J.P.; Boudou-Rouquette, P.; Michels, J.; Ropert, S.; Vidal, M.; Pol, S.; Chaussade, S.; et al. Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PLoS ONE 2012, 7, e37563. [Google Scholar] [CrossRef]
- da Cunha, L.P.; Silveira, M.N.; Mendes, M.C.S.; Costa, F.O.; Macedo, L.T.; de Siqueira, N.S.; Carvalheira, J.B.C. Sarcopenia as an independent prognostic factor in patients with metastatic colorectal cancer: A retrospective evaluation. Clin. Nutr. ESPEN 2019, 32, 107–112. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Bye, A.; Sjoblom, B.; Wentzel-Larsen, T.; Gronberg, B.H.; Baracos, V.E.; Hjermstad, M.J.; Aass, N.; Bremnes, R.M.; Flotten, O.; Jordhoy, M. Muscle mass and association to quality of life in non-small cell lung cancer patients. J. Cachexia Sarcopenia Muscle 2017, 8, 759–767. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Jette, M.; Hendricks, S. Guide for Anthropometric Measurements of Canadian Adults; C.T. Management & Consultant: Montreal, QC, Canada, 1982. [Google Scholar]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Pamoukdjian, F.; Bouillet, T.; Levy, V.; Soussan, M.; Zelek, L.; Paillaud, E. Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: A systematic review. Clin. Nutr. 2018, 37, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Deluche, E.; Leobon, S.; Desport, J.C.; Venat-Bouvet, L.; Usseglio, J.; Tubiana-Mathieu, N. Impact of body composition on outcome in patients with early breast cancer. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2018, 26, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Caan, B.J.; Weltzien, E.; Cespedes Feliciano, E.M.; Kroenke, C.H.; Meyerhardt, J.A.; Baracos, V.E.; Kwan, M.L.; Castillo, A.L.; Prado, C.M. Associations of pre-existing co-morbidities with skeletal muscle mass and radiodensity in patients with non-metastatic colorectal cancer. J. Cachexia Sarcopenia Muscle 2018, 9, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Aleixo, G.F.P.; Shachar, S.S.; Nyrop, K.A.; Muss, H.B.; Malpica, L.; Williams, G.R. Myosteatosis and prognosis in cancer: Systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2020, 145, 102839. [Google Scholar] [CrossRef] [PubMed]
- Kilgour, R.D.; Vigano, A.; Trutschnigg, B.; Lucar, E.; Borod, M.; Morais, J.A. Handgrip strength predicts survival and is associated with markers of clinical and functional outcomes in advanced cancer patients. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2013, 21, 3261–3270. [Google Scholar] [CrossRef]
- Pedersen, J.K.; Engholm, G.; Skytthe, A.; Christensen, K.; Academy of Geriatric Cancer Research. Cancer and aging: Epidemiology and methodological challenges. Acta Oncol. 2016, 55, 7–12. [Google Scholar] [CrossRef]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Prado, C.M.; Purcell, S.A.; Laviano, A. Nutrition interventions to treat low muscle mass in cancer. J. Cachexia Sarcopenia Muscle 2020, 11, 366–380. [Google Scholar] [CrossRef]
- Zambrano, D.N.; Xiao, J.; Prado, C.M.; Gonzalez, M.C. Patient-Generated Subjective Global Assessment and Computed Tomography in the assessment of malnutrition and sarcopenia in patients with cirrhosis: Is there any association? Clin. Nutr. 2020, 39, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Moreau, J.; Ordan, M.A.; Barbe, C.; Mazza, C.; Perrier, M.; Botsen, D.; Brasseur, M.; Portefaix, C.; Renard, Y.; Talliere, B.; et al. Correlation between muscle mass and handgrip strength in digestive cancer patients undergoing chemotherapy. Cancer Med. 2019, 8, 3677–3684. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.C.; Segal, R.J.; McKenzie, D.C.; Vallerand, J.R.; Morielli, A.R.; Mackey, J.R.; Gelmon, K.; Friedenreich, C.M.; Reid, R.D.; Courneya, K.S. Impact of resistance and aerobic exercise on sarcopenia and dynapenia in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. Breast Cancer Res. Treat. 2016, 158, 497–507. [Google Scholar] [CrossRef]
- de Rooy, C.; Grossmann, M.; Zajac, J.D.; Cheung, A.S. Targeting muscle signaling pathways to minimize adverse effects of androgen deprivation. Endocr. Relat. Cancer 2016, 23, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Botsen, D.; Ordan, M.A.; Barbe, C.; Mazza, C.; Perrier, M.; Moreau, J.; Brasseur, M.; Renard, Y.; Tailliere, B.; Slimano, F.; et al. Dynapenia could predict chemotherapy-induced dose-limiting neurotoxicity in digestive cancer patients. BMC Cancer 2018, 18, 955. [Google Scholar] [CrossRef]
- Chen, C.H.; Ho, C.; Huang, Y.Z.; Hung, T.T. Hand-grip strength is a simple and effective outcome predictor in esophageal cancer following esophagectomy with reconstruction: A prospective study. J. Cardiothorac. Surg. 2011, 6, 98. [Google Scholar] [CrossRef]
- Contreras-Bolivar, V.; Sanchez-Torralvo, F.J.; Ruiz-Vico, M.; Gonzalez-Almendros, I.; Barrios, M.; Padin, S.; Alba, E.; Olveira, G. GLIM criteria using hand grip strength adequately predict six-month mortality in cancer inpatients. Nutrients 2019, 11, 2043. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hermoso, A.; Ramirez-Velez, R.; Peterson, M.D.; Lobelo, F.; Cavero-Redondo, I.; Correa-Bautista, J.E.; Martinez-Vizcaino, V. Handgrip and knee extension strength as predictors of cancer mortality: A systematic review and meta-analysis. Scand. J. Med. Sci. Sports 2018, 28, 1852–1858. [Google Scholar] [CrossRef]
- Kilgour, R.D.; Vigano, A.; Trutschnigg, B.; Hornby, L.; Lucar, E.; Bacon, S.L.; Morais, J.A. Cancer-related fatigue: The impact of skeletal muscle mass and strength in patients with advanced cancer. J. Cachexia Sarcopenia Muscle 2010, 1, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Stobaus, N.; Smoliner, C.; Zocher, D.; Scheufele, R.; Valentini, L.; Lochs, H.; Pirlich, M. Determinants of hand grip strength, knee extension strength and functional status in cancer patients. Clin. Nutr. 2010, 29, 586–591. [Google Scholar] [CrossRef]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E.; Turri, A.; Klersy, C.; Cappello, S.; Ferrari, A.; Filippi, A.R.; Brugnatelli, S.; Caraccia, M.; Chiellino, S.; Borioli, V.; et al. Whey protein isolate supplementation improves body composition, muscle strength, and treatment tolerance in malnourished advanced cancer patients undergoing chemotherapy. Cancer Med. 2019, 8, 6923–6932. [Google Scholar] [CrossRef]
- Madeddu, C.; Macciò, A.; Astara, G.; Massa, E.; Dessì, M.; Antoni, G.; Panzone, F.; Serpe, R.; Mantovani, G. Open phase II study on efficacy and safety of an oral amino acid functional cluster supplementation in cancer cachexia. Mediterr. J. Nutr. Metab. 2010, 3, 165–172. [Google Scholar] [CrossRef]
- Dawson, J.K.; Dorff, T.B.; Todd Schroeder, E.; Lane, C.J.; Gross, M.E.; Dieli-Conwright, C.M. Impact of resistance training on body composition and metabolic syndrome variables during androgen deprivation therapy for prostate cancer: A pilot randomized controlled trial. BMC Cancer 2018, 18, 368. [Google Scholar] [CrossRef]
- Lonbro, S.; Dalgas, U.; Primdahl, H.; Overgaard, J.; Overgaard, K. Feasibility and efficacy of progressive resistance training and dietary supplements in radiotherapy treated head and neck cancer patients—The DAHANCA 25A study. Acta Oncol. 2013, 52, 310–318. [Google Scholar] [CrossRef]
- Madzima, T.A.; Ormsbee, M.J.; Schleicher, E.A.; Moffatt, R.J.; Panton, L.B. Effects of Resistance Training and Protein Supplementation in Breast Cancer Survivors. Med. Sci. Sports Exerc. 2017, 49, 1283–1292. [Google Scholar] [CrossRef]
- Mantovani, G.; Maccio, A.; Madeddu, C.; Serpe, R.; Massa, E.; Dessi, M.; Panzone, F.; Contu, P. Randomized phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist 2010, 15, 200–211. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, J.; Li, M.; Yi, S.; Xie, J.; Zhang, H.; Wang, J. Protein blend ingestion before allogeneic stem cell transplantation improves protein-energy malnutrition in patients with leukemia. Nutr. Res. 2017, 46, 68–77. [Google Scholar] [CrossRef]
- Wada, N.; Kurokawa, Y.; Tanaka, K.; Miyazaki, Y.; Makino, T.; Takahashi, T.; Wada, H.; Yamasaki, M.; Yamasaki, M.; Nakajima, K.; et al. Perioperative Nutritional Support With Beta-hydroxy-beta-methylbutyrate, Arginine, and Glutamine in Surgery for Abdominal Malignancies. Wounds 2018, 30, 251–256. [Google Scholar] [PubMed]
- Arribas, L.; Hurtos, L.; Taberna, M.; Peiro, I.; Vilajosana, E.; Lozano, A.; Vazquez, S.; Mesia, R.; Virgili, N. Nutritional changes in patients with locally advanced head and neck cancer during treatment. Oral Oncol. 2017, 71, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Caccialanza, R.; Cereda, E.; Caraccia, M.; Klersy, C.; Nardi, M.; Cappello, S.; Borioli, V.; Turri, A.; Imarisio, I.; Lasagna, A.; et al. Early 7-day supplemental parenteral nutrition improves body composition and muscle strength in hypophagic cancer patients at nutritional risk. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2019, 27, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E.; Cappello, S.; Colombo, S.; Klersy, C.; Imarisio, I.; Turri, A.; Caraccia, M.; Borioli, V.; Monaco, T.; Benazzo, M.; et al. Nutritional counseling with or without systematic use of oral nutritional supplements in head and neck cancer patients undergoing radiotherapy. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2018, 126, 81–88. [Google Scholar] [CrossRef]
- Obling, S.R.; Wilson, B.V.; Pfeiffer, P.; Kjeldsen, J. Home parenteral nutrition increases fat free mass in patients with incurable gastrointestinal cancer. Results of a randomized controlled trial. Clin. Nutr. 2019, 38, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Schink, K.; Herrmann, H.J.; Schwappacher, R.; Meyer, J.; Orlemann, T.; Waldmann, E.; Wullich, B.; Kahlmeyer, A.; Fietkau, R.; Lubgan, D.; et al. Effects of whole-body electromyostimulation combined with individualized nutritional support on body composition in patients with advanced cancer: A controlled pilot trial. BMC Cancer 2018, 18, 886. [Google Scholar] [CrossRef]
- Uster, A.; Ruefenacht, U.; Ruehlin, M.; Pless, M.; Siano, M.; Haefner, M.; Imoberdorf, R.; Ballmer, P.E. Influence of a nutritional intervention on dietary intake and quality of life in cancer patients: A randomized controlled trial. Nutrition 2013, 29, 1342–1349. [Google Scholar] [CrossRef]
- Antoun, S.; Baracos, V.E.; Birdsell, L.; Escudier, B.; Sawyer, M.B. Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2010, 21, 1594–1598. [Google Scholar] [CrossRef]
- Barret, M.; Antoun, S.; Dalban, C.; Malka, D.; Mansourbakht, T.; Zaanan, A.; Latko, E.; Taieb, J. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr. Cancer 2014, 66, 583–589. [Google Scholar] [CrossRef]
- Prado, C.M.; Baracos, V.E.; McCargar, L.J.; Mourtzakis, M.; Mulder, K.E.; Reiman, T.; Butts, C.A.; Scarfe, A.G.; Sawyer, M.B. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin. Cancer Res. 2007, 13, 3264–3268. [Google Scholar] [CrossRef]
- Prado, C.M.; Baracos, V.E.; McCargar, L.J.; Reiman, T.; Mourtzakis, M.; Tonkin, K.; Mackey, J.R.; Koski, S.; Pituskin, E.; Sawyer, M.B. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin. Cancer Res 2009, 15, 2920–2926. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R.; Deal, A.M.; Muss, H.B.; Weinberg, M.S.; Sanoff, H.K.; Nyrop, K.A.; Pergolotti, M.; Shachar, S.S. Skeletal muscle measures and physical function in older adults with cancer: Sarcopenia or myopenia? Oncotarget 2017, 8, 33658–33665. [Google Scholar] [CrossRef] [PubMed]
- Morishita, S.; Tsubaki, A.; Fu, J.B.; Mitobe, Y.; Onishi, H.; Tsuji, T. Cancer survivors exhibit a different relationship between muscle strength and health-related quality of life/fatigue compared to healthy subjects. Eur. J. Cancer Care 2018, 27, e12856. [Google Scholar] [CrossRef] [PubMed]
- Ness, K.K.; Mertens, A.C.; Hudson, M.M.; Wall, M.M.; Leisenring, W.M.; Oeffinger, K.C.; Sklar, C.A.; Robison, L.L.; Gurney, J.G. Limitations on physical performance and daily activities among long-term survivors of childhood cancer. Ann. Intern. Med. 2005, 143, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Kritchevsky, S.B.; Goodpaster, B.H.; Newman, A.B.; Nevitt, M.; Stamm, E.; Harris, T.B. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: The health, aging and body composition study. J. Am. Geriatr. Soc. 2002, 50, 897–904. [Google Scholar] [CrossRef]
- Srdic, D.; Plestina, S.; Sverko-Peternac, A.; Nikolac, N.; Simundic, A.M.; Samarzija, M. Cancer cachexia, sarcopenia and biochemical markers in patients with advanced non-small cell lung cancer-chemotherapy toxicity and prognostic value. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2016, 24, 4495–4502. [Google Scholar] [CrossRef]
- Rong, Y.D.; Bian, A.L.; Hu, H.Y.; Ma, Y.; Zhou, X.Z. Study on relationship between elderly sarcopenia and inflammatory cytokine IL-6, anti-inflammatory cytokine IL-10. BMC Geriatr. 2018, 18, 308. [Google Scholar] [CrossRef]
- Bian, A.L.; Hu, H.Y.; Rong, Y.D.; Wang, J.; Wang, J.X.; Zhou, X.Z. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-alpha. Eur. J. Med. Res. 2017, 22, 25. [Google Scholar] [CrossRef]
- Patel, H.J.; Patel, B.M. TNF-alpha and cancer cachexia: Molecular insights and clinical implications. Life Sci. 2017, 170, 56–63. [Google Scholar] [CrossRef]
- An, J.M.; Kang, E.A.; Han, Y.M.; Oh, J.Y.; Lee, D.Y.; Choi, S.H.; Kim, D.H.; Hahm, K.B. Dietary intake of probiotic kimchi ameliorated IL-6-driven cancer cachexia. J. Clin. Biochem. Nutr. 2019, 65, 109–117. [Google Scholar] [CrossRef]
- Stene, G.B.; Balstad, T.R.; Leer, A.S.M.; Bye, A.; Kaasa, S.; Fallon, M.; Laird, B.; Maddocks, M.; Solheim, T.S. Deterioration in Muscle Mass and Physical Function Differs According to Weight Loss History in Cancer Cachexia. Cancers 2019, 11, 1925. [Google Scholar] [CrossRef]
| Database | Search String |
|---|---|
| PubMed | ((neoplasia* OR neoplasm* OR tumor OR tumors OR tumour OR tumours OR cancer OR cancers OR malignan*) NOT necrosis) AND supplement* AND (protein OR proteins OR “amino acid*” OR aminoacid* OR BCAA* OR “branched chain amino acid*” OR leucine OR methylbutyr* OR “carnitine” OR “arginine” OR “glutamine”) AND (asthenia OR fatigue OR “muscle strength*” OR “muscular strength*” OR “handgrip strength*” OR “hand grip strength*” OR “hand-grip strength*” OR “grip strength*” OR “muscle mass” OR ffm OR “fat free mass” OR “lean mass” OR “lean body mass” OR dynapaenia OR myopenia) AND “last 10 years”[PDat] Filters: English |
| SCOPUS | (TITLE-ABS-KEY (neoplasia* OR neoplasm* OR tumor OR tumors OR tumour OR tumours OR cancer OR cancers OR malignanc*) AND TITLE-ABS-KEY (supplement*) AND TITLE-ABS-KEY (protein OR proteins OR “amino acid*” OR aminoacid* OR bcaa* OR “branched chain amino acid*” OR leucine OR methylbutyr* OR “carnitine” OR “arginine” OR “glutamine”) AND TITLE-ABS-KEY (asthenia OR fatigue OR “muscle strength*” OR “muscular strength*” OR “handgrip strength*” OR “hand grip strength*” OR “hand-grip strength*” OR “grip strength*” OR “muscle mass” OR ffm OR “fat free mass” OR “lean mass” OR “lean body mass” OR dynapaenia OR myopenia) AND NOT TITLE-ABS-KEY (necrosis)) AND DOCTYPE (ar) AND PUBYEAR > 2009 AND (LIMIT-TO (LANGUAGE, “English”)) |
| Country Author Year Published | Design Follow up time Days (d) Weeks (w) Months (m) | Population Male/Female (M/F) Age (y) Patient Characteristics Cancer type Treatment Modality | Nutritional Intervention | Endpoints | Main Results |
|---|---|---|---|---|---|
| Spain Arribas, 2017 [43] | Prospective Follow up: 3 m | N = 20 M/F: 19/1 Age: 53.7 ± 7.11 Outpatients Head and neck squamous carcinoma Chemoradiotherapy (CRT) | Dietetic counseling and nutritional supplementation according to the individual needs estimated by standard formulas. Protein requirement: 1.5 g/kg/d. Enteral nutrition (EN) by nasogastric tube (NGT) used in 35% of patients. | Changes in Patient Generated- Subjective Global Assessment (PG-SGA), body weight (BW), body mass index (BMI), muscle strength (MS), fat free mass (FFM), serum albumin, and energy and protein intake. | Significant decreases in BW, BMI, MS, and FFM; no significant changes in serum albumin, protein, or energy intake. |
| Italy Caccialanza, 2019 [44] | Single-arm clinical trial Follow up: 7 d | N = 118 M/F: 76/42 Age: 59.9 ± 14.7 Inpatients at nutritional risk with contraindications for EN Mixed tumors Chemotherapy (CT)/Radiotherapy (RT)/Palliative care | 7-day supplemental parenteral nutrition (SPN) (glucose, amino acids, lipids, electrolytes, multivitamin, and multimineral elements) to integrate oral intake in order to meet calorie requirements estimated by standard formulas. Protein requirement: 1.5 g/kg/d. | Changes in phase angle (PhA), BW, BMI, MS, and prealbumin (PAB). | SPN resulted in significant improvements in PhA, BW, BMI, MS, and PAB. |
| Italy Cereda, 2018 [45] | Randomized controlled trial Follow up: 3 m after the end of RT | N = 159 M/F: 114/45 Age: 63.8 ± 12.7 (COUNS) 66.5 ± 14.5 COUNS+ONS) Outpatients Head and neck cancer (HNC) RT | Nutritional counseling (COUNS) with or without oral nutritional supplements (ONS) (250 mL/day of an oral formula containing 500 kcal, 23 g protein, 1.9 g omega-3 fatty acids). Calorie requirements estimated by Harris Benedict formula; protein requirement set at 1.2 g/kg/d. | Changes in BW, protein-calorie intake, MS, PhA, and quality of life (QoL); anticancer treatment tolerance. | In ONS group, minor BW loss, improved energy and protein intake, and QoL; trend toward significance for MS (p = 0.057); no significant differences for PhA. In ONS group, less (p = 0.029) need for changes in anticancer treatments due to toxicity. |
| Italy Cereda, 2019 [35] | Randomized controlled trial Follow up: 3 m | N = 166 M/F:100/66 Age: 65.7 ± 11.4 (COUNS) 65.1 ± 11.7 (COUNS+WPI) Malnourished patients Advanced mixed tumors Candidate to or undergoing CT | Nutritional counseling (COUNS) with or without whey protein isolate (WPI) supplementation (20 g/d). Calorie requirements estimated by Harris Benedict formula; protein requirement set at 1.5 g/kg/d. | Changes in PhA, standardized phase angle (SPhA),fat-free mass index (FFMI), BW, MS, and CT toxicity. | Significantly improved PhA, SPhA, FFMI, BW, and MS, reduced risk of CT toxicity in WPI as compared with COUNS. |
| US Dawson, 2018 [37] | Randomized controlled trial Follow up: 12 w | N = 37 M/F: 37/0 Age: 66.3 ± 9.0 (PRO and control), 68.6 ± 8.4 (TRAINPRO and TRAIN) Outpatients Prostate cancer Androgen deprivation therapy | Pts assigned to resistance training and protein supplementation (TRAINPRO), resistance training (TRAIN), protein supplementation (PRO), or control. TRAINPRO and PRO: 50 g/day of WPI. | Changes in lean mass (LM), appendicular skeletal muscle (ASM) index, body fat %, MS, physical function, QoL, metabolic syndrome (MetS) score, and MetS components. | Resistance training significantly increased LM, appendicular skeletal mass, and sarcopenic index, and decreased body fat %. No interaction effects of TRAIN and PRO for any outcome. |
| Denmark Lonbro, 2013 [38] | Randomized controlled trial Follow up: 2 m | N = 30 M/F: 23/7 Age: 56 (PROCR), 59 (PLA) Outpatients HNC Terminated RT ± CT ± Immunotherapy | Creatine (5 g) and protein (30 g) supplementation (PROCR) or placebo (PLA) in close relation to progressive resistance training session. | Changes over time and group differences in lean body mass (LBM), MS, and functional performance. | Significant LBM, MS, and functional performance increase in both groups. No significant group differences in any endpoints. |
| Italy Madeddu, 2010 [36] | Uncontrolled trial Follow up: 8 w | N = 25 M/F: 13/12 Age: 65.8 ± 11.4 Cachectic pts stage IV Mixed tumors at any site Active antineoplastic treatment | Oral amino acid functional cluster (AFC) containing 4 g of essential amino acids. | Changes in BW, BMI, LBM, MS, fatigue, and laboratory (albumin, fibrinogen, C-reactive protein (CRP), tumor necrosis alpha (TNFα), leptin, and reactive oxygen species (ROS)) variables. | Significant increases in MS and serum albumin and decrease (p = 0.001) in ROS levels. Trend toward increased body weight (p = 0.056) and leptin (p = 0.052). No significant changes in CRP, IL-6, or TNFα. |
| US Madzima, 2017 [39] | Cohort Follow up: 12 w | N = 33 M/F: 0/33 Age: 59 ± 8 Survivors Breast cancer Terminated treatment | Patients assigned to resistance training (RT) or RT + whey/casein protein isolate (W/CPI) supplementation (40/d). | Changes in MS, LBM, fat body mass (FBM), insulin growth factor 1 (IGF-1), adiponectin, and CRP. | Both groups significantly increased IGF-1, MS, and LBM, and decreased FBM. No difference between groups. No change in adiponectin or CRP. |
| Italy Mantovani, 2010 [40] | Randomized controlled trial Follow up: 4 m | N = 332 M/F: 181/151 Age: 61.5 ± 9.7 (arm 1) 60.6 ± 13.5 (arm 2) 62.8 ± 11.5 (arm 3) 62.4 ± 11.9 (arm 4) 62.4 ± 9.4 (arm 5) Cancer-related anorexia/cachexia syndrome (CACS) Advanced tumors at any site CT or hormone therapy (HT) | Patients assigned to five arms: 1) medroxyprogesterone (500 mg/d) or megestrol acetate (320 mg/d); 2) oral high calorie and protein supplementation with eicosapentaenoic acid (EPA) (2.2 g/d); 3) L-carnitine 4 g/d; 4) thalidomide (200 mg/d); 5) combination of the above. | Change in LBM, resting energy expenditure (REE), fatigue, MS, appetite, proinflammatory cytokines, total energy expenditure (TEE), active energy expenditure (AEE), appetite, QoL, and Glasgow prognostic score (GPS). | In arm 5, LBM, REE, AEE, and MS were all significantly increased. Fatigue, GPS and Eastern Cooperative Oncology Group-Performance Status (ECOG-PS) were decreased. Appetite, IL-6, and TNFα were unchanged. |
| Denmark Obling, 2019 [46] | Randomized controlled trial Follow up: 24 w | N = 47 M/F: 20/27 Age: 66.9 (41.5–88.2) Outpatients at nutritional risk Gastrointestinal cancers Candidate for or undergoing CT | Patients assigned to two groups: 1) nutritional care and dietetic counselling; 2) nutritional care, dietetic counseling, and supplemental home parenteral nutrition (PN). Estimated requirements: energy 125 kJ/kg, protein 1.5 g/kg/d. | Changes in FFM, MS, QoL, and survival. | FFM and QoL increase in intervention group. MS increase in both groups, no difference between groups. No difference in survival. |
| China Ren, 2017 [41] | Randomized controlled trial Follow up: 30 d (pre transplantation) 30 d (post transplantation) | N = 24 M/F: 16/8 Age: 29.2 ± 18 (BP group) 31.6 ± 12 (ND group) Outpatients Acute leukemia Bone marrow transplantation | Natural diet + soy-whey protein blend (50% protein from whey and 50% from soy protein isolate) (BP) compared with natural diet (ND). Calorie and protein targets set at 35/kcal/kg/d and 1.5 g/kg/d in both groups. | Changes in BMI, upper arm muscle circumference (AMC), MS, serum albumin, time to stem cell engrafment. | In BP group, significant increases in AMC, MS, and serum albumin. Significantly shorter time to stem cell engrafment in BP. |
| Germany Schink, 2018 [47] | Non-randomized controlled trial Follow up: 12 w | N = 82 M/F: 74/57 Age: 59.9 ± 12.7 Advanced solid tumors at any site CT/RT/HT/other | Whole-body electromyostimulation (WB-EMS) physical exercise program twice a week vs CON (no exercise). In both groups, individualized nutrition counseling (energy intake ≥25 kcal/kg/die, protein: intake >1 g/kg/die), protein/amino acid-rich oral supplements, or EN or PN. | Change in SMM, body composition, BW, MS, QoL, fatigue, albumin, CRP | In WB-EMS group, significantly higher SMM and BW, improved physical function, and performance status. No significant differences in QoL, fatigue, albumin, or CRP. MS increased similarly in both groups. |
| Switzerland Uster, 2013 [48] | Randomized controlled trial Follow up: 3 m | N = 58 M/F: 46/12 Age: 63.8 ± 13.3 (NT) 66.2 ± 8.9 (CON) Outpatients, undernourished, or at high risk for malnutrition Mixed tumors at any site | Individualized nutritional intervention (counseling + food fortification and ONS if required (NT)) versus no intervention (CON). | Changes in dietary intake, BW, performance status, MS, and QoL. | In intervention group, significantly higher energy and protein intake. No significant improvements in nutritional status, MS, physical functioning, or QoL. |
| Japan Wada, 2018 [42] | Randomized controlled trial Follow up: 3 d pre-operatively 7 d post-operatively | N = 60 M/F: 34/26 Age: 66 (40–681) (NT); 69 (25–81) (CON) Inpatients Abdominal cancers Surgery | NT: Beta-hydroxy-beta-methylbutyrate (HMB) (1.2 g)/arginine (Arg) (7 g)/glutamine (Gln) (7 g) once daily preop and postop. CON: Equivalent amount of isocaloric juice. | Wound complications, length of hospital stay (LOS), skeletal muscle mass (SMM), MS, and skin water content. | No significant differences between groups for any of the explored endpoints. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanetti, M.; Gortan Cappellari, G.; Barazzoni, R.; Sanson, G. The Impact of Protein Supplementation Targeted at Improving Muscle Mass on Strength in Cancer Patients: A Scoping Review. Nutrients 2020, 12, 2099. https://doi.org/10.3390/nu12072099
Zanetti M, Gortan Cappellari G, Barazzoni R, Sanson G. The Impact of Protein Supplementation Targeted at Improving Muscle Mass on Strength in Cancer Patients: A Scoping Review. Nutrients. 2020; 12(7):2099. https://doi.org/10.3390/nu12072099
Chicago/Turabian StyleZanetti, Michela, Gianluca Gortan Cappellari, Rocco Barazzoni, and Gianfranco Sanson. 2020. "The Impact of Protein Supplementation Targeted at Improving Muscle Mass on Strength in Cancer Patients: A Scoping Review" Nutrients 12, no. 7: 2099. https://doi.org/10.3390/nu12072099
APA StyleZanetti, M., Gortan Cappellari, G., Barazzoni, R., & Sanson, G. (2020). The Impact of Protein Supplementation Targeted at Improving Muscle Mass on Strength in Cancer Patients: A Scoping Review. Nutrients, 12(7), 2099. https://doi.org/10.3390/nu12072099





