Vitamin D Metabolites and Binding Protein Predict Preeclampsia in Women with Type 1 Diabetes
Abstract
1. Introduction
2. Research Design and Methods
2.1. Study Design and Participants
2.2. Laboratory Measures
2.3. Statistical Analysis
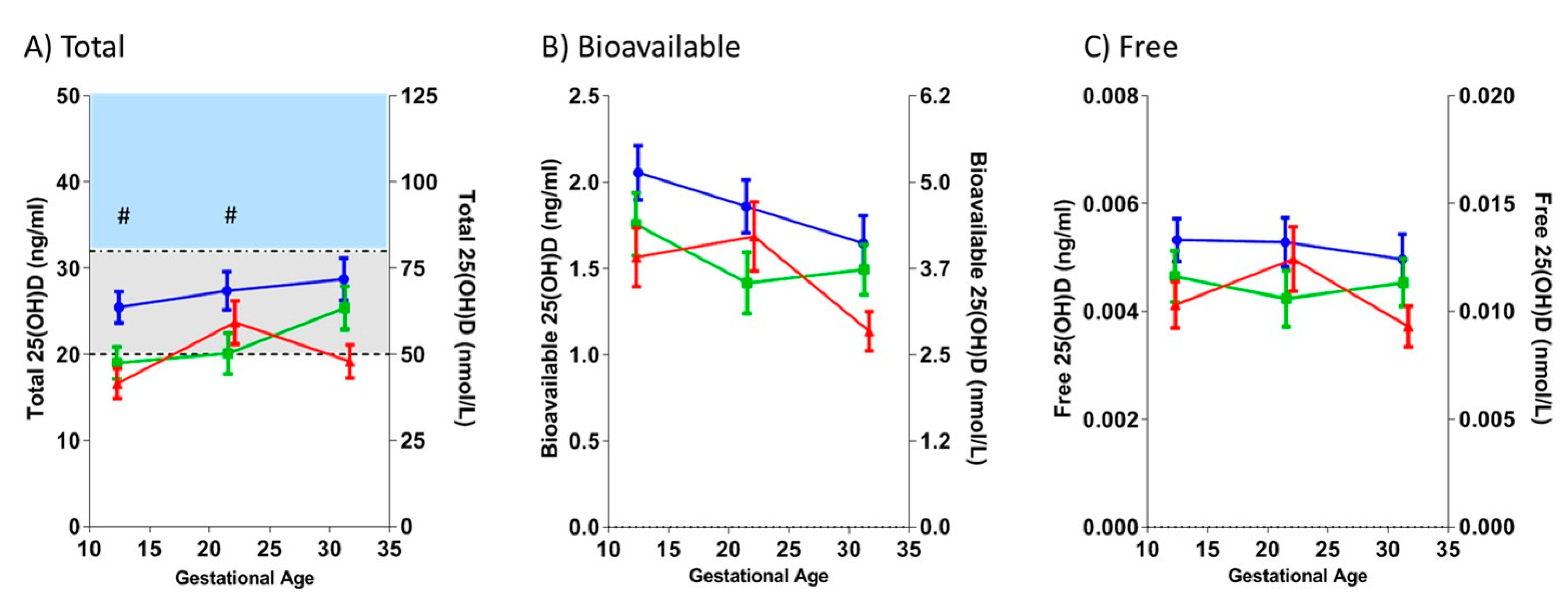
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethics Approval
Data Availability
Additional Information
Role of the Funder/Sponsor
Abbreviations
| BMI | Body Mass Index; |
| DM+PE+ | Women with type 1 diabetes who subsequently developed PE; |
| DM+PE− | Women with type 1 diabetes who did not develop PE; |
| DM− | Non-diabetic, normotensive women; |
| eGFR | Estimated glomerular filtration rate; |
| HbA1c | Glycated hemoglobin; |
| MAMPED | Markers And Mechanisms for PreEclampsia in Type 1 Diabetes; |
| MAP | Mean Arterial Pressure; |
| PE | Preeclampsia; |
| T1DM | Type 1 diabetes mellitus; |
| uNGALcc | Urinary neutrophil-gelatinase associated lipocalin (creatinine corrected); |
| VDBP | Vitamin D Binding Protein; |
| 1,25(OH)2D | 1,25-dihydroxyvitamin D; |
| 25(OH)D | 25-hydroxyvitamin D. |
References
- Roth, D.E.; Abrams, S.A.; Aloia, J.; Bergeron, G.; Bourassa, M.W.; Brown, K.H.; Calvo, M.S.; Cashman, K.D.; Combs, G.; De-Regil, L.M.; et al. Global prevalence and disease burden of vitamin D deficiency: A roadmap for action in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2018, 1430, 44–79. [Google Scholar] [CrossRef]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Johnson, D.; Hulsey, T.C.; Ebeling, M.; Wagner, C.L. Vitamin D supplementation during pregnancy: Double-blind, randomized clinical trial of safety and effectiveness. J. Bone Miner. Res. 2011, 26, 2341–2357. [Google Scholar] [CrossRef]
- Ganguly, A.; Tamblyn, J.A.; Finn-Sell, S.; Chan, S.Y.; Westwood, M.; Gupta, J.; Kilby, M.D.; Gross, S.R.; Hewison, M. Vitamin D, the placenta and early pregnancy: Effects on trophoblast function. J. Endocrinol. 2018, 236, R93–R103. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Cohen, W.R.; Silva, P.; Epstein, F.H. Elevated 1,25-dihydroxyvitamin D plasma levels in normal human pregnancy and lactation. J. Clin. Investig. 1979, 63, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Ma, S.; Wang, Y.; Hou, L.; Shi, Y.; Yao, M.; Wang, X.; Zhang, H.; Jiang, L. Effects of Placental Ischemia Are Attenuated by 1,25-Dihydroxyvitamin D Treatment and Associated with Reduced Apoptosis and Increased Autophagy. DNA Cell Biol. 2016, 35, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D, Calcium. Dietary Reference Intakes for Calcium and Vitamin, D; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; National Academies Press (US): Washington, DC, USA, 2011.
- Halhali, A.; Villa, A.R.; Madrazo, E.; Soria, M.C.; Mercado, E.; Diaz, L.; Avila, E.; Garabédian, M.; Larrea, F. Longitudinal changes in maternal serum 1,25-dihydroxyvitamin D and insulin like growth factor I levels in pregnant women who developed preeclampsia: Comparison with normotensive pregnant women. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Catov, J.M.; Simhan, H.N.; Holick, M.F.; Powers, R.W.; Roberts, J.M. Maternal vitamin D deficiency increases the risk of preeclampsia. J. Clin. Endocrinol. Metab. 2007, 92, 3517–3522. [Google Scholar] [CrossRef]
- Robinson, C.J.; Alanis, M.C.; Wagner, C.L.; Hollis, B.W.; Johnson, D.D. Plasma 25-hydroxyvitamin D levels in early-onset severe preeclampsia. Am. J. Obstet. Gynecol. 2010, 203, e361–e366. [Google Scholar] [CrossRef]
- Ma, R.; Gu, Y.; Zhao, S.; Sun, J.; Groome, L.J.; Wang, Y. Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E928–E935. [Google Scholar] [CrossRef]
- Wei, S.Q.; Audibert, F.; Hidiroglou, N.; Sarafin, K.; Julien, P.; Wu, Y.; Luo, Z.C.; Fraser, W.D. Longitudinal vitamin D status in pregnancy and the risk of pre-eclampsia. BJOG 2012, 119, 832–839. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Simhan, H.N.; Catov, J.M.; Roberts, J.M.; Platt, R.W.; Diesel, J.C.; Klebanoff, M.A. Maternal vitamin D status and the risk of mild and severe preeclampsia. Epidemiology 2014, 25, 207–214. [Google Scholar] [CrossRef]
- Smith, T.A.; Kirkpatrick, D.R.; Kovilam, O.; Agrawal, D.K. Immunomodulatory role of vitamin D in the pathogenesis of preeclampsia. Expert Rev. Clin. Immunol. 2015, 11, 1055–1063. [Google Scholar] [CrossRef]
- Kiely, M.E.; Zhang, J.Y.; Kinsella, M.; Khashan, A.S.; Kenny, L.C. Vitamin D status is associated with uteroplacental dysfunction indicated by pre-eclampsia and small-for-gestational-age birth in a large prospective pregnancy cohort in Ireland with low vitamin D status. Am. J. Clin. Nutr. 2016, 104, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Tamblyn, J.A.; Susarla, R.; Jenkinson, C.; Jeffery, L.E.; Ohizua, O.; Chun, R.F.; Chan, S.Y.; Kilby, M.D.; Hewison, M. Dysregulation of maternal and placental vitamin D metabolism in preeclampsia. Placenta 2017, 50, 70–77. [Google Scholar] [CrossRef]
- Vestgaard, M.; Secher, A.L.; Ringholm, L.; Jensen, J.B.; Damm, P.; Mathiesen, E.R. Vitamin D insufficiency, preterm delivery and preeclampsia in women with type 1 diabetes—An observational study. Acta Obstet. Gynecol. Scand. 2017, 96, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Mirzakhani, H.; Carey, V.J.; McElrath, T.F.; Laranjo, N.; O’Connor, G.; Iverson, R.E.; Lee-Parritz, A.; Strunk, R.C.; Bacharier, L.B.; Macones, G.A.; et al. The Association of Maternal Asthma and Early Pregnancy Vitamin D with Risk of Preeclampsia: An Observation from Vitamin D Antenatal Asthma Reduction Trial (VDAART). J. Allergy Clin. Immunol. Pract. 2018, 6, 600–608. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, K.M.; Kiely, M. Systematic Review of Vitamin D and Hypertensive Disorders of Pregnancy. Nutrients 2018, 10, 294. [Google Scholar] [CrossRef]
- Mäkinen, M.; Löyttyniemi, E.; Koskinen, M.; Vähä-Mäkilä, M.; Siljander, H.; Nurmio, M.; Mykkänen, J.; Virtanen, S.M.; Simell, O.; Hyöty, H.; et al. Serum 25-Hydroxyvitamin D Concentrations at Birth in Children Screened for HLA-DQB1 Conferred Risk for Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 2277–2285. [Google Scholar] [CrossRef]
- Tapia, G.; Marild, K.; Dahl, S.R.; Lund-Blix, N.A.; Viken, M.K.; Lie, B.A.; Njølstad, P.R.; Joner, G.; Skrivarhaug, T.; Cohen, A.S.; et al. Maternal and Newborn Vitamin D-Binding Protein, Vitamin D Levels, Vitamin D Receptor Genotype, and Childhood Type 1 Diabetes. Diabetes Care 2019, 42, 553–559. [Google Scholar] [CrossRef]
- Bikle, D.D.; Siiteri, P.K.; Ryzen, E.; Haddad, J.G. Serum protein binding of 1,25-dihydroxyvitamin D: A reevaluation by direct measurement of free metabolite levels. J. Clin. Endocrinol. Metab. 1985, 61, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Gee, E.; Halloran, B.; Kowalski, M.A.; Ryzen, E.; Haddad, J.G. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J. Clin. Endocrinol. Metab. 1986, 63, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Chun, R.F.; Peercy, B.E.; Orwoll, E.S.; Nielson, C.M.; Adams, J.S.; Hewison, M. Vitamin D and DBP: The free hormone hypothesis revisited. J. Steroid Biochem. Mol. Biol. 2014, 144, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.B.; Lai, J.; Lizaola, B.; Kane, L.; Weyland, P.; Terrault, N.A.; Stotland, N.; Bikle, D. Variability in free 25(OH) vitamin D levels in clinical populations. J. Steroid Biochem. Mol. Biol. 2014, 144, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Xue, H.; Wang, L.; Chen, Q.; Chen, X.; Zhang, Y.; Hu, G.; Ling, W. Serum Bioavailable and Free 25-Hydroxyvitamin D Levels, but Not Its Total Level, Are Associated With the Risk of Mortality in Patients With Coronary Artery Disease. Circ. Res. 2018, 123, 996–1007. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Task Force on Hypertension in Pregnancy Report. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar] [CrossRef]
- Persson, M.; Norman, M.; Hanson, U. Obstetric and perinatal outcomes in type 1 diabetic pregnancies: A large, population-based study. Diabetes Care 2009, 32, 2005–2009. [Google Scholar] [CrossRef]
- Bennett, S.E.; McPeake, J.; McCance, D.R.; Manderson, J.G.; Johnston, P.; McGalliard, R.; McGinty, A. Maternal vitamin D status in type 1 diabetic pregnancy: Impact on neonatal vitamin D status and association with maternal glycaemic control. PLoS ONE 2013, 8, e74068. [Google Scholar] [CrossRef]
- Azar, M.; Basu, A.; Jenkins, A.J.; Nankervis, A.J.; Hanssen, K.F.; Scholz, H.; Henriksen, T.; Garg, S.K.; Hammad, S.M.; Scardo, J.A.; et al. Serum carotenoids and fat-soluble vitamins in women with type 1 diabetes and preeclampsia: A longitudinal study. Diabetes Care 2011, 34, 1258–1264. [Google Scholar] [CrossRef]
- Yu, Y.; Jenkins, A.J.; Nankervis, A.J.; Hanssen, K.F.; Scholz, H.; Henriksen, T.; Lorentzen, B.; Clausen, T.; Garg, S.K.; Menard, M.K.; et al. Anti-angiogenic factors and pre-eclampsia in type 1 diabetic women. Diabetologia 2009, 52, 160–168. [Google Scholar] [CrossRef]
- Du, M.; Basu, A.; Fu, D.; Wu, M.; Centola, M.; Jenkins, A.J.; Hanssen, K.F.; Garg, S.K.; Hammad, S.M.; Scardo, J.A.; et al. Serum inflammatory markers and preeclampsia in type 1 diabetes: A prospective study. Diabetes Care 2013, 36, 2054–2061. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Yu, J.Y.; Jenkins, A.J.; Nankervis, A.J.; Hanssen, K.F.; Henriksen, T.; Lorentzen, B.; Garg, S.K.; Menard, M.K.; Hammad, S.M.; et al. Trace elements as predictors of preeclampsia in type 1 diabetic pregnancy. Nutr. Res. 2015, 35, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.B.; Hookham, M.B.; Yu, J.Y.; Lockhart, S.M.; Du, M.; Jenkins, A.J.; Nankervis, A.; Hanssen, K.F.; Henriksen, T.; Garg, S.K.; et al. Circulating adipokines are associated with pre-eclampsia in women with type 1 diabetes. Diabetologia 2017, 60, 2514–2524. [Google Scholar] [CrossRef]
- Kelly, C.B.; Hookham, M.B.; Yu, J.Y.; Jenkins, A.J.; Nankervis, A.J.; Hanssen, K.F.; Garg, S.K.; Scardo, J.A.; Hammad, S.M.; Menard, M.K.; et al. Subclinical First Trimester Renal Abnormalities Are Associated with Preeclampsia in Normoalbuminuric Women With Type 1 Diabetes. Diabetes Care 2018, 41, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.B.; Yu, J.Y.; Jenkins, A.J.; Nankervis, A.J.; Hanssen, K.F.; Garg, S.K.; Scardo, J.A.; Basu, A.; Hammad, S.M.; Aston, C.E.; et al. Haptoglobin phenotype modulates lipoprotein-associated risk for preeclampsia in women with Type 1 diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 4743–4755. [Google Scholar] [CrossRef]
- Bhan, I.; Powe, C.E.; Berg, A.H.; Ankers, E.; Wenger, J.B.; Karumanchi, S.A.; Thadhani, R.I. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney Int. 2012, 82, 84–89. [Google Scholar] [CrossRef]
- Karras, S.N.; Polyzos, S.A.; Newton, D.A.; Wagner, C.L.; Hollis, B.W.; Ouweland, J.V.D.; Dursun, E.; Gezen-Ak, D.; Kotsa, K.; Annweiler, C.; et al. Adiponectin and vitamin D-binding protein are independently associated at birth in both mothers and neonates. Endocrine 2018, 59, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Gee, E.; Halloran, B.; Haddad, J.G. Free 1,25-dihydroxyvitamin D levels in serum from normal subjects, pregnant subjects, and subjects with liver disease. J. Clin. Investig. 1984, 74, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R. Chapter 5—The Vitamin D Binding Protein DBP. In Vitamin D, 3rd ed.; Feldman, D., Pike, J.W., Adams, J.S., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 57–72. [Google Scholar]
- Purswani, J.M.; Gala, P.; Dwarkanath, P.; Larkin, H.M.; Kurpad, A.; Mehta, S. The role of vitamin D in pre-eclampsia: A systematic review. BMC Pregnancy Childbirth 2017, 17, 231. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.B.; Gallagher, J.C.; Jorde, R.; Berg, V.; Walsh, J.; Eastell, R.; Evans, A.L.; Bowles, S.; Naylor, K.E.; Jones, K.S.; et al. Determination of free 25(OH)D concentrations and their relationships to total 25(OH)D in multiple clinical populations. J. Clin. Endocrinol. Metab. 2018, 103, 3278–3288. [Google Scholar] [CrossRef]
- Newton, D.A.; Baatz, J.E.; Kindy, M.S.; Gattoni-Celli, S.; Shary, J.R.; Hollis, B.W.; Wagner, C.L. Vitamin D binding protein polymorphisms significantly impact vitamin D status in children. Pediatric Res. 2019, 86, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Pittard, W.B., III. Evaluation of the total fetomaternal vitamin D relationships at term: Evidence for racial differences. J. Clin. Endocinol. Metab. 1984, 59, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Ganz, A.B.; Park, H.; Malysheva, O.V.; Caudill, M.A. Vitamin D binding protein rs7041 genotype alters vitamin D metabolism in pregnant women. FASEB J. 2018, 32, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Schwartz, J. Vitamin D binding protein, total and free vitamin D levels in different physiological and pathophysiological conditions. Front. Endocrinol. 2019, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Hewison, M. Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch. Biochem. Biophys. 2012, 523, 95–102. [Google Scholar] [CrossRef]
- Al-Badr, W.; Martin, K.J. Vitamin D and kidney disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1555–1560. [Google Scholar] [CrossRef]
- Kim, C.S.; Kim, S.W. Vitamin D and chronic kidney disease. Korean J. Intern. Med. 2014, 29, 416–427. [Google Scholar] [CrossRef]
- Noyola-Martinez, N.; Diaz, L.; Avila, E.; Halhali, A.; Larrea, F.; Barrera, D. Calcitriol downregulates TNF-alpha and IL-6 expression in cultured placental cells from preeclamptic women. Cytokine 2013, 61, 245–250. [Google Scholar] [CrossRef]
- Olmos-Ortiz, A.; Noyola-Martinez, N.; Barrera, D.; Zaga-Clavellina, V.; Avila, E.; Halhali, A.; Biruete, B.; Larrea, F.; Díaz, L. IL-10 inhibits while calcitriol reestablishes placental antimicrobial peptides gene expression. J. Steroid Biochem. Mol. Biol. 2015, 148, 187–193. [Google Scholar] [CrossRef] [PubMed]
- August, P.; Marcaccio, B.; Gertner, J.M.; Druzin, M.L.; Resnick, L.M.; Laragh, J.H. Abnormal 1,25-dihydroxyvitamin D metabolism in preeclampsia. Am. J. Obstet. Gynecol. 1992, 166, 1295–1299. [Google Scholar] [CrossRef]
- Corcoy, R.; Mendoza, L.C.; Simmons, D.; Desoye, G.; Adelantado, J.M.; Chico, A.; Devlieger, R.; van Assche, A.; Galjaard, S.; Timmerman, D.; et al. The DALI vitamin D randomized controlled trial for gestational diabetes mellitus prevention: No major benefit shown besides vitamin D sufficiency. Clin. Nutr. 2020, 39, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Legarth, C.; Grimm, D.; Wehland, M.; Bauer, J.; Kruger, M. The Impact of Vitamin D in the Treatment of Essential Hypertension. Int. J. Mol. Sci. 2018, 19, 455. [Google Scholar] [CrossRef] [PubMed]

| Clinical Characteristics | DM+PE+ (n = 23) | p Value a | DM+PE− (n = 24) | p Value b | DM− (n = 19) |
|---|---|---|---|---|---|
| Age of woman (years) | 28.5 ± 5.6 | 0.31 | 29.9 ± 3.8 | 0.25 | 31.4 ± 4.5 |
| Ethnicity (%) | |||||
| White non-Hispanic | 91 | 0.51 | 92 | 0.44 | 100 |
| Australian | 0 | 4 | 0 | ||
| White Australian | 9 | 4 | 0 | ||
| BMI (kg/m2) | 27.9 ± 5.9 | 0.028 | 24.6 ± 4.1 | 0.50 | 23.8 ± 3.8 |
| Smoking (%) c | |||||
| No | 91 | 0.69 | 88 | 0.55 | 100 |
| Stopped in pregnancy | 5 | 4 | 0 | ||
| Gravida (n) | 1.3 ± 0.7 | 1.00 | 1.3 ± 0.7 | 0.19 | 1.7 ± 1.0 |
| Para (n) | 0.2 ± 0.5 | 0.91 | 0.2 ± 0.5 | 0.13 | 0.5 ± 1.0 |
| Duration of T1DM (years) | 16.8 ± 6.8 | 0.32 | 14.8 ± 7.0 | - | - |
| HbA1c (%) | 7.4 ± 1.2 | 0.046 | 6.7 ± 1.0 | <0.0001 | 5.3 ± 0.3 |
| HbA1c (mmol/mol) | 57 ± 14 | 0.046 | 50 ± 11 | <0.0001 | 35 ± 3 |
| Blood pressure (mmHg) | |||||
| BP systolic | 113.1 ± 12.4 | 0.26 | 109.4 ± 9.6 | 0.23 | 113.3 ± 8.7 |
| BP diastolic | 66.6 ± 9.0 | 0.27 | 63.8 ± 8.1 | 0.24 | 66.9 ± 7.6 |
| Mean arterial pressure | 82.1 ± 9.0 | 0.21 | 79.0 ± 7.7 | 0.14 | 82.7 ± 6.2 |
| Total daily insulin (IU/d) | 62.2 ± 19.7 | 0.009 | 47.9 ± 14.2 | - | - |
| Total cholesterol (mmol/L) | 4.7 ± 0.7 | 0.53 | 4.5 ± 0.9 | 0.18 | 4.9 ± 0.7 |
| HDL cholesterol (mmol/L) | 1.9 ± 0.4 | 0.029 | 2.2 ± 0.5 | 0.71 | 2.1 ± 0.6 |
| LDL cholesterol (mmol/L) | 2.4 ± 0.7 | 0.08 | 2.0 ± 0.7 | 0.18 | 2.3 ± 0.8 |
| Triacylglycerol (mmol/L) | 1.0 ± 0.3 | 0.27 | 0.8 ± 0.3 | 0.09 | 1.1 ± 0.4 |
| Gestational age (weeks) | |||||
| Visit 1 | 12.3 ± 2.1 | 0.94 | 12.3 ± 1.7 | 0.49 | 12.6 ± 1.7 |
| Visit 2 | 22.1 ± 1.6 | 0.18 | 21.5 ± 1.3 | 0.95 | 21.5 ± 1.3 |
| Visit 3 | 31.7 ± 1.7 | 0.39 | 31.3 ± 1.5 | 0.82 | 31.2 ± 1.1 |
| DM+PE+ | DM+PE− | DM− | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Visit Number | N | Mean | SD | p Value a | N | Mean | SD | p Value b | N | Mean | SD | ||||
| Total 1,25(OH)2D/Total 25(OH)D (×103) | 1 | 20 | 7.2 | ± | 2.6 | 0.14 | 23 | 5.9 | ± | 2.9 | 0.14 | 16 | 4.7 | ± | 1.8 |
| 2 | 22 | 6.3 | ± | 2.2 | 0.99 | 21 | 6.3 | ± | 3.0 | 0.21 | 18 | 5.3 | ± | 1.6 | |
| 3 | 19 | 10.1 | ± | 6.6 | 0.021 | 23 | 6.1 | ± | 3.8 | 0.47 | 17 | 5.4 | ± | 2.2 | |
| Bioavailable 1,25(OH)2D/Bioavailable 25(OH)D (×103) | 1 | 20 | 11.7 | ± | 4.3 | 0.15 | 23 | 9.7 | ± | 4.9 | 0.18 | 15 | 7.7 | ± | 3.2 |
| 2 | 21 | 10.3 | ± | 3.6 | 0.99 | 21 | 10.5 | ± | 4.9 | 0.21 | 18 | 8.9 | ± | 2.7 | |
| 3 | 19 | 16.9 | ± | 11.2 | 0.010 | 22 | 9.6 | ± | 5.5 | 0.74 | 17 | 9.0 | ± | 3.8 | |
| Free 1,25(OH)2D/Free 25(OH)D (×103) | 1 | 20 | 127 | ± | 47 | 0.15 | 23 | 105 | ± | 53 | 0.18 | 15 | 84 | ± | 34 |
| 2 | 21 | 111 | ± | 39 | 0.88 | 21 | 114 | ± | 53 | 0.21 | 18 | 96 | ± | 29 | |
| 3 | 19 | 182 | ± | 120 | 0.010 | 22 | 103 | ± | 59 | 0.74 | 17 | 97 | ± | 41 | |
| VDBP (μmol/L) | 1 | 23 | 5.3 | ± | 1.1 | 0.54 | 23 | 5.6 | ± | 1.8 | 0.15 | 18 | 6.3 | ± | 1.1 |
| 2 | 23 | 6.5 | ± | 0.9 | 0.95 | 21 | 6.5 | ± | 1.2 | 0.20 | 18 | 7.1 | ± | 1.6 | |
| 3 | 23 | 7.0 | ± | 1.1 | 0.032 | 24 | 7.9 | ± | 1.6 | 0.79 | 19 | 8.0 | ± | 1.8 | |
| Total 25(OH)D/VDBP (×103) | 1 | 22 | 8.0 | ± | 4.0 | 0.42 | 23 | 9.0 | ± | 4.5 | 0.29 | 18 | 10.3 | ± | 3.2 |
| 2 | 22 | 9.3 | ± | 5.1 | 0.38 | 21 | 8.0 | ± | 4.5 | 0.15 | 18 | 9.9 | ± | 3.7 | |
| 3 | 22 | 6.9 | ± | 3.3 | 0.25 | 24 | 8.2 | ± | 4.1 | 0.40 | 18 | 9.2 | ± | 3.8 | |
| [25(OH)D bound to VDBP *]/VDBP (×103) | 1 | 22 | 7.2 | ± | 3.5 | 0.41 | 23 | 8.1 | ± | 4.0 | 0.31 | 17 | 9.3 | ± | 2.9 |
| 2 | 21 | 8.7 | ± | 4.8 | 0.36 | 21 | 7.4 | ± | 4.2 | 0.14 | 18 | 9.2 | ± | 3.4 | |
| 3 | 22 | 6.5 | ± | 3.1 | 0.16 | 23 | 7.9 | ± | 3.7 | 0.52 | 18 | 8.7 | ± | 3.5 | |
| Total 1,25(OH)2D/VDBP (×105) | 1 | 21 | 4.6 | ± | 0.9 | 0.33 | 23 | 4.3 | ± | 1.0 | 0.66 | 16 | 4.5 | ± | 1.1 |
| 2 | 23 | 5.2 | ± | 1.4 | 0.005 | 21 | 4.1 | ± | 1.0 | 0.025 | 18 | 4.9 | ± | 1.2 | |
| 3 | 20 | 5.4 | ± | 1.9 | 0.005 | 23 | 4.0 | ± | 1.0 | 0.20 | 18 | 4.5 | ± | 1.0 | |
| [1,25(OH)2D bound to VDBP *]/VDBP (×105) | 1 | 21 | 3.9 | ± | 0.8 | 0.33 | 23 | 3.7 | ± | 0.9 | 0.63 | 15 | 3.8 | ± | 0.9 |
| 2 | 22 | 4.5 | ± | 1.3 | 0.007 | 21 | 3.6 | ± | 0.8 | 0.018 | 18 | 4.3 | ± | 1.0 | |
| 3 | 20 | 4.8 | ± | 1.7 | 0.009 | 22 | 3.7 | ± | 1.0 | 0.25 | 18 | 4.0 | ± | 0.9 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelly, C.B.; Wagner, C.L.; Shary, J.R.; Leyva, M.J.; Yu, J.Y.; Jenkins, A.J.; Nankervis, A.J.; Hanssen, K.F.; Garg, S.K.; Scardo, J.A.; et al. Vitamin D Metabolites and Binding Protein Predict Preeclampsia in Women with Type 1 Diabetes. Nutrients 2020, 12, 2048. https://doi.org/10.3390/nu12072048
Kelly CB, Wagner CL, Shary JR, Leyva MJ, Yu JY, Jenkins AJ, Nankervis AJ, Hanssen KF, Garg SK, Scardo JA, et al. Vitamin D Metabolites and Binding Protein Predict Preeclampsia in Women with Type 1 Diabetes. Nutrients. 2020; 12(7):2048. https://doi.org/10.3390/nu12072048
Chicago/Turabian StyleKelly, Clare B., Carol L. Wagner, Judith R. Shary, Misti J. Leyva, Jeremy Y. Yu, Alicia J. Jenkins, Alison J. Nankervis, Kristian F. Hanssen, Satish K. Garg, James A. Scardo, and et al. 2020. "Vitamin D Metabolites and Binding Protein Predict Preeclampsia in Women with Type 1 Diabetes" Nutrients 12, no. 7: 2048. https://doi.org/10.3390/nu12072048
APA StyleKelly, C. B., Wagner, C. L., Shary, J. R., Leyva, M. J., Yu, J. Y., Jenkins, A. J., Nankervis, A. J., Hanssen, K. F., Garg, S. K., Scardo, J. A., Hammad, S. M., Aston, C. E., & Lyons, T. J. (2020). Vitamin D Metabolites and Binding Protein Predict Preeclampsia in Women with Type 1 Diabetes. Nutrients, 12(7), 2048. https://doi.org/10.3390/nu12072048





