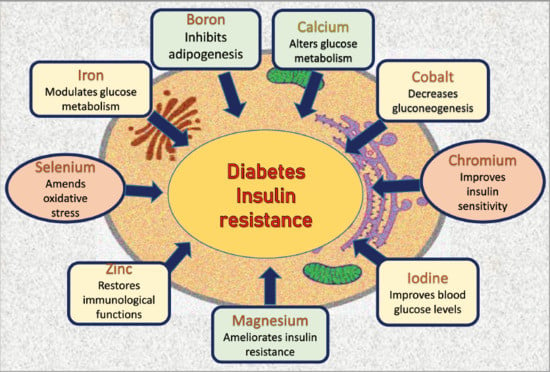
Role of Minerals and Trace Elements in Diabetes and Insulin Resistance
Abstract
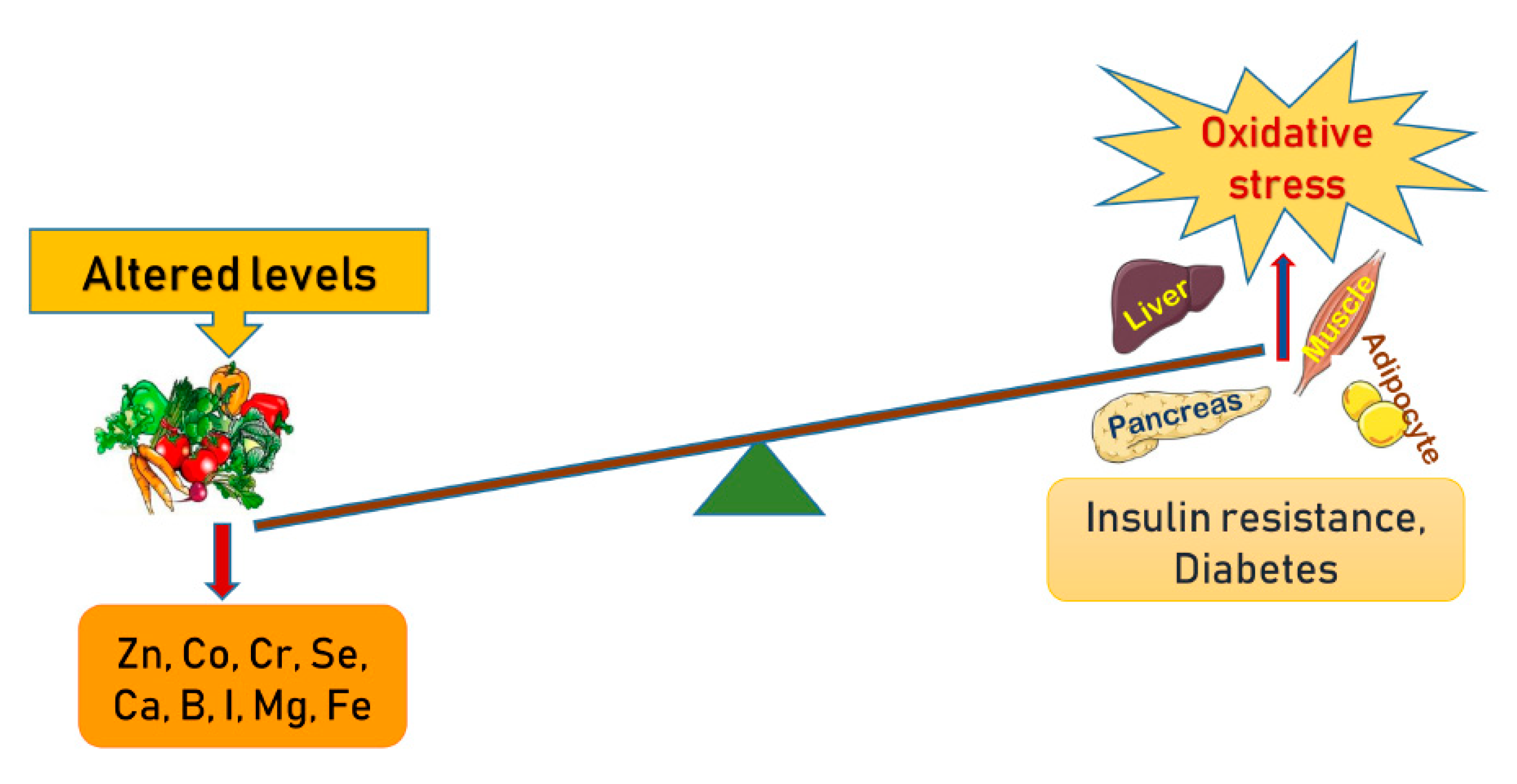
1. Introduction
2. Boron
3. Calcium
4. Cobalt
5. Chromium
6. Iodine
7. Iron
8. Magnesium
9. Selenium
10. Zinc (Zn)
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Calabrese, E.J.; Canada, A.T.; Sacco, C. Trace Elements and Public Health. Annu. Rev. Public Health 1985, 6, 131–146. [Google Scholar] [CrossRef]
- Young, V.R. Trace element biology: The knowledge base and its application for the nutrition of individuals and populations. J. Nutr. 2003, 133 (Suppl. 1), 1581S–1587S. [Google Scholar] [CrossRef]
- Nordberg, M.; Nordberg, G.F. Trace element research-historical and future aspects. J. Trace Elements Med. Boil. 2016, 38, 46–52. [Google Scholar] [CrossRef]
- Uğurlu, V.; Binay, C.; Şimşek, E.; Bal, C. Cellular Trace Element Changes in Type 1 Diabetes Patients. J. Clin. Res. Pediatr. Endocrinol. 2016, 8, 180–186. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, C.; Yang, Z.; Zhang, W.; Niu, Y.; Li, X.; Qin, L.; Su, Q. Alterations of serum trace elements in patients with type 2 diabetes. J. Trace Elements Med. Boil. 2017, 40, 91–96. [Google Scholar] [CrossRef]
- Siddiqui, K.; Bawazeer, N.; Joy, S.S. Variation in Macro and Trace Elements in Progression of Type 2 Diabetes. Sci. World J. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Badran, M.; Morsy, R.; Soliman, H.; Elnimr, T. Assessment of trace elements levels in patients with Type 2 diabetes using multivariate statistical analysis. J. Trace Elements Med. Boil. 2016, 33, 114–119. [Google Scholar] [CrossRef]
- Wolide, A.D.; Zawdie, B.; Alemayehu, T.; Tadesse, S. Association of trace metal elements with lipid profiles in type 2 diabetes mellitus patients: A cross sectional study. BMC Endocr. Disord. 2017, 17, 64. [Google Scholar] [CrossRef]
- Sun, W.; Yang, J.; Wang, W.; Hou, J.; Cheng, Y.; Fu, Y.; Xu, Z.; Cai, L. The beneficial effects of Zn on Akt-mediated insulin and cell survival signaling pathways in diabetes. J. Trace Elements Med. Boil. 2018, 46, 117–127. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, X.; Xiao, X.; Zheng, J.; Li, M.; Yu, M.; Ping, F.; Wang, Z.; Qi, C.; Wang, T.; et al. Dietary Chromium Restriction of Pregnant Mice Changes the Methylation Status of Hepatic Genes Involved with Insulin Signaling in Adult Male Offspring. PLoS ONE 2017, 12, e0169889. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Sinitskii, A.; Popova, E.; Nemereshina, O.; Gatiatulina, E.; Skalnaya, M.G.; Skalny, A.V.; Nikonorov, A. Alteration of local adipose tissue trace element homeostasis as a possible mechanism of obesity-related insulin resistance. Med. Hypotheses 2015, 85, 343–347. [Google Scholar] [CrossRef]
- Kashiv, Y.; Austin, J.R.; Lai, B.; Rose, V.; Vogt, S.; El-Muayed, M. Imaging trace element distributions in single organelles and subcellular features. Sci. Rep. 2016, 6, 21437. [Google Scholar] [CrossRef]
- Koekkoek, W.A.; Van Zanten, A.R. Antioxidant Vitamins and Trace Elements in Critical Illness. Nutr. Clin. Pract. 2016, 31, 457–474. [Google Scholar] [CrossRef]
- Derakhshanian, H.; Javanbakht, M.; Zarei, M.; Djalali, E.; Djalali, M. Vitamin D increases IGF-I and insulin levels in experimental diabetic rats. Growth Horm. IGF Res. 2017, 36, 57–59. [Google Scholar] [CrossRef]
- Sujatha, P. Trace Elements in Diabetes Mellitus. J. Clin. Diagn. Res. 2013, 7, 1863–1865. [Google Scholar] [CrossRef]
- Uluisik, I.; Karakaya, H.Ç.; Koc, A. The importance of boron in biological systems. J. Trace Elements Med. Boil. 2018, 45, 156–162. [Google Scholar] [CrossRef]
- Khaliq, H.; Juming, Z.; Ke-Mei, P. The Physiological Role of Boron on Health. Boil. Trace Element Res. 2018, 186, 31–51. [Google Scholar] [CrossRef]
- Dessordi, R.; Spirlandeli, A.L.; Zamarioli, A.; Volpon, J.B.; Navarro, A.M. Boron supplementation improves bone health of non-obese diabetic mice. J. Trace Elements Med. Boil. 2017, 39, 169–175. [Google Scholar] [CrossRef]
- Zofkova, I.; Nemcikova, P.; Matucha, P. Trace elements and bone health. Clin. Chem. Lab. Med. 2013, 51, 1–7. [Google Scholar] [CrossRef]
- Bakken, N.A.; Hunt, C.D. Dietary Boron Decreases Peak Pancreatic In Situ Insulin Release in Chicks and Plasma Insulin Concentrations in Rats Regardless of Vitamin D or Magnesium Status. J. Nutr. 2003, 133, 3577–3583. [Google Scholar] [CrossRef]
- Ablikim, M.; Achasov, M.N.; Albayrak, O.; Ambrose, D.J.; An, F.; An, Q.; Bai, J.Z.; Ferroli, R.B.; Ban, Y.; Becker, J.; et al. Observation of a Charged(DD¯*) ± Mass Peak ine + e−→πDD¯*ats = 4.26 GeV. Phys. Rev. Lett. 2014, 112, 022001. [Google Scholar] [CrossRef]
- Caglar, G.S.; Çakal, G.Ö.; Yüce, E.; Pabuccu, R. Evaluation of serum boron levels and lipid profile in pregnancies with or without gestational diabetes. J. Périnat. Med. 2012, 40. [Google Scholar] [CrossRef]
- Doğan, A.; Demirci, S.; Apdik, H.; Bayrak, O.F.; Gulluoglu, S.; Tuysuz, E.C.; Gusev, O.; Rizvanov, A.A.; Nikerel, E.; Sahin, F. A new hope for obesity management: Boron inhibits adipogenesis in progenitor cells through the Wnt/β-catenin pathway. Metabolism 2017, 69, 130–142. [Google Scholar] [CrossRef]
- Coban, F.K.; Ince, S.; Kucukkurt, I.; Demirel, H.H.; Hazman, Ö. Boron attenuates malathion-induced oxidative stress and acetylcholinesterase inhibition in rats. Drug Chem. Toxicol. 2014, 38, 391–399. [Google Scholar] [CrossRef]
- Ozcan, L.; Tabas, I. Calcium signalling and ER stress in insulin resistance and atherosclerosis. J. Intern. Med. 2016, 280, 457–464. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, W.; Zhong, N.; Wu, J.; Jiang, H.; Du, J.; Li, Y.; Ma, X.; Zhao, M.; Hashimoto, K.; et al. Impaired processing speed and attention in first-episode drug naive schizophrenia with deficit syndrome. Schizophr. Res. 2014, 159, 478–484. [Google Scholar] [CrossRef]
- Kawasaki, N.; Matsui, K.; Ito, M.; Nakamura, T.; Yoshimura, T.; Ushijima, H.; Maeyama, M. Effect of calcium supplementation on the vascular sensitivity to angiotensin II in pregnant women. Am. J. Obstet. Gynecol. 1985, 153, 576–582. [Google Scholar] [CrossRef]
- Pittas, A.G.; Lau, J.; Hu, F.B.; Dawson-Hughes, B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2007, 92, 2017–2029. [Google Scholar] [CrossRef]
- Abbas, W.A.; Al-Zubaidi, M.A.; Al-Khazraji, S.K. Estimation of Serum Calcium and Parathyroid Hormone (Pth) Levels in Diabetic Patients in Correlation with Age and Duration of Disease. Clin. Chem. Lab. Med. 2011, 49, S365. [Google Scholar]
- Kanchana, N.; Saikumar, P. Serum Calcium Levels In Type 2 Diabetes Mellitus. IOSR J. Dent. Med Sci. 2014, 13, 1–3. [Google Scholar] [CrossRef]
- Hassan, S.A.E. Serum Calcium Levels in Correlation with Glycated Hemoglobin in Type 2 Diabetic Sudanese Patients. Adv. Diabetes Metab. 2016, 4, 59–64. [Google Scholar]
- Chen, S.; Itoh, Y.; Masuda, T.; Shimizu, S.; Zhao, J.; Ma, J.; Nakamura, S.; Okuro, K.; Noguchi, H.; Uosaki, K.; et al. Subnanoscale hydrophobic modulation of salt bridges in aqueous media. Science 2015, 348, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Kim, G.; Jang, E.H.; Kwon, H.-S.; Baek, K.H.; Oh, K.W.; Lee, J.H.; Yoon, K.-H.; Lee, W.C.; Lee, K.W.; et al. Altered calcium homeostasis is correlated with the presence of metabolic syndrome and diabetes in middle-aged and elderly Korean subjects: The Chungju Metabolic Disease Cohort study (CMC study). Atherosclerosis 2010, 212, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Vasdev, S.; Martin, G.; Gadag, V.; Zhang, H. Altered Calcium Homeostasis Is Correlated With Abnormalities of Fasting Serum Glucose, Insulin Resistance, and -Cell Function in the Newfoundland Population. Diabetes 2005, 54, 3336–3339. [Google Scholar] [CrossRef] [PubMed]
- Ly, L.D.; Xu, S.; Choi, S.-K.; Ha, C.-M.; Thoudam, T.; Cha, S.-K.; Wiederkehr, A.; Wollheim, C.B.; Lee, I.-K.; Park, K.-S. Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Exp. Mol. Med. 2017, 49, e291. [Google Scholar] [CrossRef] [PubMed]
- Henquin, J.-C. The dual control of insulin secretion by glucose involves triggering and amplifying pathways in β-cells. Diabetes Res. Clin. Pract. 2011, 93, S27–S31. [Google Scholar] [CrossRef]
- Aune, D.; Norat, T.; Romundstad, P.R.; Vatten, L.J. Dairy products and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Am. J. Clin. Nutr. 2013, 98, 1066–1083. [Google Scholar] [CrossRef]
- Gijsbers, L.; Ding, E.L.; Malik, V.S.; De Goede, J.; Geleijnse, J.M.; Soedamah-Muthu, S.S. Consumption of dairy foods and diabetes incidence: A dose-response meta-analysis of observational studies. Am. J. Clin. Nutr. 2016, 103, 1111–1124. [Google Scholar] [CrossRef]
- Saker, F.; Ybarra, J.; Leahy, P.; Hanson, R.W.; Kalhan, S.C.; Ismail-Beigi, F. Glycemia-lowering effect of cobalt chloride in the diabetic rat: Role of decreased gluconeogenesis. Am. J. Physiol. Content 1998, 274, E984–E991. [Google Scholar] [CrossRef]
- Yildirim, Ö.; Buyukbingol, Z. Effect of cobalt on the oxidative status in heart and aorta of streptozotocin-induced diabetic rats. Cell Biochem. Funct. 2002, 21, 27–33. [Google Scholar] [CrossRef]
- Cao, J.; Vecoli, C.; Neglia, D.; Tavazzi, B.; Lazzarino, G.; Novelli, M.; Masiello, P.; Wang, Y.-T.; Puri, N.; Paolocci, N.; et al. Cobalt-Protoporphyrin Improves Heart Function by Blunting Oxidative Stress and Restoring NO Synthase Equilibrium in an Animal Model of Experimental Diabetes. Front. Physiol. 2012, 3. [Google Scholar] [CrossRef][Green Version]
- Anjum, A. Comparative study on calcium, magnesium and cobalt in diabetic and non diabetic patients (males) in Punjab, Pakistan. Afr. J. Biotechnol. 2012, 11, 7258–7262. [Google Scholar]
- Flores, C.R.; Puga, M.P.; Wrobel, K.; Garay-Sevilla, M.E.; Wrobel, K. Trace elements status in diabetes mellitus type 2: Possible role of the interaction between molybdenum and copper in the progress of typical complications. Diabetes Res. Clin. Pract. 2011, 91, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Tsubamoto, Y.; Eto, K.; Noda, M.; Daniel, S.; Suga, S.; Yamashita, S.; Kasai, H.; Wakui, M.; Sharp, G.W.G.; Kimura, S.; et al. Hexamminecobalt(III) Chloride Inhibits Glucose-induced Insulin Secretion at the Exocytotic Process. J. Boil. Chem. 2000, 276, 2979–2985. [Google Scholar] [CrossRef] [PubMed]
- Mertz, W.; Schwarz, K. Impaired intravenous glucose tolerance as an early sign of dietary necrotic liver degeneration. Arch. Biochem. Biophys. 1955, 58, 504–506. [Google Scholar] [CrossRef]
- Anderson, R.A.; Polansky, M.M.; Bryden, N.A.; Roginski, E.E.; Mertz, W.; Glinsmann, W. Chromium supplementation of human subjects: Effects on glucose, insulin, and lipid variables. Metabolism 1983, 32, 894–899. [Google Scholar] [CrossRef]
- Chen, Z.C.; Ma, J.F. Improving Nitrogen Use Efficiency in Rice through Enhancing Root Nitrate Uptake Mediated by a Nitrate Transporter, NRT1.1B. J. Genet. Genom. 2015, 42, 463–465. [Google Scholar] [CrossRef]
- Cheng, Y.; Ma, W.; Li, X.; Miao, W.; Zheng, L.; Cheng, B. Polyamines stimulate hyphal branching and infection in the early stage of Glomus etunicatum colonization. World J. Microbiol. Biotechnol. 2011, 28, 1615–1621. [Google Scholar] [CrossRef]
- Anderson, R.A. Nutritional factors influencing the glucose/insulin system: Chromium. J. Am. Coll. Nutr. 1997, 16, 404–410. [Google Scholar] [CrossRef]
- Sherman, L.; Glennon, J.; Brech, W.; Klomberg, G.; Gordon, E. Failure of trivalent chromium to improve hyperglycemia in diabetes mellitus. Metab. Clin. Exp. Clin. Exp. 1968, 17, 439–442. [Google Scholar] [CrossRef]
- Offenbacher, E.G.; Rinko, C.J.; Pi-Sunyer, F.X. The effects of inorganic chromium and brewer’s yeast on glucose tolerance, plasma lipids, and plasma chromium in elderly subjects. Am. J. Clin. Nutr. 1985, 42, 454–461. [Google Scholar] [CrossRef]
- Rabinowitz, M.B.; Gonick, H.C.; Levin, S.R.; Davidson, M.B. Clinical trial of chromium and yeast supplements on carbohydrate and lipid metabolism in diabetic men. Boil. Trace Element Res. 1983, 5, 449–466. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.S.; Brooks, B.A.; Eylath, U. The effects of chromium supplementation on serum glucose and lipids in patients with and without non-insulin-dependent diabetes. Metab. Clin. Exp. 1992, 41, 768–771. [Google Scholar] [CrossRef]
- Cefalu, W.T.; Rood, J.; Pinsonat, P.; Qin, J.; Sereda, O.; Levitan, L.; Anderson, R.A.; Zhang, X.H.; Martin, J.M.; Martin, C.K.; et al. Characterization of the metabolic and physiologic response to chromium supplementation in subjects with type 2 diabetes mellitus. Metab. Clin. Exp. 2009, 59, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Potter, J.F.; Levin, P.; Anderson, R.A.; Freiberg, J.; Andres, R.; Elahi, D. Glucose metabolism in glucose-intolerant older people during chromium supplementation. Metab. Clin. Exp. 1985, 34, 199–204. [Google Scholar] [CrossRef]
- Preuss, H.G.; Anderson, R.A. Chromium update: Examining recent literature 1997–1998. Curr. Opin. Clin. Nutr. Metab. Care 1998, 1, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Vrtovec, M.; Vrtovec, B.; Briski, A.; Kocijančič, A.; Anderson, R.A.; Radovancevic, B. Chromium supplementation shortens QTc interval duration in patients with type 2 diabetes mellitus. Am. Heart J. 2005, 149, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Qin, J.; Martin, J.; Zhang, X.H.; Sereda, O.; Anderson, R.A.; Pinsonat, P.; Cefalu, W.T. Phenotype of subjects with type 2 diabetes mellitus may determine clinical response to chromium supplementation. Metab. Clin. Exp. 2007, 56, 1652–1655. [Google Scholar] [CrossRef]
- Wells, I.C.; Claassen, J.P.; Anderson, R.J. A test for adequacy of chromium nutrition in humans—Relation to Type 2 diabetes mellitus. Biochem. Biophys. Res. Commun. 2003, 303, 825–827. [Google Scholar] [CrossRef]
- Glinsmann, W.H.; Feldman, F.J.; Mertz, W. Plasma Chromium after Glucose Administration. Science 1966, 152, 1243–1245. [Google Scholar] [CrossRef]
- Saner, G.; Yüksel, T.; Gurson, C.T. Effect of chromium on insulin secretion and glucose removal rate in the newborn. Am. J. Clin. Nutr. 1980, 33, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Mertz, W. Chromium in Human Nutrition: A Review. J. Nutr. 1993, 123, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.B. Elucidating a biological role for chromium at a molecular level. Acc. Chem. Res. 2000, 33, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Mccarty, M.F. The therapeutic potential of Glucose Tolerance Factor. Med. Hypotheses 1980, 6, 1177–1189. [Google Scholar] [CrossRef]
- Kazi, T.G.; Afridi, H.I.; Kazi, N.; Jamali, M.K.; Arain, M.B.; Jalbani, N.; Kandhro, G.A. Copper, Chromium, Manganese, Iron, Nickel, and Zinc Levels in Biological Samples of Diabetes Mellitus Patients. Boil. Trace Element Res. 2008, 122, 1–18. [Google Scholar] [CrossRef]
- Balk, E.; Tatsioni, A.; Lichtenstein, A.H.; Lau, J.; Pittas, A.G. Effect of Chromium Supplementation on Glucose Metabolism and Lipids: A systematic review of randomized controlled trials. Diabetes Care 2007, 30, 2154–2163. [Google Scholar] [CrossRef]
- Anderson, R.A. Chromium, glucose intolerance and diabetes. J. Am. Coll. Nutr. 1998, 17, 548–555. [Google Scholar] [CrossRef]
- Rajendran, K.; Manikandan, S.; Nair, L.; Karuthodiyil, R.; Vijayarajan, N.; Gnanasekar, R.; Kapil, V.V.; Mohamed, A.S. Serum Chromium Levels in Type 2 Diabetic Patients and Its Association with Glycaemic Control. J. Clin. Diagn. Res. 2015, 9, OC05–OC08. [Google Scholar] [CrossRef]
- Sundaram, B.; Aggarwal, A.; Sandhir, R. Chromium picolinate attenuates hyperglycemia-induced oxidative stress in streptozotocin-induced diabetic rats. J. Trace Elements Med. Boil. 2013, 27, 117–121. [Google Scholar] [CrossRef]
- Markou, K.; Georgopoulos, N.; Kyriazopoulou, V.; Vagenakis, A. Iodine-Induced Hypothyroidism. Thyroid. 2001, 11, 501–510. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Chen, J.; Duan, P.; Wang, J.; Liu, Y.; Guo, H. Effects of iodine excess on islet beta cells(beta-TC-6) function and the mechanism. J. Hyg. Res. 2017, 46, 610–614. [Google Scholar]
- Nederstigt, C.; Corssmit, E.P.; De Koning, E.J.P.; Dekkers, O.M. Incidence and prevalence of thyroid dysfunction in type 1 diabetes. J. Diabetes Complicat. 2016, 30, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Subekti, I.; Pramono, L.A.; Dewiasty, E.; Harbuwono, D.S. Thyroid Dysfunction in Type 2 Diabetes Mellitus Patients. Acta Med. Indones. 2017, 49, 314–323. [Google Scholar] [PubMed]
- Al-Attas, O.S.; Al-Daghri, N.; Alkharfy, K.M.; Alokail, M.S.; Al-Johani, N.J.; Abd-Alrahman, S.H.; Yakout, S.M.; Draz, H.; Sabico, S. Urinary Iodine is Associated with Insulin Resistance in Subjects with Diabetes Mellitus Type 2. Exp. Clin. Endocrinol. Diabetes 2012, 120, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Gierach, M.; Gierach, J.; Junik, R. Insulinooporność a choroby tarczycy. Endokrynol. Polska 2014, 65, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Rogowicz-Frontczak, A.; Pilacinski, S.; Chwialkowska, A.T.; Naskręt, D.; Zozulinska-Ziolkiewicz, R. Insulin resistance is associated with larger thyroid volume in adults with type 1 diabetes independently from presence of thyroid autoimmunity. Scand. J. Clin. Lab. Investig. 2018, 78, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Cooppan, R.; Kozak, G.P. Hyperthyroidism and diabetes mellitus. An analysis of 70 patients. Arch. Intern. Med. 1980, 140, 370–373. [Google Scholar] [CrossRef]
- Anil, C.; Akkurt, A.; Ayturk, S.; Kut, A.; Gursoy, A. Impaired glucose metabolism is a risk factor for increased thyroid volume and nodule prevalence in a mild-to-moderate iodine deficient area. Metab. Clin. Exp. 2013, 62, 970–975. [Google Scholar] [CrossRef]
- Michalek, A.M.; Mahoney, M.C.; Calebaugh, D. Hypothyroidism and diabetes mellitus in an American Indian population. J. Fam. Pract. 2000, 49, 638. [Google Scholar]
- Fernandez-Real, J.-M.; McClain, D.; Manco, M. Mechanisms Linking Glucose Homeostasis and Iron Metabolism Toward the Onset and Progression of Type 2 Diabetes. Diabetes Care 2015, 38, 2169–2176. [Google Scholar] [CrossRef]
- Fernandez-Real, J.-M.; López-Bermejo, A.; Ricart-Engel, W. Cross-talk between iron metabolism and diabetes. Diabetes 2002, 51, 2348–2354. [Google Scholar] [CrossRef] [PubMed]
- Lao, T.T.; Chan, P.L.; Tam, K.F. Gestational diabetes mellitus in the last trimester—A feature of maternal iron excess? Diabetic Med. J. Br. Diabet. Assoc. 2001, 18, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; MacIsaac, R.J.; Tsalamandris, C.; Power, D.; Jerums, G. Unrecognized anemia in patients with diabetes: A cross-sectional survey. Diabetes Care 2003, 26, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; MacIsaac, R.J.; Tsalamandris, C.; Jerums, G. Elevated iron indices in patients with diabetes. Diabet. Med. 2004, 21, 798–802. [Google Scholar] [CrossRef]
- Jiang, R.; Manson, J.E.; Meigs, J.B.; Ma, J.; Rifai, N.; Hu, F.B. Body Iron Stores in Relation to Risk of Type 2 Diabetes in Apparently Healthy Women. JAMA 2004, 291, 711–717. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Ruscica, M.; Rametta, R.; Recalcati, S.; Steffani, L.; Gatti, S.; Girelli, D.; Cairo, G.; Magni, P.; Fargion, S.; et al. Dietary Iron Overload Induces Visceral Adipose Tissue Insulin Resistance. Am. J. Pathol. 2013, 182, 2254–2263. [Google Scholar] [CrossRef]
- Krisai, P.; Leib, S.; Aeschbacher, S.; Kofler, T.; Assadian, M.; Maseli, A.; Todd, J.; Estis, J.; Risch, M.; Risch, L.; et al. Relationships of iron metabolism with insulin resistance and glucose levels in young and healthy adults. Eur. J. Intern. Med. 2016, 32, 31–37. [Google Scholar] [CrossRef]
- Rajpathak, S.N.; Crandall, J.P.; Wylie-Rosett, J.; Kabat, G.C.; Rohan, T.E.; Hu, F.B. The role of iron in type 2 diabetes in humans. Biochim. Biophys. Acta (BBA) Gen. Subj. 2009, 1790, 671–681. [Google Scholar] [CrossRef]
- Afkhami-Ardekani, M.; Rashidi, M. Iron status in women with and without gestational diabetes mellitus. J. Diabetes Complicat. 2009, 23, 194–198. [Google Scholar] [CrossRef]
- Sharif, A.; Younus, S.; Baig, K.; Ali, N.H. Prevalence and Risk of Anemia in Type-2 Diabetic Patients. Health 2014, 6, 1415–1419. [Google Scholar] [CrossRef]
- Huth, C.; Beuerle, S.; Zierer, A.; Heier, M.; Herder, C.; Kaiser, T.; Koenig, W.; Kronenberg, F.; Oexle, K.; Rathmann, W.; et al. Biomarkers of iron metabolism are independently associated with impaired glucose metabolism and type 2 diabetes: The KORA F4 study. Eur. J. Endocrinol. 2015, 173, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Ridaura, R.; Willett, W.C.; Rimm, E.B.; Liu, S.; Stampfer, M.J.; Manson, J.E.; Hu, F.B. Magnesium intake and risk of type 2 diabetes in men and women. Diabetes Care 2004, 27, 134–140. [Google Scholar] [CrossRef]
- Hans, C.P.; Chaudhary, D.P.; Bansal, D.D. Magnesium deficiency increases oxidative stress in rats. Indian J. Exp. Boil. 2002, 40, 1275–1279. [Google Scholar]
- Hata, A.; Doi, Y.; Ninomiya, T.; Mukai, N.; Hirakawa, Y.; Hata, J.; Ozawa, M.; Uchida, K.; Shirota, T.; Kitazono, T.; et al. Magnesium intake decreases Type 2 diabetes risk through the improvement of insulin resistance and inflammation: The Hisayama Study. Diabet. Med. 2013, 30, 1487–1494. [Google Scholar] [CrossRef]
- Dibaba, D.T.; Xun, P.; Fly, A.D.; Yokota, K.; He, K. Dietary magnesium intake and risk of metabolic syndrome: A meta-analysis. Diabet. Med. 2014, 31, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jeong, E.-M.; Liu, H.; Xie, A.; So, E.Y.; Shi, G.; Jeong, G.E.; Zhou, A.; Dudley, S.C. Magnesium supplementation improves diabetic mitochondrial and cardiac diastolic function. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Blumberg, D.; Bonetti, A.; Jacomella, V.; Capillo, S.; Truttmann, A.C.; Lüthy, C.M.; Colombo, J.P.; Bianchetti, M.G. Free circulating magnesium and renal magnesium handling during acute metabolic acidosis in humans. Am. J. Nephrol. 1998, 18, 233–236. [Google Scholar] [CrossRef]
- Pham, P.-C.T.; Pham, S.V.; Miller, J.M. Hypomagnesemia in Patients with Type 2 Diabetes. Clin. J. Am. Soc. Nephrol. 2007, 2, 366–373. [Google Scholar] [CrossRef]
- Paolisso, G.; Ravussin, E. Intracellular magnesium and insulin resistance: Results in Pima Indians and Caucasians. J. Clin. Endocrinol. Metab. 1995, 80, 1382–1385. [Google Scholar] [CrossRef]
- Djurhuus, M.; Klitgaard, N.; Pedersen, K.; Blaabjerg, O.; Altura, B.; Altura, B.; Henriksen, J.E. Magnesium reduces insulin-stimulated glucose uptake and serum lipid concentrations in type 1 diabetes. Metab. Clin. Exp. 2001, 50, 1409–1417. [Google Scholar] [CrossRef]
- Rosique-Esteban, N.; Guasch-Ferré, M.; Hernández-Alonso, P.; Salas-Salvadó, J. Dietary Magnesium and Cardiovascular Disease: A Review with Emphasis in Epidemiological Studies. Nutrients 2018, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Kao, W.H.; Folsom, A.R.; Nieto, F.J.; Mo, J.P.; Watson, R.L.; Brancati, F.L. Serum and dietary magnesium and the risk for type 2 diabetes mellitus: The Atherosclerosis Risk in Communities Study. Arch. Intern. Med. 1999, 159, 2151–2159. [Google Scholar] [CrossRef] [PubMed]
- ESaris, N.; Mervaala, E.; Karppanen, H.; AKhawaja, J.; Lewenstam, A. Magnesium. An update on physiological, clinical and analytical aspects. Clin. Chim. Acta 2000, 294, 1–26. [Google Scholar]
- Peacock, J.M.; Folsom, A.R.; Arnett, D.K.; Eckfeldt, J.H.; Szklo, M. Relationship of serum and dietary magnesium to incident hypertension: The Atherosclerosis Risk in Communities (ARIC) Study. Ann. Epidemiol. 1999, 9, 159–165. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L.-J. Magnesium metabolism in type 2 diabetes mellitus, metabolic syndrome and insulin resistance. Arch. Biochem. Biophys. 2007, 458, 40–47. [Google Scholar] [CrossRef]
- Viktorinova, A.; Tošerová, E.; Križko, M.; Ďuračková, Z.; Tǒserová, E. Altered metabolism of copper, zinc, and magnesium is associated with increased levels of glycated hemoglobin in patients with diabetes mellitus. Metab. Clin. Exp. 2009, 58, 1477–1482. [Google Scholar] [CrossRef]
- Sales, C.H.; Pedrosa, L.F.C.; Lima, J.; Lemos, T.; Colli, C. Influence of magnesium status and magnesium intake on the blood glucose control in patients with type 2 diabetes. Clin. Nutr. 2011, 30, 359–364. [Google Scholar] [CrossRef]
- Shah, G.; Pinnas, J.L.; Lung, C.C.; Mahmoud, S.; Mooradian, A.D. Tissue-specific distribution of malondialdehyde modified proteins in diabetes mellitus. Life Sci. 1994, 55, 1343–1349. [Google Scholar] [CrossRef]
- Manal Kamal, M.S.; Naglaa, K.; Khadega, A. Evaluation of trace elements and Malondialdehyde levels in type II diabetes mellitus. Diabetes Metab. Syndr. Clin. Res. Rev. 2009, 3, 214–218. [Google Scholar] [CrossRef]
- Wei, J.; Zeng, C.; Gong, Q.-Y.; Yang, H.-B.; Li, X.-X.; Lei, G.-H.; Yang, T.-B. The association between dietary selenium intake and diabetes: A cross-sectional study among middle-aged and older adults. Nutr. J. 2015, 14, 18. [Google Scholar] [CrossRef]
- Bleys, J.; Navas-Acien, A.; Guallar, E. Serum Selenium and Diabetes in U.S. Adults. Diabetes Care 2007, 30, 829–834. [Google Scholar] [CrossRef]
- Bleys, J.; Navas-Acien, A.; Guallar, E. Selenium and diabetes: More bad news for supplements. Ann. Intern. Med. 2007, 147, 271–272. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.Y.; Seo, S.; Kim, Y.; Kim, C.; Shim, S.; Jee, S.; Lee, S.; Jang, M.; Kim, M.; Yim, S.; et al. Selenium acts as an insulin-like molecule for the down-regulation of diabetic symptoms via endoplasmic reticulum stress and insulin signalling proteins in diabetes-induced non-obese diabetic mice. J. Biosci. 2007, 32, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Stranges, S.; Marshall, J.R.; Natarajan, R.; Donahue, R.P.; Trevisan, M.; Combs, G.F.; Cappuccio, F.P.; Ceriello, A.; Reid, E.M. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: A randomized trial. Ann. Intern. Med. 2007, 147, 217–223. [Google Scholar] [CrossRef]
- Stranges, S.; Sieri, S.; Vinceti, M.; Grioni, S.; Guallar, E.; Laclaustra, M.; Muti, P.; Berrino, F.; Krogh, V. A prospective study of dietary selenium intake and risk of type 2 diabetes. BMC Public Health 2010, 10, 564. [Google Scholar] [CrossRef] [PubMed]
- Priya, K.; Dhas, T.K.M.; Sylvia, J.; Rita, M.A. Selenium and glutathione peroxidase in diabetes mellitus. Int. J. Pharma Biosci. 2015, 6, 496–501. [Google Scholar]
- De Vega, R.G.; Fernández-Sánchez, M.L.; Fernández, J.C.; Álvarez Menéndez, F.V.; Sanz-Medel, A. Selenium levels and Glutathione peroxidase activity in the plasma of patients with type II diabetes mellitus. J. Trace Elements Med. Boil. 2016, 37, 44–49. [Google Scholar] [CrossRef]
- Park, K.; Rimm, E.B.; Siscovick, D.S.; Spiegelman, N.; Manson, J.E.; Morris, J.S.; Hu, F.B.; Mozaffarian, D. Toenail Selenium and Incidence of Type 2 Diabetes in U.S. Men and Women. Diabetes Care 2012, 35, 1544–1551. [Google Scholar] [CrossRef]
- Lu, C.-W.; Chang, H.-H.; Yang, K.-C.; Kuo, C.-S.; Lee, L.-T.; Huang, K.-C. High serum selenium levels are associated with increased risk for diabetes mellitus independent of central obesity and insulin resistance. BMJ Open Diabetes Res. Care 2016, 4. [Google Scholar] [CrossRef]
- Foster, M.; Samman, S. Zinc and Regulation of Inflammatory Cytokines: Implications for Cardiometabolic Disease. Nutrients 2012, 4, 676–694. [Google Scholar] [CrossRef]
- Kloubert, V.; Rink, L. Zinc as a micronutrient and its preventive role of oxidative damage in cells. Food Funct. 2015, 6, 3195–3204. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.V. Zinc and insulin in pancreatic beta-cells. Endocrine 2013, 45, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.; Karges, W.; Rink, L. Zinc and diabetes—Clinical links and molecular mechanisms. J. Nutr. Biochem. 2009, 20, 399–417. [Google Scholar] [CrossRef] [PubMed]
- El Dib, R.; Gameiro, O.L.F.; Ogata, M.S.P.; Módolo, N.S.P.; Braz, L.G.; Jorge, E.C.; Junior, P.D.N.; Beletate, V.; Nascimento, P.D. Zinc supplementation for the prevention of type 2 diabetes mellitus in adults with insulin resistance. Cochrane Database Syst. Rev. 2015, CD005525. [Google Scholar] [CrossRef]
- Bandeira, V.D.S.; Pires, L.V.; Hashimoto, L.L.; De Alencar, L.L.; Almondes, K.G.S.; Lottenberg, S.A.; Cozzolino, S.M.F. Association of reduced zinc status with poor glycemic control in individuals with type 2 diabetes mellitus. J. Trace Elements Med. Boil. 2017, 44, 132–136. [Google Scholar] [CrossRef]
- Maher, M.; Ahmed, S.R.H. A Study of Serum Magnesium, Zinc, Copper and Glycohemoglobin In Children With Type 1 Diabetes Mellitus. Alex. J. Pediatr. 2002, 16, 285–289. [Google Scholar]
- Aly, H.F.; Mantawy, M.M. Comparative effects of zinc, selenium and vitamin E or their combination on carbohydrate metabolizing enzymes and oxidative stress in streptozotocin induced-diabetic rats. Eur. Rev. Med Pharmacol. Sci. 2012, 16, 66–78. [Google Scholar]
- Abou-Seif, M.A.; Youssef, A.-A. Evaluation of some biochemical changes in diabetic patients. Clin. Chim. Acta 2004, 346, 161–170. [Google Scholar] [CrossRef]
- El-Zebda, G.A. Significance of serum levels of copper and zinc in Type II diabetic, hypertensive, and diabetic hypertensive patients in Gaza City. Available online: http://library.iugaza.edu.ps/thesis/69220.pdf (accessed on 22 June 2020).
- Estakhri, M.; Djazayery, A.; Eshraghian, M.R.; Jalali, M.; Karamizadeh, Z.; Chamari, M.; Milani, M.P. Serum Zinc Levels in Children and Adolescents with Type-1 Diabetes Mellitus. Iran. J. Public Heal. 2011, 40, 83–88. [Google Scholar]
- Xu, J.; Zhou, Q.; Liu, G.; Tan, Y.; Cai, L. Analysis of Serum and Urinal Copper and Zinc in Chinese Northeast Population with the Prediabetes or Diabetes with and without Complications. Oxid. Med. Cell. Longev. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Meenakshi, P.U.G.; Nayyar, S.B. Comparative Study of Serum Zinc, Magnesium and Copper Levels among Patients of Type 2 Diabetes Mellitus with and without Microangiopathic Complications. Innov. J. Med. Health Sci. 2013, 3, 274–278. [Google Scholar]
- Anil Kumar, V.S.P.D.; Jaiprabhu, J.; Krishnan, R. Serum copper and zinc levels significance in type 2 diabetic patients. J. Med. Sci. Tech. 2014, 3, 79–81. [Google Scholar]
- Gagandeep, D.S.J.; Shailaza, S.; Rahul, R. Evaluation of Trace Elements and Glycated Hemoglobin in Type 2 Diabetes Mellitus. World J. Pharm. Pharm. Sci. 2015, 4, 940–947. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubey, P.; Thakur, V.; Chattopadhyay, M. Role of Minerals and Trace Elements in Diabetes and Insulin Resistance. Nutrients 2020, 12, 1864. https://doi.org/10.3390/nu12061864
Dubey P, Thakur V, Chattopadhyay M. Role of Minerals and Trace Elements in Diabetes and Insulin Resistance. Nutrients. 2020; 12(6):1864. https://doi.org/10.3390/nu12061864
Chicago/Turabian StyleDubey, Pallavi, Vikram Thakur, and Munmun Chattopadhyay. 2020. "Role of Minerals and Trace Elements in Diabetes and Insulin Resistance" Nutrients 12, no. 6: 1864. https://doi.org/10.3390/nu12061864
APA StyleDubey, P., Thakur, V., & Chattopadhyay, M. (2020). Role of Minerals and Trace Elements in Diabetes and Insulin Resistance. Nutrients, 12(6), 1864. https://doi.org/10.3390/nu12061864