Vitamin K2 Needs an RDI Separate from Vitamin K1
Abstract
1. Introduction
2. Generally Accepted Definition
3. Reliable Analysis Method Complies with Definition
4. Food Databases on Vitamin K2
5. Prospective Cohort Studies
6. Clinical Trials on Metabolic Processes
7. Clinical Trials on Efficacy and Dose–Response
8. Safety Data
9. Systematic Reviews and/or Meta-Analyses
10. Plausible Biological Rationale
11. Conclusions and Next Steps
Funding
Acknowledgments
Conflicts of Interest
Disclosures
References
- Dam, H.; Schønheyder, F. The occurrence and chemical nature of Vitamin K. Biochem. J. 1936, 30, 897–901. [Google Scholar] [CrossRef]
- Halder, M.; Petsophonsakul, P.; Akbulut, A.C.; Pavlic, A.; Bohan, F.; Anderson, E.; Maresz, K.; Kramann, R.; Schurgers, L.J. Vitamin K: Double bonds beyond coagulation insights into differences between Vitamin K1 and K2 in health and disease. Int. J. Mol. Sci. 2019, 20, 896. [Google Scholar] [CrossRef] [PubMed]
- Shenkin, A. Dietary reference values for Vitamin A, Vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. J. Hum. Nutr. Diet. 2003, 16, 199–200. [Google Scholar] [CrossRef]
- Schwalfenberg, G. Vitamins K1 and K2: The emerging group of vitamins required for human health. J. Nutr. Metab. 2017, 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gröber, U.; Reichrath, J.; Holick, M.; Kisters, K. Vitamin K: An old vitamin in a new perspective. DermatoEndocrinology 2015, 6, e968490. [Google Scholar] [CrossRef] [PubMed]
- Dismore, M.L.; Haytowitz, D.B.; Gebhardt, S.E.; Peterson, J.W.; Booth, S.L. Vitamin K content of nuts and fruits in the US diet. J. Am. Diet. Assoc. 2003, 103, 1650–1652. [Google Scholar] [CrossRef] [PubMed]
- Tarento, T.D.C.; McClure, D.D.; Talbot, A.M.; Regtop, H.L.; Biffin, J.R.; Valtchev, P.; Dehghani, F.; Kavanagh, J. A potential biotechnological process for the sustainable production of Vitamin K1. Crit. Rev. Biotechnol. 2018, 69, 1–19. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Vermeer, C. Determination of phylloquinone and menaquinones in food. Haemostasis 2000, 30, 298–307. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Teunissen, K.J.F.; Hamulyák, K.; Knapen, M.H.J.; Vik, H.; Vermeer, C. Vitamin K–containing dietary supplements: comparison of synthetic Vitamin K1 and natto-derived menaquinone-7. Blood 2007, 109, 3279–3283. [Google Scholar] [CrossRef]
- Vermeer, C.; Raes, J.; van’t Hoofd, C.; Knapen, M.H.J.; Xanthoulea, S. Menaquinone content of cheese. Nutrients 2018, 10, 446. [Google Scholar] [CrossRef]
- Sato, T.; Schurgers, L.J.; Uenishi, K. Comparison of menaquinone-4 and menaquinone-7 bioavailability in healthy women. Nutr. J. 2012, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- McCann, J.C.; Ames, B.N. Vitamin K, an example of triage theory: is micronutrient inadequacy linked to diseases of aging? Am. J. Clin. Nutr. 2009, 90, 889–907. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academy Press: Washington, DC, USA, 2000. [Google Scholar]
- Hara, K.; Akiyama, Y.; Nakamura, T.; Murota, S.; Morita, I. The inhibitory effect of Vitamin K2 (menatet-renone) on bone resorption may be related to its side chain. Bone 1995, 16, 179–184. [Google Scholar] [CrossRef]
- Wu, W.-J.; Kim, M.S.; Ahn, B.-Y. The inhibitory effect of Vitamin K on RANKL-induced osteoclast differentiation and bone resorption. Food Funct. 2015, 6, 3351–3358. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Shearer, M.J.; Hamulyák, K.; Stoöcklin, E.; Vermeer, C. Effect of Vitamin K intake on the stability of oral anticoagulant treatment: Dose-response relationships in healthy subjects. Blood 2004, 104, 2682–2689. [Google Scholar] [CrossRef][Green Version]
- Theuwissen, E.; Teunissen, K.J.; Spronk, H.M.H.; Hamulyak, K.; ten Cate, H.; Shearer, M.J.; Vermeer, C.; Schurgers, L.J. Effect of low-dose supplements of menaquinone-7 (Vitamin K2) on the stability of oral anticoagulant treatment: Dose–response relationship in healthy volun-teers. J. Thromb. Haemost. 2013, 11, 1085–1092. [Google Scholar] [CrossRef]
- Marles, R.J.; Roe, A.L.; Oketch-Rabah, H.A. US Pharmacopeial Convention safety evaluation of menaquinone-7, a form of Vitamin K. Nutr. Rev. 2017, 75, 553–578. [Google Scholar] [CrossRef] [PubMed]
- Beulens, J.W.J.; Booth, S.L.; van den Heuvel, E.G.; Stoecklin, E.; Baka, A.; Vermeer, C. The role of menaquinones (Vitamin K₂) in human health. Br. J. Nutr. 2013, 110, 1357–1368. [Google Scholar] [CrossRef]
- Lupton, J.R.; Atkinson, S.A.; Chang, N.; Fraga, C.G.; Levy, J.; Messina, M.; Richardson, D.P.; Van Ommen, B.; Yang, Y.; Griffiths, J.C.; et al. Exploring the benefits and challenges of establishing a DRI-like process for bioactives. Eur. J. Nutr. 2014, 28, 1–9. [Google Scholar] [CrossRef]
- Thijssen, H.H.W.; Drittij-Reijnders, M.J. Vitamin K distribution in rat tissues: Dietary phylloquinone is a source of tissue menaquinone-4. Br. J. Nutr. 1994, 72, 415–425. [Google Scholar] [CrossRef]
- Hodges, S.; Bejui, J.; Leclercq, M.; Delmas, P. Detection and measurement of Vitamins K1 and K2 in human cortical and trabecular bone. J. Bone Miner. Res. 2009, 8, 1005–1008. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Ohtani, Y.; Yamada, Y.; Saitoh, S.; Harada, H. Difference in the metabolism of Vitamin K between liver and bone in Vitamin K-deficient rats. Br. J. Nutr. 2002, 87, 307–314. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Doisy, E.A.; Binkley, S.B.; Thayer, S.A.; McKee, R.W. Vitamin K. Science 1940, 91, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Buitenhuis, H.C.; Soute, B.A.; Vermeer, C. Comparison of the Vitamins K1, K2 and K3 as cofactors for the hepatic Vitamin K-dependent carboxylase. Biochim. Biophys. Acta 1990, 1034, 170–175. [Google Scholar] [CrossRef]
- Manoury, E.; Jourdon, K.; Boyaval, P.; Fourcassie, P. Quantitative measurement of Vitamin K2 (menaquinones) in various fermented dairy products using a reliable high-performance liquid chromatography method. J. Dairy Sci. 2013, 96, 1335–1346. [Google Scholar] [CrossRef] [PubMed]
- Hojo, K.; Watanabe, R.; Mori, T.; Taketomo, N. Quantitative measurement of Tetrahydromenaquinone-9 in cheese fermented by propionibacteria. J. Dairy Sci. 2007, 90, 4078–4083. [Google Scholar] [CrossRef] [PubMed]
- Elder, S.J.; Haytowitz, D.B.; Howe, J.; Peterson, J.W.; Booth, S.L. Vitamin K contents of meat, dairy, and fast food in the U.S. diet. J. Agric. Food Chem. 2006, 54, 463–467. [Google Scholar] [CrossRef]
- Koivu-Tikkanen, T.J.; Ollilainen, V.; Piironen, V.I. Determination of phylloquinone and menaquinones in animal products with fluorescence detection after postcolumn reduction with metallic zinc. J. Agric. Food Chem. 2000, 48, 6325–6331. [Google Scholar] [CrossRef]
- Kamao, M.; Suhara, Y.; Tsugawa, N.; Uwano, M.; Yamaguchi, N.; Uenishi, K.; Ishida, H.; Sasaki, S.; Okano, T. Vitamin K content of foods and dietary Vitamin K intake in Japanese young women. J. Nutr. Sci. Vitaminol. 2007, 53, 464–470. [Google Scholar] [CrossRef]
- Indyk, H.E.; Woollard, D.C. Vitamin K in milk and infant formulas: determination and distribution of phylloquinone and menaquinone-4. Analyst 1997, 122, 465–469. [Google Scholar] [CrossRef]
- Card, D.J.; Shearer, M.J.; Schurgers, L.J.; Harrington, D.J. The external quality assurance of phylloquinone (Vitamin K1) analysis in human serum. Biomed. Chromatogr. 2009, 23, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guo, L.; Bu, C. Vitamin K status and cardiovascular events or mortality: A meta-analysis. Eur. J. Prev. Cardiol. 2018, 26, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Kamao, M.; Suhara, Y.; Tsugawa, N.; Okano, T. Determination of plasma Vitamin K by high-performance liquid chromatography with fluorescence detection using Vitamin K analogs as internal standards. J. Chromatogr. B 2005, 816, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Dunovska, K.; Klapkova, E.; Sopko, B.; Cepova, J.; Prusa, R. LC-MS/MS quantitative analysis of phylloquinone, menaquinone-4 and menaquinone-7 in the human serum of a healthy population. PeerJ 2019, 7, e7695. [Google Scholar] [CrossRef]
- Fu, X.; Peterson, J.W.; Hdeib, M.; Booth, S.L.; Grusak, M.A.; Lichtenstein, A.H.; Dolnikowski, G. Measurement of Deuterium-Labeled Phylloquinone in plasma by high-performance liquid chromatography/mass spectrometry. Anal. Chem. 2009, 81, 5421–5425. [Google Scholar] [CrossRef]
- Suhara, Y.; Kamao, M.; Tsugawa, N.; Okano, T. Method for the determination of Vitamin K homologues in human plasma using high-performance liquid chromatography-tandem mass spectrometry. Anal. Chem. 2005, 77, 757–763. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Geleijnse, J.M.; Grobbee, D.E.; Pols, H.; Hofman, A.; Witteman, J.C.; Vermeer, C. Nutritional intake of Vitamins K1 (Phylloquinone) and K2 (Menaquinone) in The Netherlands. J. Nutr. Environ. Med. 1999, 9, 115–122. [Google Scholar] [CrossRef]
- Cundiff, D.; Agutter, P.S. Cardiovascular disease death before age 65 in 168 countries correlated statistically with biometrics, socioeconomic status, tobacco, gender, exercise, macronutrients, and Vitamin K. Cureus 2016, 8. [Google Scholar] [CrossRef]
- Ferreira, D.W.; Haytowitz, D.B.; Tassinari, M.A.; Peterson, J.W.; Booth, S.L. Vitamin K contents of grains, cereals, fast-food breakfasts, and baked goods. J. Food Sci. 2006, 71, S66–S70. [Google Scholar] [CrossRef]
- Fu, X.; Harshman, S.G.; Shen, X.; Haytowitz, D.B.; Karl, J.P.; Wolfe, B.E.; Booth, S.L. Multiple Vitamin K forms exist in dairy foods. Curr. Dev. Nutr. 2017, 1, e000638. [Google Scholar] [CrossRef]
- Hirauchi, K.; Sakano, T.; Notsumoto, S.; Nagaoka, A.; Morimoto, A.; Fujimoto, K.; Masuda, S.; Suzuki, Y. Measurement of k vitamins in animal tissues by high-performance liquid chromatography with fluorimetric detection. J. Chromatogr. B Biomed. Sci. Appl. 1989, 497, 131–137. [Google Scholar] [CrossRef]
- William, S.; Akiko, A. History of Natto and its Relatives (1405–2012); Soyinfo Center: Lafayette, CA, USA, 2012; ISBN 978-1-928914-42-6. [Google Scholar]
- Rohde, D.L.T.; Olson, S.; Chang, J.T. Modelling the recent common ancestry of all living humans. Nature 2004, 431, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Iki, M.; Morita, A.; Kajita, E.; Kagamimori, S.; Kagawa, Y.; Yoneshima, H. Intake of fermented soybeans, natto, is associated with reduced bone loss in postmenopausal women: Japanese Population-Based Osteoporosis (JPOS) Study. J. Nutr. 2006, 136, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Orimo, H.; Fujita, T.; Onomura, T.; Inoue, T.; Kushida, K.; Shiraki, M. Clinical evaluation of soft capsule menatetrenone (Ea-0167) in the treatment of osteoporosis. Late Phase II Dose Study. J. New Rem. Clin. 1992, 41, 1249–1279. [Google Scholar]
- Asakura, H.; Myou, S.; Ontachi, Y.; Kato, M.; Saito, M.; Yamazaki, M.; Nakao, S.; Mizutani, T.; Morishita, E. Vitamin K administration to elderly patients with Osteoporosis induces no hemostatic activation, even in those with suspected Vitamin K deficiency. Osteoporos. Int. 2001, 12, 996–1000. [Google Scholar] [CrossRef]
- Kojima, A.; Ikehara, S.; Kamiya, K.; Kajita, E.; Sato, Y.; Kouda, K.; Tamaki, J.; Kagamimori, S.; Iki, M. Natto intake is inversely associated with Osteoporotic fracture risk in postmenopausal Japanese women. J. Nutr. 2019, 150, 599–605. [Google Scholar] [CrossRef]
- Fusaro, M.; Noale, M.; Viola, V.; Galli, F.; Tripepi, G.; Vajente, N.; Plebani, M.; Zaninotto, M.; Guglielmi, G.; Miotto, D.; et al. VItamin K Italian (VIKI) dialysis study investigators. Vitamin K, vertebral fractures, vascular calcifications, and mortality: VItamin K Italian (VIKI) dialysis study. J. Bone Miner. Res. 2012, 27, 2271–2278. [Google Scholar] [CrossRef]
- Evenepoel, P.; Claes, K.; Meijers, B.; Laurent, M.R.; Bammens, B.; Naesens, M.; Sprangers, B.; Pottel, H.; Cavalier, E.; Kuypers, D. Poor Vitamin K status is associated with low bone mineral density and increased fracture risk in end-stage renal disease. J. Bone Miner. Res. 2018, 34, 262–269. [Google Scholar] [CrossRef]
- Knapen, M.H.J.; Braam, L.A.J.L.M.; Drummen, N.E.; Bekers, O.; Hoeks, A.P.G.; Vermeer, C. Menaquinone-7 supplementation improves arterial stiffness in healthy postmenopausal women. A double-blind randomised clinical trial. Thromb. Haemost. 2015, 113, 1135–1144. [Google Scholar] [CrossRef]
- Knapen, M.H.J.; Drummen, N.E.; Smit, E.; Vermeer, C.; Theuwissen, E. Three-year low-dose menaquinone-7 supplementation helps decrease bone loss in healthy postmenopausal women. Osteoporos. Int. 2013, 24, 2499–2507. [Google Scholar] [CrossRef]
- Westenfeld, R.; Krueger, T.; Schlieper, G.; Cranenburg, E.C.; Magdeleyns, E.J.; Heidenreich, S.; Holzmann, S.; Vermeer, C.; Jahnen-Dechent, W.; Ketteler, M.; et al. Effect of Vitamin K2 supplementation on functional Vitamin K deficiency in hemodialysis patients: A randomized trial. Am. J. Kidney Dis. 2012, 59, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Gast, G.-C.M.; De Roos, N.; Sluijs, I.; Bots, M.; Beulens, J.; Geleijnse, J.; Witteman, J.; Grobbee, D.; Peeters, P.; Van Der Schouw, Y.; et al. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Evatt, M.L.; McDermott, M.P.; Delong, M.R.; Kumari, M.; Auinger, P.; Tangpricha, V. High prevalence of hypovitaminosis D status in patients with early parkinson disease. Arch. Neurol. 2011, 68, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Apalset, E.M.; Gjesdal, C.G.; Eide, G.E.; Tell, G.S. Intake of Vitamin K1 and K2 and risk of hip fractures: The Hordaland Health Study. Bone 2011, 49, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Beulens, J.W.J.; van der A, D.L.; Grobbee, D.E.; Sluijs, I.; Spijkerman, A.M.; Van Der Schouw, Y.T. Dietary phylloquinone and menaquinones intakes and risk of type 2 Diabetes. Diabetes Care 2010, 33, 1699–1705. [Google Scholar] [CrossRef]
- Iwamoto, J.; Sato, Y.; Takeda, T.; Matsumoto, H. High-dose Vitamin K supplementation reduces fracture incidence in postmenopausal women: A review of the literature. Nutr. Res. 2009, 29, 221–228. [Google Scholar] [CrossRef]
- Bulló, M.; Estruch, R.; Salas-Salvadó, J. Dietary Vitamin K intake is associated with bone quantitative ultrasound measurements but not with bone peripheral biochemical markers in elderly men and women. Bone 2011, 48, 1313–1318. [Google Scholar] [CrossRef]
- Fusaro, M.; D’Angelo, A.V.; Gallieni, M. Consequences of Vitamin K2 deficiency in hemodialysis patients. Am. J. Kidney Dis. 2012, 60, 169. [Google Scholar] [CrossRef]
- Kohlmeier, M.; Saupe, J.; Shearer, M.J.; Schaefer, K.; Asmus, G. Bone health of adult hemodialysis patients is related to Vitamin K status. Kidney Int. 1997, 51, 1218–1221. [Google Scholar] [CrossRef]
- Pilkey, R.M.; Morton, A.R.; Boffa, M.B.; Noordhof, C.; Day, A.G.; Su, Y.; Miller, L.M.; Koschinsky, M.L.; Booth, S.L. Subclinical Vitamin K deficiency in hemodialysis patients. Am. J. Kidney Dis. 2007, 49, 432–439. [Google Scholar] [CrossRef]
- Holden, R.M.; Morton, A.R.; Garland, J.S.; Pavlov, A.; Day, A.G.; Booth, S.L. Vitamins K and D status in stages 3–5 chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Willems, B.A.G.; Vermeer, C.; Reutelingsperger, C.P.M.; Schurgers, L.J. The realm of Vitamin K dependent proteins: Shifting from coagulation toward calcification. Mol. Nutr. Food Res. 2014, 58, 1620–1635. [Google Scholar] [CrossRef]
- Shearer, M.J.; Newman, P. Metabolism and cell biology of Vitamin K. Thromb. Haemost. 2017, 22, 530–547. [Google Scholar] [CrossRef]
- Hussain, M.M.; Goldberg, I.J.; Weisgraber, K.H.; Mahley, R.W.; Innerarity, T.L. Uptake of chylomicrons by the liver, but not by the bone marrow, is modulated by lipoprotein lipase activity. Arter. Thromb. Vasc. Boil. 1997, 17, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Shearer, M.J.; Bach, A.; Kohlmeier, M. Chemistry, nutritional sources, tissue distribution and metabolism of Vitamin K with special reference to bone health. J. Nutr. 1996, 126, 1181S–1186S. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Vermeer, C. Differential lipoprotein transport pathways of K-Vitamins in healthy subjects. Biochim. Biophys. Acta (BBA) 2002, 1570, 27–32. [Google Scholar] [CrossRef]
- Hollander, D.; Rim, E.; Ruble, P.E. Vitamin K2 colonic and ileal in vivo absorption: bile, fatty acids, and pH effects on transport. Am. J. Physiol. 1977, 233, E124–E129. [Google Scholar] [CrossRef]
- Uematsu, T.; Nagashima, S.; Niwa, M.; Kohno, K.; Sassa, T.; Ishii, M.; Tomono, Y.; Yamato, C.; Kanamaru, M. Effect of dietary fat content on oral bioavailability of menatetrenone in humans. J. Pharm. Sci. 1996, 85, 1012–1016. [Google Scholar] [CrossRef]
- Booth, S.L.; Tucker, K.L.; McKeown, N.M.; Davidson, K.W.; Dallal, G.; Sadowski, J.A. Relationships between dietary intakes and fasting plasma concentrations of fat-soluble vitamins in humans. J. Nutr. 1997, 127, 587–592. [Google Scholar] [CrossRef][Green Version]
- Shearer, M.J.; Fu, X.; Booth, S.L. Vitamin K nutrition, metabolism, and requirements: Current concepts and future research. Adv. Nutr. 2012, 3, 182–195. [Google Scholar] [CrossRef]
- Shearer, M.J.; McBurney, A.; Barkhan, P. Studies on the absorption and metabolism of phylloquinone (Vitamin K1) in man. Vitam. Horm. 1974, 32, 513–542. [Google Scholar] [PubMed]
- Gijsbers, B.L.M.G.; Jie, K.-S.G.; Vermeer, C. Effect of food composition on Vitamin K absorption in human volunteers. Br. J. Nutr. 1996, 76, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Konishi, T.; Baba, S.; Sone, H. Whole-body autoradiographic study of Vitamin K distribution in Rat. Chem. Pharm. Bull. 1973, 21, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Okano, T.; Shimomura, Y.; Yamane, M.; Suhara, Y.; Kamao, M.; Sugiura, M.; Nakagawa, K. Conversion of phylloquinone (Vitamin K1) into menaquinone-4 (Vitamin K2) in mice: Two possible routes for menaquinone-4 accumulation in cerebra of mice. J. Biol. Chem. 2008, 283, 11270–11279. [Google Scholar] [CrossRef] [PubMed]
- Berkner, K. Vitamin K-Dependent carboxylation. In Vitamin K; Vitamins & Hormones: San Diego, CA, USA, 2008; Volume 78, pp. 131–156. [Google Scholar] [CrossRef]
- Bahney, C.S.; Do, R.L.Z.; Allison, P.; Theologis, A.; Ashley, J.; Ahn, J.; Miclau, T.; Marcucio, R.S.; Hankenson, K.D. Cellular biology of fracture healing. J. Orthop. Res. 2018, 37, 35–50. [Google Scholar] [CrossRef]
- Aoun, M.; Makki, M.; Azar, H.; Matta, H.; Chelala, D.N. High dephosphorylated-uncarboxylated MGP in Hemodialysis patients: Risk factors and response to Vitamin K2, A pre-post intervention clinical trial. BMC Nephrol. 2017, 18, 191. [Google Scholar] [CrossRef]
- Caluwe, R.; Vandecasteele, S.; Van Vlem, B.; Vermeer, C.; De Vriese, A.S. Vitamin K2 supplementation in haemodialysis patients: A randomized dose-finding study. Nephrol. Dial. Transplant. 2014, 29, 1385–1390. [Google Scholar] [CrossRef]
- Dituri, F.; Buonocore, G.; Pietravalle, A.; Naddeo, F.; Cortesi, M.; Pasqualetti, P.; Tataranno, M.L.; Agostino, R. PIVKA-II plasma levels as markers of subclinical Vitamin K deficiency in term infants. J. Matern. Fetal Neonatal Med. 2012, 25, 1660–1663. [Google Scholar] [CrossRef]
- Shiraki, M.; Shiraki, Y.; Aoki, C.; Miura, M. Vitamin K2 (Menatetrenone) effectively prevents fractures and sustains lumbar bone mineral density in Osteoporosis. J. Bone Miner. Res. 2010, 15, 515–521. [Google Scholar] [CrossRef]
- Booth, S.L.; Martini, L.; Peterson, J.W.; Saltzman, E.; Dallal, G.E.; Wood, R.J. Dietary phylloquinone depletion and repletion in older women. J. Nutr. 2003, 133, 2565–2569. [Google Scholar] [CrossRef]
- Rehder, U.S.; Gundberg, C.M.; Booth, S.L.; Borges, C.R. Gamma-carboxylation and fragmentation of osteocalcin in human serum defined by mass spectrometry. Mol. Cell. Proteom. 2015, 14, 1546–1555. [Google Scholar] [CrossRef]
- Inaba, N.; Sato, T.; Yamashita, T. Low-dose daily intake of Vitamin K2 (Menaquinone-7) improves osteocalcin?-Carboxylation: A double-blind, randomized controlled trials. J. Nutr. Sci. Vitaminol. 2015, 61, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, E.; Aoki, M.; Watanabe, F.; Kamimura, A. Low-dose menaquinone-4 improves γ-carboxylation of osteocalcin in young males: a non-placebo-controlled dose-response study. Nutr. J. 2014, 13, 85. [Google Scholar] [CrossRef] [PubMed][Green Version]
- FDA. Menaquinone-7/MenaQ7GRAS Assessment-NattoPharma ASA. 2008. Available online: http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-foodsgen/documents/document/ucm264117.pdf (accessed on 19 October 2019).
- European Food Safety Authority (EFSA). Vitamin K2 added for nutritional purposes in foods for particular nutritional uses, food supplements and foods intended for the general population and Vitamin K2 as a source of Vitamin K added for nutritional purposes to foodstuffs, in the context of Regu. EFSA J. 2008, 6, 822. [Google Scholar] [CrossRef]
- Pucaj, K.; Rasmussen, H.; Møller, M.; Preston, T. Safety and toxicological evaluation of a synthetic Vitamin K2, Menaquinone-7. Toxicol. Mech. Methods 2011, 21, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-B.; Wan, S.-L.; Lü, Y.-J.; Ning, L.; Liu, C.; Fan, S.-W. Does Vitamin K2 play a role in the prevention and treatment of Osteoporosis for postmenopausal women: A meta-analysis of randomized controlled trials. Osteoporos. Int. 2014, 26, 1175–1186. [Google Scholar] [CrossRef]
- Cockayne, S.; Adamson, J.; Lanham-New, S.; Shearer, M.J.; Gilbody, S.; Torgerson, D. Vitamin K and the prevention of fractures. Arch. Intern. Med. 2006, 166, 1256. [Google Scholar] [CrossRef]
- Lees, J.S.; A Chapman, F.; Witham, M.D.; Jardine, A.G.; Mark, P.B. Vitamin K status, supplementation and vascular disease: A systematic review and meta-analysis. Heart 2019, 105, 938–945. [Google Scholar] [CrossRef]
- Rees, K.; Guraewal, S.; Wong, Y.L.; Majanbu, D.L.; Mavrodaris, A.; Stranges, S.; Kandala, N.-B.; Clarke, A.; Franco, O.H. Is Vitamin K consumption associated with cardio-metabolic disorders? A systematic review. Maturitas 2010, 67, 121–128. [Google Scholar] [CrossRef]
- Moher, D.; Tricco, A.C. Issues related to the conduct of systematic reviews: A focus on the nutrition field. Am. J. Clin. Nutr. 2008, 88, 1191–1199. [Google Scholar]
- Russell, R.; Chung, M.; Balk, E.M.; Atkinson, S.; Giovannucci, E.L.; Ip, S.; Lichtenstein, A.H.; Mayne, S.T.; Raman, G.; Ross, A.C.; et al. Opportunities and challenges in conducting systematic reviews to support the development of nutrient reference values: Vitamin A as an example. Am. J. Clin. Nutr. 2009, 89, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Shearer, M.J.; Okano, T. Key pathways and regulators of Vitamin K function and intermediary metabolism. Annu. Rev. Nutr. 2018, 38, 127–151. [Google Scholar] [CrossRef] [PubMed]
- Shearer, M. Vitamin K metabolism and nutriture. Blood Rev. 1992, 6, 92–104. [Google Scholar] [CrossRef]
- Bell, R.G. Metabolism of Vitamin K and prothrombin synthesis: Anticoagulants and the Vitamin K--epoxide cycle. Fed. Proc. 1978, 37, 2599–2604. [Google Scholar] [PubMed]
- Lacombe, J.; Ferron, M. VKORC1L1, An enzyme mediating the effect of Vitamin K in liver and extrahepatic tissues. Nutrients 2018, 10, 970. [Google Scholar] [CrossRef]
- Wasilewski, G.B.; Vervloet, M.G.; Schurgers, L.J. The bone—vasculature axis: Calcium supplementation and the role of Vitamin K. Front. Cardiovasc. Med. 2019, 6, 6. [Google Scholar] [CrossRef]
- Maresz, K. Proper calcium use: Vitamin K2 as a promoter of bone and cardiovascular health. Integr. Med. 2015, 14, 34–39. [Google Scholar]
- Dahlberg, S.; Ede, J.; Schurgers, L.J.; Vermeer, C.; Kander, T.; Klarin, B.; Schött, U. Desphospho-uncarboxylated matrix-gla protein is increased postoperatively in cardiovascular risk patients. Nutrients 2018, 10, 46. [Google Scholar] [CrossRef]
- Huang, Y. Combined treatment of Vitamin K and teriparatide on bone metabolism and biomechanics in rats with Osteoporosis. Exp. Ther. Med. 2018, 15, 315–319. [Google Scholar] [CrossRef]
- Manna, P.; Kalita, J. Beneficial role of Vitamin K supplementation on insulin sensitivity, glucose metabolism, and the reduced risk of type 2 diabetes: A review. Nutrition 2016, 32, 732–739. [Google Scholar] [CrossRef]
- Møller, M.; Gjelstad, I.M.F.; Baksaas, I.; Grande, T.; Aukrust, I.R.; Drevon, C.A.; Møller, M.; Fange, G.I.M.; Ingebjørg, B.; Tone, G.; et al. Bioavailability and chemical/functional aspects of synthetic MK-7 vs. fermentation-derived MK-7 in randomised controlled trials. Int. J. Vitam. Nutr. Res. 2016, 87, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kanellakis, S.; Moschonis, G.; Tenta, R.; Schaafsma, A.; Heuvel, E.V.D.; Papaioannou, N.; Lyritis, G.; Manios, Y. Changes in parameters of bone metabolism in postmenopausal women following a 12-month intervention period using dairy products enriched with Calcium, Vitamin D, and Phylloquinone (Vitamin K1) or Menaquinone-7 (Vitamin K2): The postmenopausal health study II. Calcif. Tissue Int. 2012, 90, 251–262. [Google Scholar] [CrossRef]
- Brandenburg, V.M.; Reinartz, S.; Kaesler, N.; Krüger, T.; Dirrichs, T.; Kramann, R.; Peeters, F.; Floege, J.; Keszei, A.; Marx, N.; et al. Slower progress of aortic valve calcification with Vitamin K supplementation: Results from a prospective interventional proof-of-concept study. Circulation 2017, 135, 2081–2083. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.M.; Tile, L.; Lee, Y.; Tomlinson, G.; Hawker, G.; Scher, J.; Hu, H.; Vieth, R.; Thompson, L.; Jamal, S.; et al. Vitamin K supplementation in postmenopausal women with osteopenia (ECKO trial): A randomized controlled trial. PLoS Med. 2008, 5, e196. [Google Scholar] [CrossRef] [PubMed]
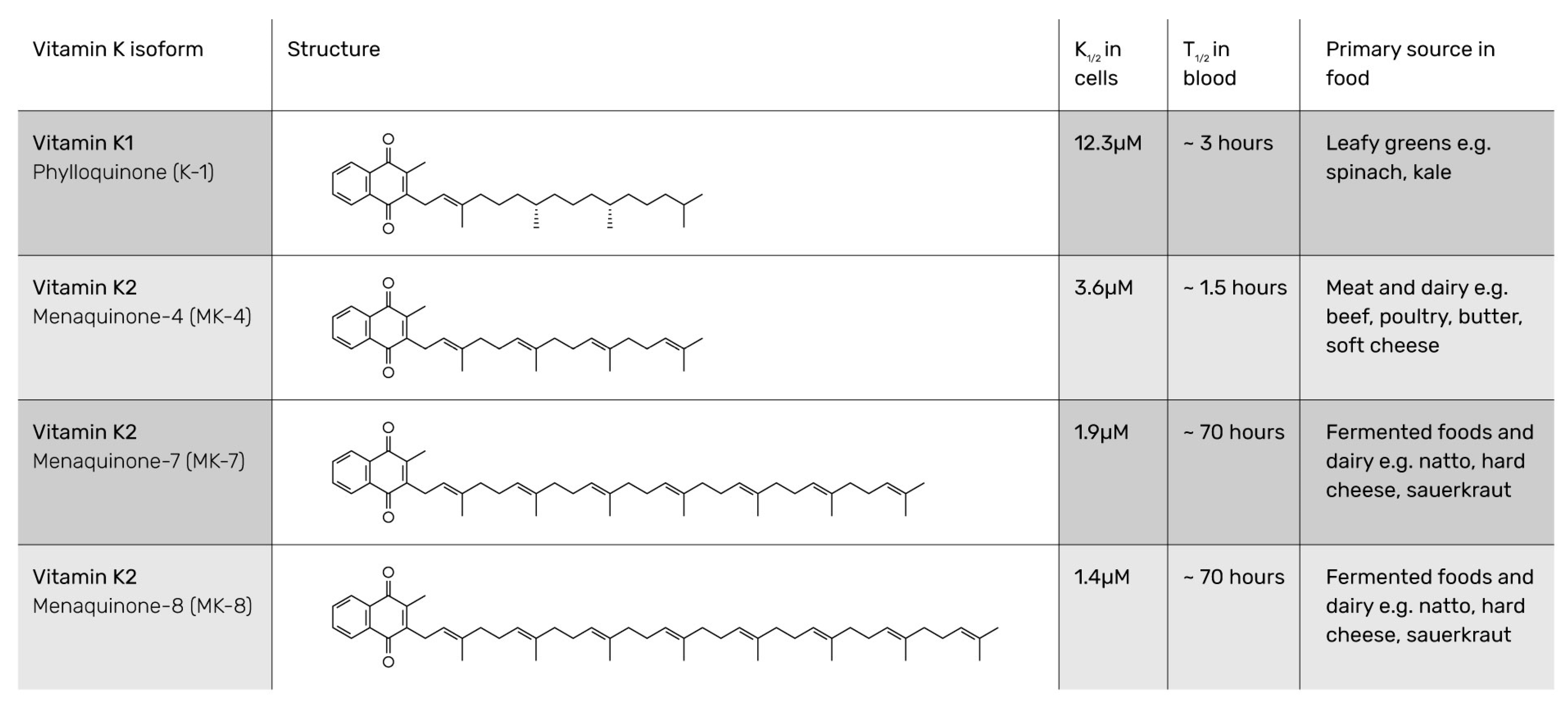
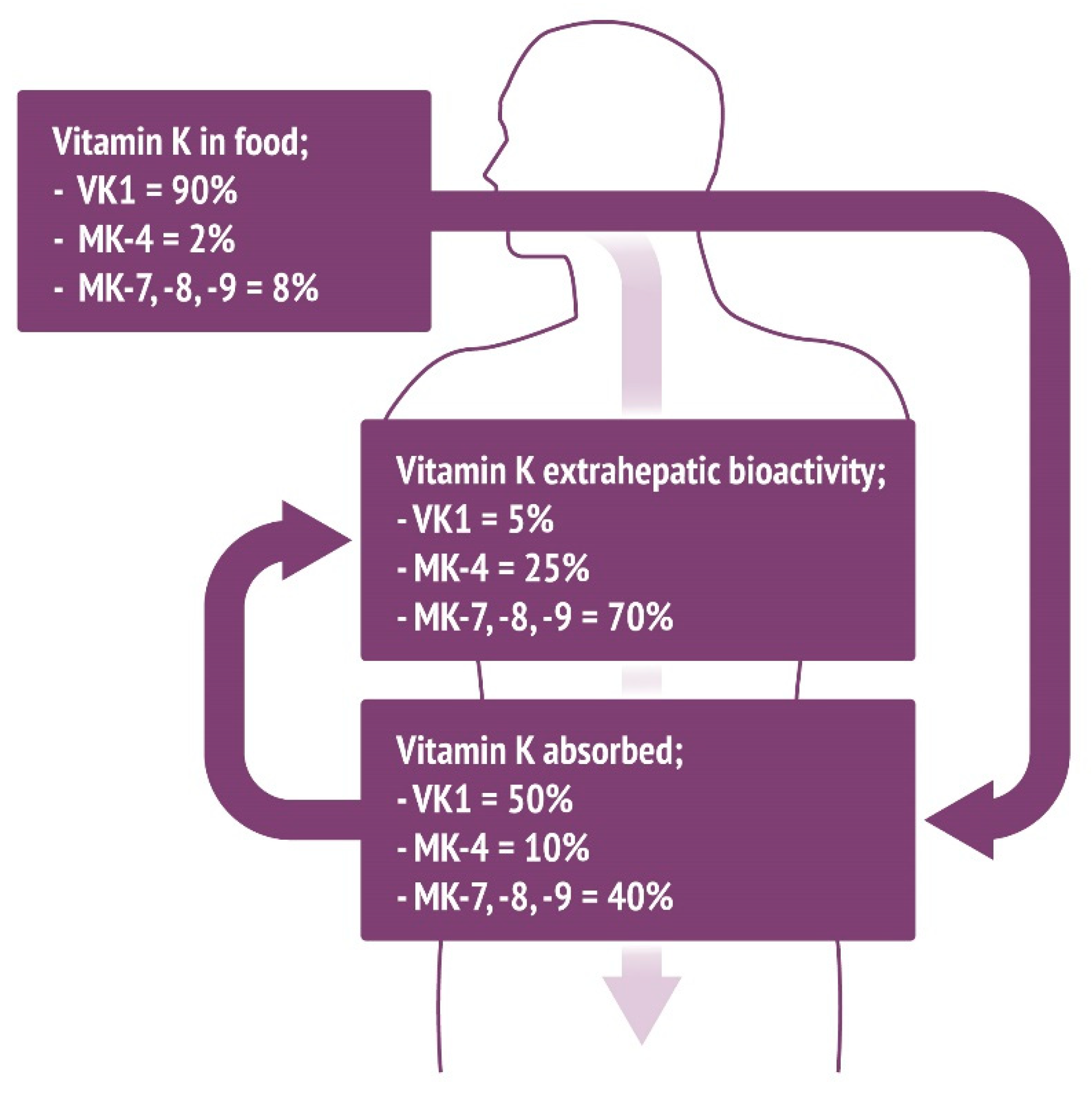
| Food Category | Food Source | Vitamin K1 Content per 100 g of Food Sample (µg) | Vitamin K2 Content per 100 g of Food Sample (µg) |
|---|---|---|---|
| Prepared vegetables | Natto (fermented soybeans) | 32.1 | 108.9 |
| Roasted soybeans | 57.3 | Not compared in the study | |
| Sauerkraut | 22.4 | 5.5 | |
| Vegetables | Collards | 706 | Not compared in the study |
| Turnip | 568 | Not compared in the study | |
| Broccoli | 146.7 | Not compared in the study | |
| Spinach | 96.7 | Not compared in the study | |
| Kale | 73.3 | Not compared in the study | |
| Carrot | 25.5 | Not compared in the study | |
| Fruits | Dried prunes | 51.1–68.1 | Not compared in the study |
| Kiwifruit | 33.9–50.3 | Not compared in the study | |
| Avocado | 15.7–27.0 | Not compared in the study | |
| Blueberries | 14.7–27.2 | Not compared in the study | |
| Blackberries | 14.7–25.1 | Not compared in the study | |
| Grapes red and green | 13.8–18.1 | Not compared in the study | |
| Dried figs | 11.4–20.0 | Not compared in the study | |
| Nuts | Pine nuts | 33.4–73.7 | Not compared in the study |
| Cashews | 19.4–64.3 | Not compared in the study | |
| Pistachios | 10.1–15.1 | Not compared in the study | |
| Cheese | Roquefort | 6.56 | 38.1 |
| Pecorino | 5.56 | 93.7 | |
| Brie | 4.92 | 12.5 | |
| Boursin | 4.55 | 11.1 | |
| Norvegia | 4.37 | 41.5 | |
| Stilton | 3.62 | 49.4 | |
| Münster | 2.1 | 80.1 | |
| Camembert | 2.5 | 68.1 | |
| Gamalost | 0.18 | 54.2 | |
| Emmental | 2.41 | 43.3 | |
| Raclette | 1.55 | 32.3 | |
| Meat | Beef liver | 2.3 | 11.2 |
| Beef meat | 0.02 | 1.89 | |
| Minced meat | 1.1 | 7.6 | |
| Chicken meat | Not detected in the study | 10.1 | |
| Pork meat | Not detected in the study | 1.4 | |
| Pork liver | Not detected in the study | 1.8 | |
| Fish | Mackerel | 0.5 | 0.6 |
| Eel | 1.3 | 63.1 | |
| Plaice | Not detected in the study | 5.3 | |
| Prawns | Not detected in the study | 0.19 | |
| Salmon | 0.13 | 0.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbulut, A.C.; Pavlic, A.; Petsophonsakul, P.; Halder, M.; Maresz, K.; Kramann, R.; Schurgers, L. Vitamin K2 Needs an RDI Separate from Vitamin K1. Nutrients 2020, 12, 1852. https://doi.org/10.3390/nu12061852
Akbulut AC, Pavlic A, Petsophonsakul P, Halder M, Maresz K, Kramann R, Schurgers L. Vitamin K2 Needs an RDI Separate from Vitamin K1. Nutrients. 2020; 12(6):1852. https://doi.org/10.3390/nu12061852
Chicago/Turabian StyleAkbulut, Asim Cengiz, Angelina Pavlic, Ploingarm Petsophonsakul, Maurice Halder, Katarzyna Maresz, Rafael Kramann, and Leon Schurgers. 2020. "Vitamin K2 Needs an RDI Separate from Vitamin K1" Nutrients 12, no. 6: 1852. https://doi.org/10.3390/nu12061852
APA StyleAkbulut, A. C., Pavlic, A., Petsophonsakul, P., Halder, M., Maresz, K., Kramann, R., & Schurgers, L. (2020). Vitamin K2 Needs an RDI Separate from Vitamin K1. Nutrients, 12(6), 1852. https://doi.org/10.3390/nu12061852






