An Assessment of the Glyconutrient Ambrotose™ on Immunity, Gut Health, and Safety in Men and Women: A Placebo-Controlled, Double-Blind, Randomized Clinical Trial
Abstract
:1. Introduction
2. Methods
2.1. Subjects
2.2. Treatment Assignment
2.3. Laboratory Test Visits
2.4. Health and Well-Being Questionnaires
2.5. Blood Collection and Analysis
2.6. Ex Vivo Stimulation LPS Stimulation and Cytokine Measurements
2.7. Activity and Dietary Intake
2.8. Data Analysis
3. Results
3.1. Heart Rate and Blood Pressure
3.2. Subjective Measures/Quality of Life
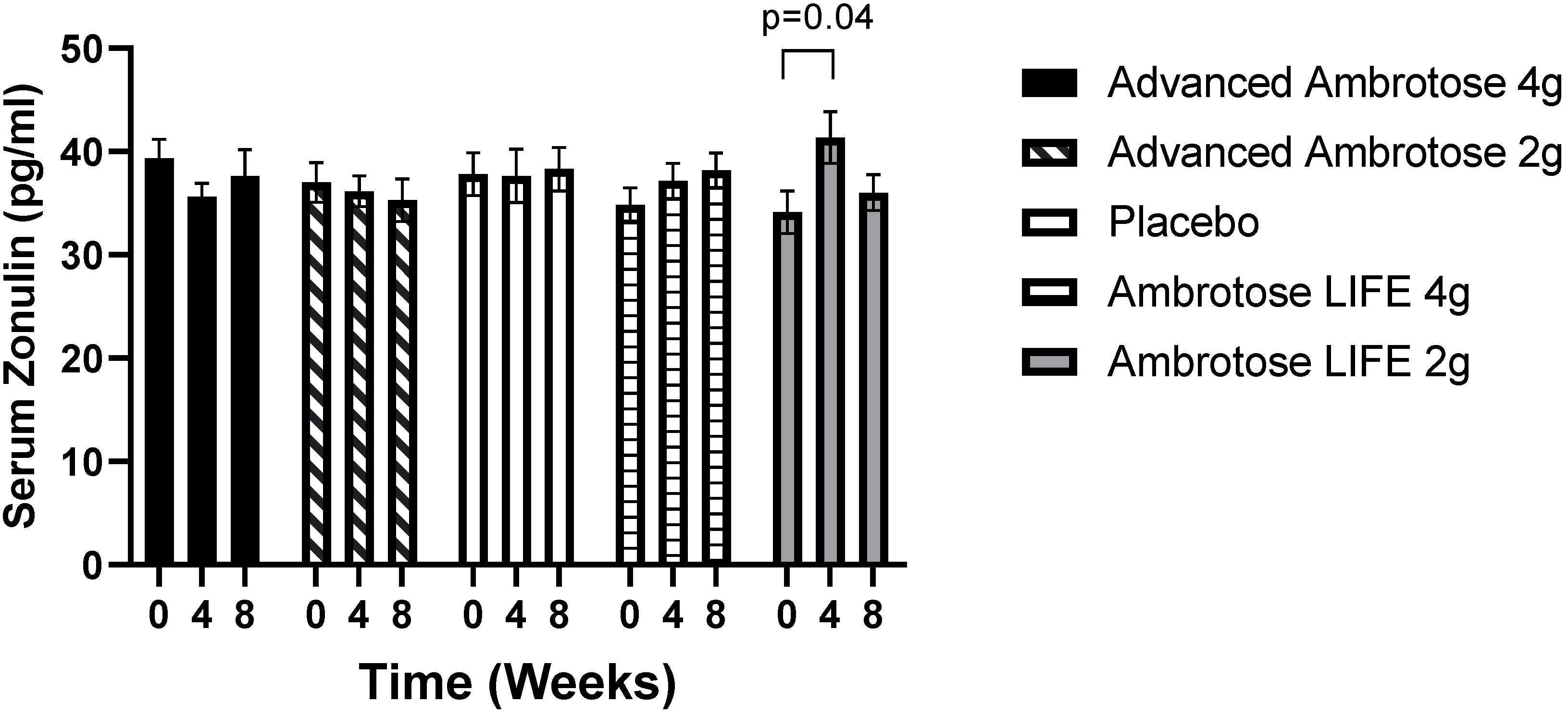
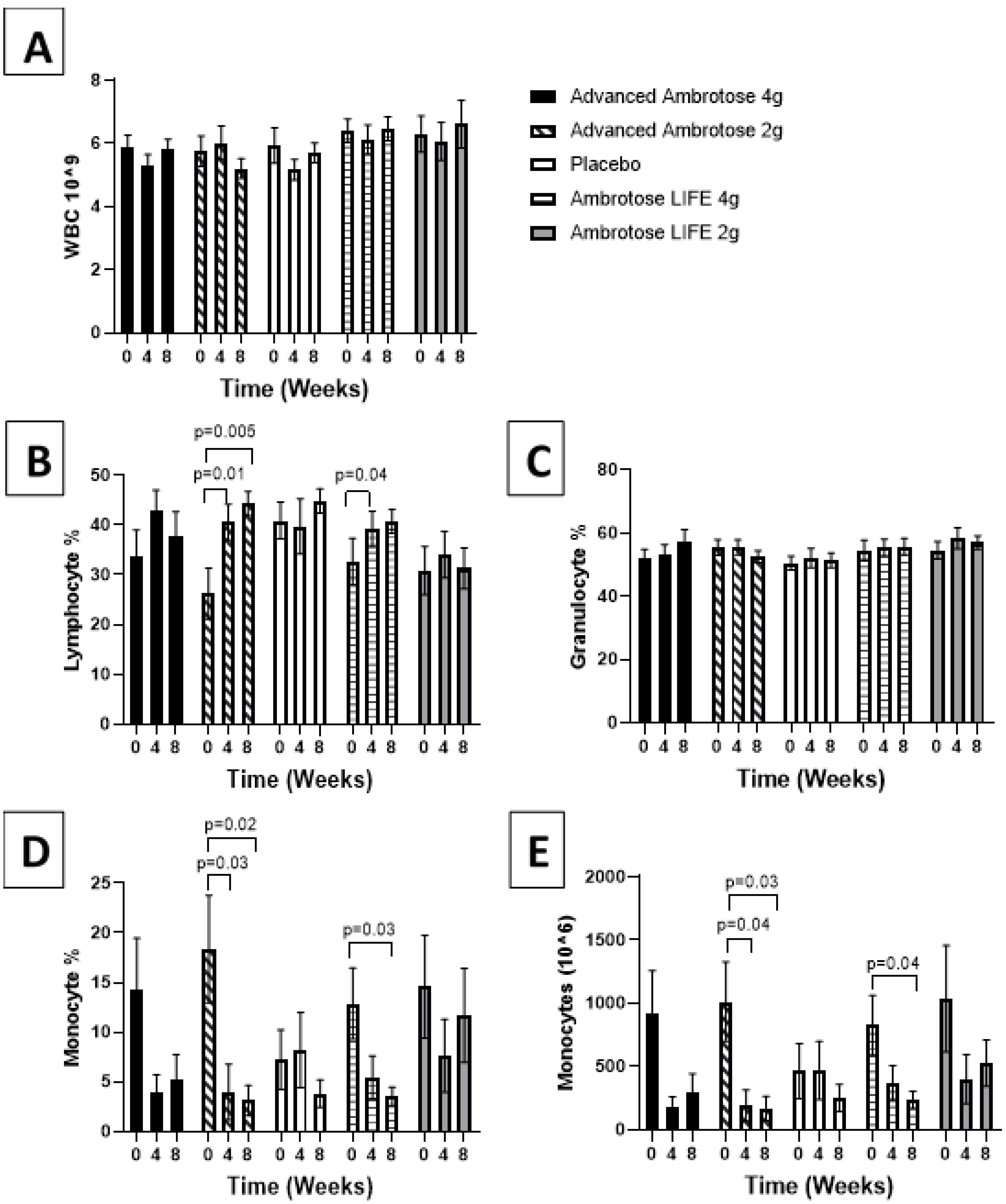
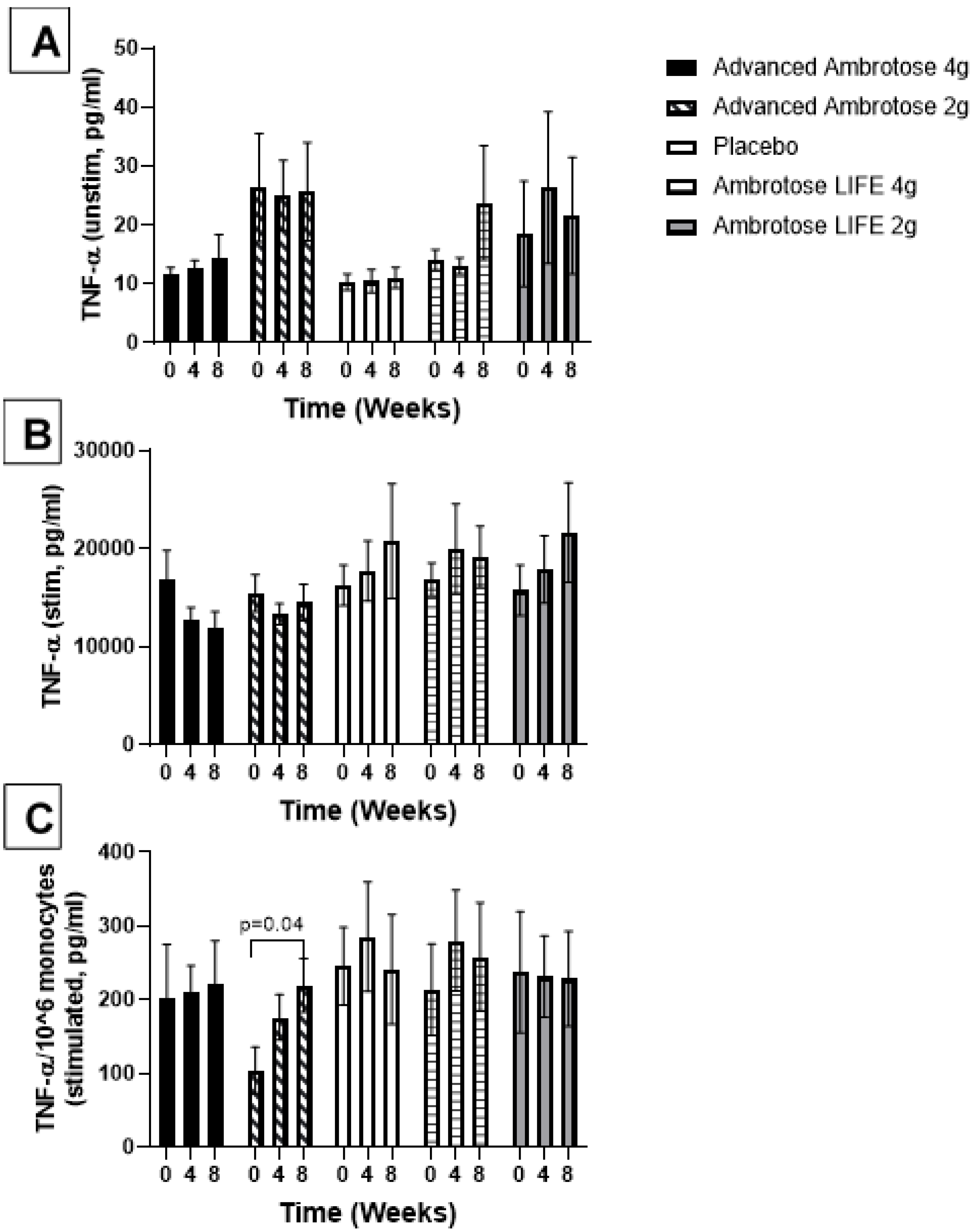
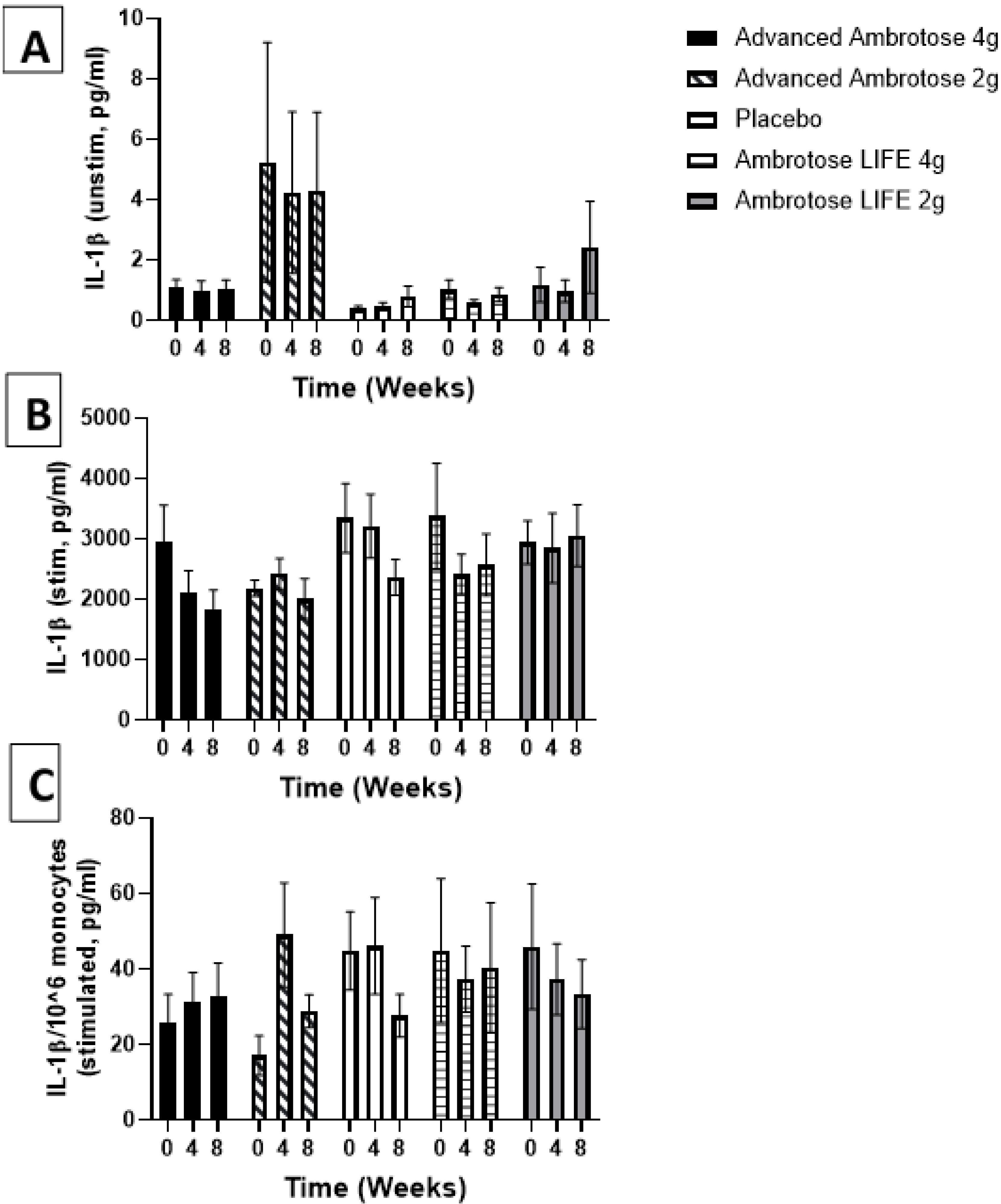
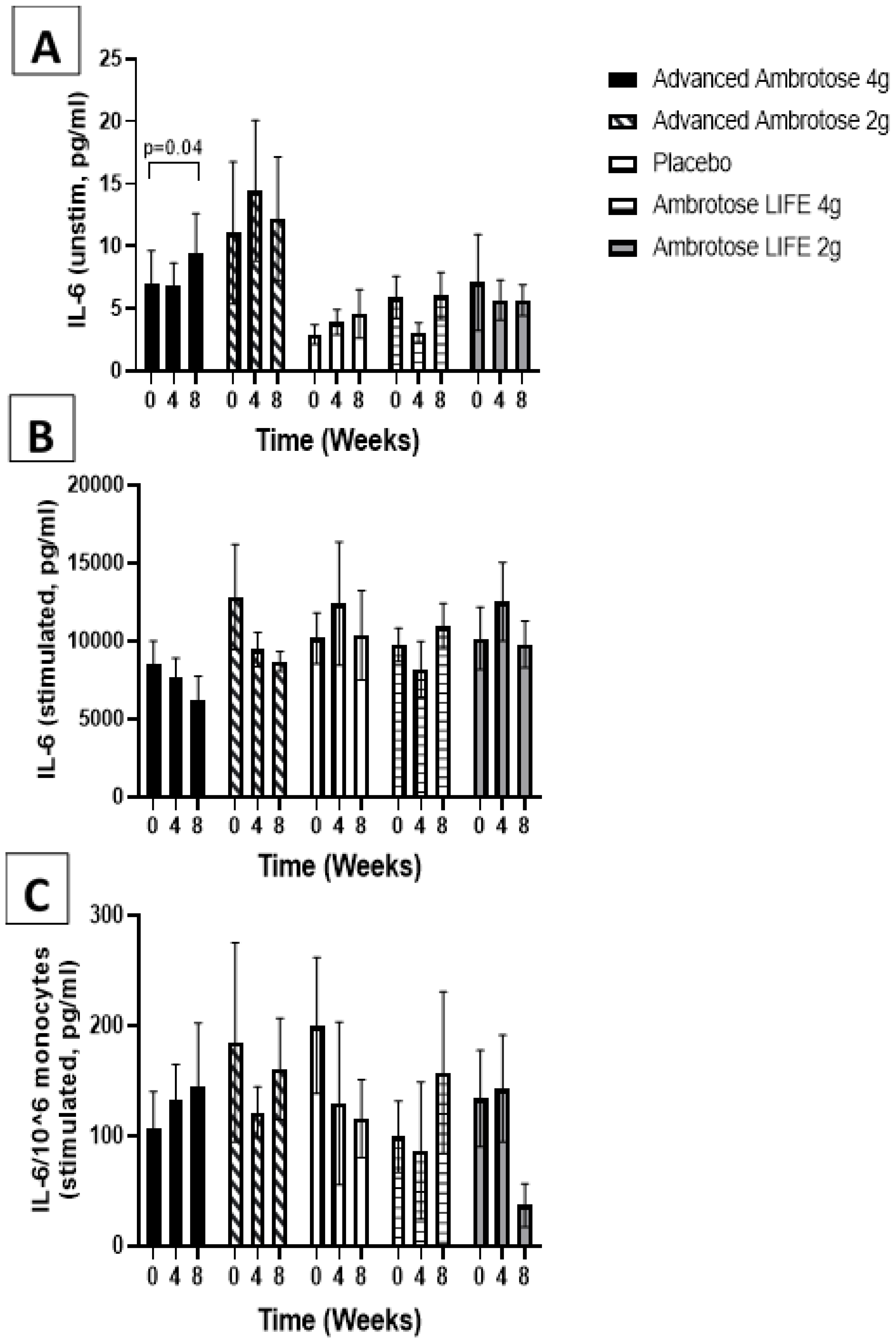
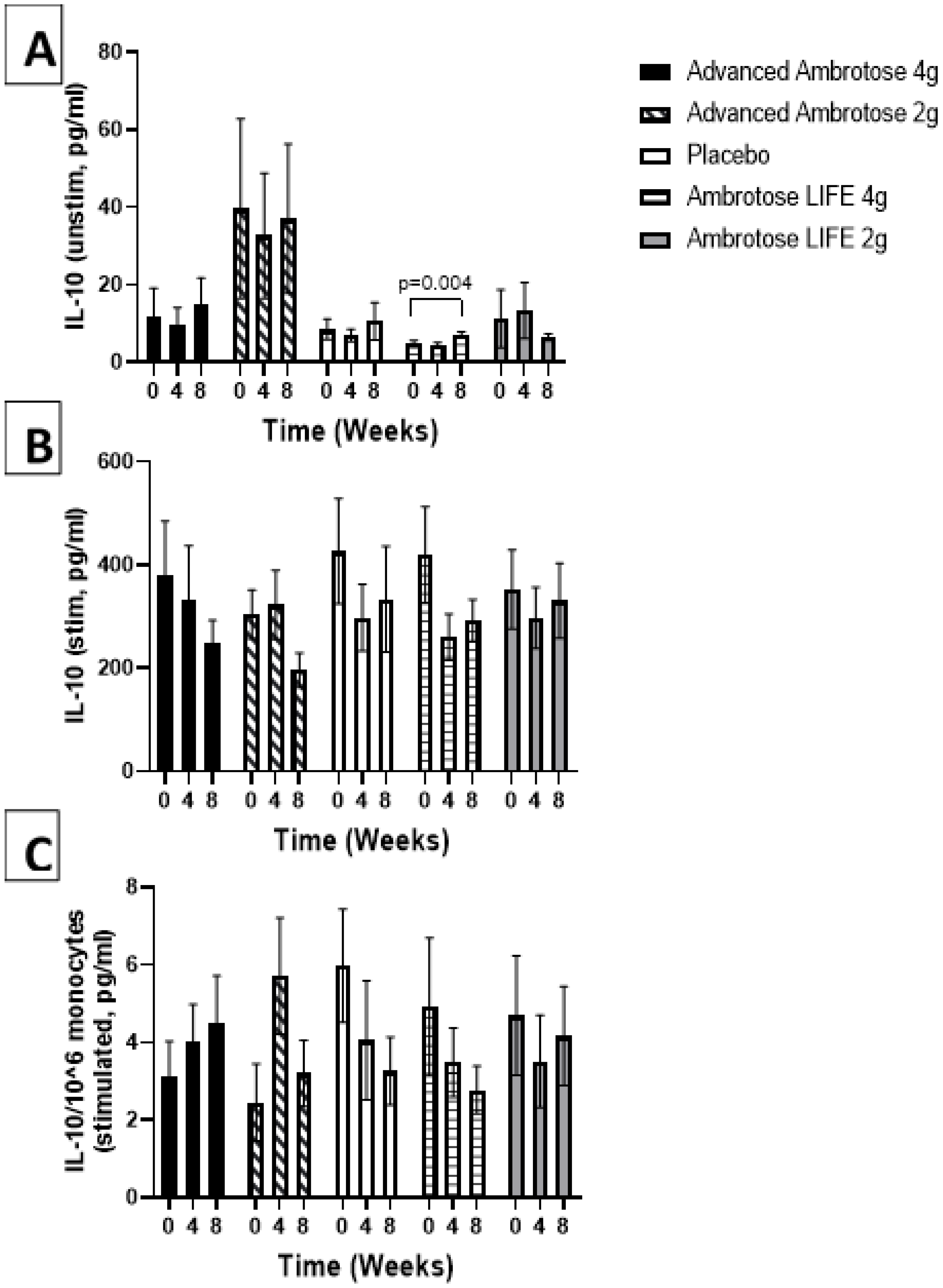
3.3. Biochemical Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, C.-S.; Chang, C.-J.; Lu, C.-C.; Martel, J.; Ojcius, D.; Ko, Y.-F.; Young, J.; Lai, H.-C. Impact of the gut microbiota, prebiotics, and probiotics on human health and disease. Biomed. J. 2014, 37, 259–268. [Google Scholar]
- Myers, S.P.; Stevenson, L.; Cheras, P.A.; O’Connor, J.; Brooks, L.; Rolfe, M.; Conellan, P.; Morris, C. A forced titration study of the antioxidant and immunomodulatory effects of Ambrotose AO supplement. BMC Complementary Altern. Med. 2010, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Best, T.; Bryan, J.; Burns, N. An investigation of the effects of saccharides on the memory performance of middle-aged adults. J. Nutr. Health Aging 2008, 12, 657–662. [Google Scholar]
- Best, T.; Howe, P.; Bryan, J.; Buckley, J.; Scholey, A. Acute effects of a dietary non-starch polysaccharide supplement on cognitive performance in healthy middle-aged adults. Nutr. Neurosci. 2015, 18, 76–86. [Google Scholar] [CrossRef]
- Best, T.; Kemps, E.; Bryan, J. Saccharide effects on cognition and well-being in middle-aged adults: A randomized controlled trial. Dev. Neuropsychol. 2009, 35, 66–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloomer, R.J.; Canale, R.E.; Blankenship, M.M.; Fisher-Wellman, K.H. Effect of Ambrotose AO® on resting and exercise-induced antioxidant capacity and oxidative stress in healthy adults. Nutr. J. 2010, 9, 49. [Google Scholar] [CrossRef] [Green Version]
- Galland, L. The gut microbiome and the brain. J Med. Food 2014, 17, 1261–1272. [Google Scholar] [CrossRef]
- Marzorati, M.; Maignien, L.; Verhelst, A.; Luta, G.; Sinnott, R.; Kerckhof, F.M.; Boon, N.; Van de Wiele, T.; Possemiers, S. Barcoded pyrosequencing analysis of the microbial community in a simulator of the human gastrointestinal tract showed a colon region-specific microbiota modulation for two plant-derived polysaccharide blends. Antonie Van Leeuwenhoek 2013, 103, 409–420. [Google Scholar] [CrossRef]
- Marzorati, M.; Verhelst, A.; Luta, G.; Sinnott, R.; Verstraete, W.; Van de Wiele, T.; Possemiers, S. In vitro modulation of the human gastrointestinal microbial community by plant-derived polysaccharide-rich dietary supplements. Int. J. Food Microbiol. 2010, 139, 168–176. [Google Scholar] [CrossRef]
- Vitetta, L.; Bambling, M.; Alford, H. The gastrointestinal tract microbiome, probiotics, and mood. Inflammopharmacology 2014, 22, 333–339. [Google Scholar] [CrossRef]
- Alavi, A.; Fraser, O.; Tarelli, E.; Bland, M.; Axford, J. An open-label dosing study to evaluate the safety and effects of a dietary plant-derived polysaccharide supplement on the N-glycosylation status of serum glycoproteins in healthy subjects. Eur. J. Clin. Nutr. 2011, 65, 648. [Google Scholar] [CrossRef]
- Alavi, A.; Goodfellow, L.; Fraser, O.; Tarelli, E.; Bland, M.; Axford, J. A double-blind, randomized, placebo-controlled study to explore the efficacy of a dietary plant-derived polysaccharide supplement in patients with rheumatoid arthritis. Rheumatol. 2011, 50, 1111–1119. [Google Scholar] [CrossRef] [Green Version]
- Turner-Bowker, D.; Hogue, S.J. Short form 12 health survey (SF-12). Encycl. Qual. Life Well-Being Res. 2014, 5954–5957. [Google Scholar]
- Grossi, E.; Groth, N.; Mosconi, P.; Cerutti, R.; Pace, F.; Compare, A.; Apolone, G. Development and validation of the short version of the Psychological General Well-Being Index (PGWB-S). Health Qual. Life Outcomes 2006, 4, 88. [Google Scholar] [CrossRef] [Green Version]
- Best, T.; Kemps, E.; Bryan, J. Perceived changes in well-being following polysaccharide intake in middle-aged adults. Appl. Res. Qual. Life 2012, 7, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Stancil, A.N.; Hicks, L.H. Glyconutrients and perception, cognition, and memory. Percept. Mot. Ski. 2009, 108, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Roca, E.; Cantó, E.; Nescolarde, L.; Perea, L.; Bayes-Genis, A.; Sibila, O.; Vidal, S. Effects of a polysaccharide-based multi-ingredient supplement on salivary immunity in non-elite marathon runners. J. Int. Soc. Sports Nutr. 2019, 16, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christ, A.; Günther, P.; Lauterbach, M.A.; Duewell, P.; Biswas, D.; Pelka, K.; Scholz, C.J.; Oosting, M.; Haendler, K.; Baßler, K. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell 2018, 172, 162–175. [Google Scholar] [CrossRef] [Green Version]
- Tak, T.; Van Groenendael, R.; Pickkers, P.; Koenderman, L. Monocyte subsets are differentially lost from the circulation during acute inflammation induced by human experimental endotoxemia. J. Innate Immun. 2017, 9, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Vozarova, B.; Weyer, C.; Lindsay, R.S.; Pratley, R.E.; Bogardus, C.; Tataranni, P.A. High white blood cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes 2002, 51, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-H.; Lee, Y.-J.; Park, B. Higher monocyte count with normal white blood cell count is positively associated with 10-year cardiovascular disease risk determined by Framingham risk score among community-dwelling Korean individuals. Medicine 2019, 98, e15340. [Google Scholar] [CrossRef] [PubMed]
- Scheinin, T.; Butler, D.M.; Salway, F.; Scallon, B.; Feldmann, M. Validation of the interleukin-10 knockout mouse model of colitis: Antitumour necrosis factor-antibodies suppress the progression of colitis. Clin. Exp. Immunol. 2003, 133, 38–43. [Google Scholar] [CrossRef]
- Andersen, V.; Ernst, A.; Christensen, J.; Østergaard, M.; Jacobsen, B.A.; Tjønneland, A.; Krarup, H.B.; Vogel, U. The polymorphism rs3024505 proximal to IL-10 is associated with risk of ulcerative colitis and Crohns disease in a Danish case-control study. Bmc Med Genet. 2010, 11, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobo-Silva, D.; Carriche, G.M.; Castro, A.G.; Roque, S.; Saraiva, M. Balancing the immune response in the brain: IL-10 and its regulation. J. Neuroinflammation 2016, 13, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, S.D.; Du, K.; Rendeiro, C.; Wang, L.; Wu, Q.; Rubakhin, S.S.; Vazhappilly, R.; Baxter, J.H.; Sweedler, J.V.; Rhodes, J.S. A unique combination of micronutrients rejuvenates cognitive performance in aged mice. Behav. Brain Res. 2017, 320, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K. T reg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232. [Google Scholar] [CrossRef]
- Gullón, B.; Gullón, P.; Tavaria, F.; Alonso, J.L.; Pintado, M. In vitro assessment of the prebiotic potential of Aloe vera mucilage and its impact on the human microbiota. Food Funct. 2015, 6, 525–531. [Google Scholar] [CrossRef]
- Quezada, M.P.; Salinas, C.; Gotteland, M.; Cardemil, L. Acemannan and Fructans from Aloe vera (Aloe barbadensis Miller) Plants as Novel Prebiotics. J. Agric. Food Chem. 2017, 65, 10029–10039. [Google Scholar] [CrossRef]
| Variable | Ambrotose Advanced 4 g (n = 15) | Ambrotose Advanced 2 g (n = 15) | Placebo (n = 15) | Ambrotose LIFE 4 g (n = 15) | Ambrotose LIFE 2 g (n = 15) | p Value |
|---|---|---|---|---|---|---|
| Age (years) | 26.9 ± 5.4 | 26.3 ± 5.7 | 29.5 ± 11.6 | 30.2 ± 11.4 | 28.1 ± 7.4 | 0.70 |
| Height (cm) | 171.3 ± 10.3 | 174.0 ± 10.6 | 171.9 ± 8.2 | 170.8 ± 9.2 | 168.8 ± 9.6 | 0.68 |
| Weight (kg) | 73.7 ± 17.1 | 73.9 ± 17.3 | 78.4 ± 15.5 | 79.1 ± 15.5 | 69.7 ± 13.1 | 0.47 |
| BMI (kg∙m−2) | 24.9 ± 4.5 | 24.2 ± 4.1 | 26.4 ± 4.3 | 27.1 ± 4.8 | 24.4 ± 4.4 | 0.30 |
| Waist Circumference (cm) | 81.3 ± 10.4 | 80.2 ± 10.0 | 86.7 ± 12.6 | 87.2 ± 13.8 | 80.2 ± 8.4 | 0.21 |
| Hip Circumference (cm) | 101.8 ± 8.6 | 100.0 ± 8.8 | 103.2 ± 12.3 | 102.9 ± 10.4 | 100.0 ± 11.0 | 0.85 |
| Waist/Hip Ratio | 0.80 ± 0.06 | 0.80 ± 0.04 | 0.84 ± 0.10 | 0.85 ± 0.08 | 0.80 ± 0.04 | 0.12 |
| Resting HR (bpm) | 68.7 ± 12.8 | 64.5 ± 12.8 | 72.0 ± 10.2 | 71.5 ± 10.6 | 66.5 ± 13.4 | 0.39 |
| Resting SBP (mm Hg) | 120.5 ± 10.4 | 122.9 ± 12.0 | 125.6 ± 10.4 | 119.9 ± 13.0 | 117.8 ± 11.2 | 0.40 |
| Resting DBP (mm Hg) | 71.6 ± 8.7 | 73.8 ± 7.7 | 78.3 ± 8.3 | 76.1 ± 10.0 | 73.2 ± 10.0 | 0.29 |
| Anaerobic Exercise (yrs) | 4.4 ± 3.8 | 4.3 ± 5.2 | 6.1 ± 6.1 | 3.4 ± 4.2 | 4.2 ± 3.5 | 0.62 |
| Anaerobic Exercise (hrs/wk) | 2.8 ± 3.1 | 1.3 ± 1.2 | 2.1 ± 2.4 | 2.2 ± 2.5 | 1.8 ± 1.7 | 0.48 |
| Aerobic Exercise (yrs) | 7.6 ± 6.1 | 8.1 ± 7.1 | 8.7 ± 10.0 | 4.8 ± 4.5 | 7.5 ± 6.2 | 0.59 |
| Aerobic Exercise (hrs/wk) | 2.4 ± 1.4 | 3.0 ± 1.7 | 3.1 ± 2.0 | 2.6 ± 1.6 | 2.7 ± 1.3 | 0.69 |
| Variable | Ambrotose Advanced 4 g (n = 15) | Ambrotose Advanced 2 g (n = 15) | Placebo (n = 15) | Ambrotose LIFE 4 g (n = 15) | Ambrotose LIFE 2 g (n = 15) |
|---|---|---|---|---|---|
| Calories | |||||
| Week 0 | 2092.1 ± 864.1 | 2058.1 ± 477.8 | 1756.2 ± 245.2 | 2122.3 ± 798.6 | 2108.9 ± 599.8 |
| Week 4 | 1836.5 ± 802.4 | 2092.3 ± 480.0 | 1687.4 ± 276.6 | 2281.8 ± 955.5 | 1994.0 ± 651.0 |
| Week 8 | 2072.0 ± 932.6 | 2066.5 ± 480.3 | 1673.7 ± 326.9 | 2191.3 ± 868.0 | 2090.8 ± 673.3 |
| Protein (g) | |||||
| Week 0 | 105.6 ± 77.1 | 88.0 ± 23.1 | 75.0 ± 26.2 | 96.6 ± 54.4 | 103.3 ± 33.2 |
| Week 4 | 94.5 ± 75.2 | 90.5 ± 29.0 | 67.8 ± 18.1 | 99.1 ± 76.3 | 88.9 ± 30.5 |
| Week 8 | 111.3 ± 79.7 | 98.6 ± 38.1 | 72.0 ± 21.7 | 108.3 ± 73.1 | 99.9 ± 37.7 |
| Carbohydrate (g) | |||||
| Week 0 | 225.9 ± 74.1 | 240.8 ± 57.6 | 191.5 ± 50.4 | 209.1 ± 102.3 | 253.6 ± 80.1 |
| Week 4 | 217.0 ± 84.5 | 245.7 ± 52.5 | 189.1 ± 40.2 | 223.8 ± 127.0 | 236.8 ± 83.3 |
| Week 8 | 216.4 ± 95.7 | 228.7 ± 44.2 | 181.6 ± 49.8 | 205.2 ± 111.4 | 254.1 ± 80.8 |
| Fiber (g) | |||||
| Week 0 | 21.4 ± 9.1 | 18.4 ± 4.5 | 15.0 ± 8.8 | 21.8 ± 12.3 | 23.9 ± 10.7 |
| Week 4 | 19.3 ± 9.0 | 18.6 ± 5.9 | 14.0 ± 5.0 | 23.0 ± 16.0 | 22.9 ± 11.0 |
| Week 8 | 19.3 ± 7.7 | 15.9 ± 4.0 | 14.7 ± 7.3 | 21.3 ± 15.3 | 22.3 ± 9.3 |
| Sugar (g) | |||||
| Week 0 | 63.9 ± 23.8 | 87.5 ± 36.2 | 65.0 ± 30.8 | 77.4 ± 53.2 | 86.3 ± 30.2 |
| Week 4 | 68.9 ± 34.3 | 84.2 ± 29.2 | 54.8 ± 22.8 | 80.2 ± 61.3 | 82.0 ± 39.5 |
| Week 8 | 68.7 ± 48.1 | 87.9 ± 23.7 | 51.0 ± 25.1 | 68.2 ± 57.5 | 89.4 ± 34.8 |
| Fat (g) | |||||
| Week 0 | 84.9 ± 37.5 | 82.8 ± 26.4 | 69.4 ± 14.5 | 84.6 ± 43.9 | 76.1 ± 29.3 |
| Week 4 | 67.9 ± 37.4 | 83.3 ± 24.6 | 69.6 ± 17.5 | 86.7 ± 44.8 | 77.7 ± 28.9 |
| Week 8 | 82.2 ± 41.8 | 84.8 ± 26.9 | 68.1 ± 18.1 | 86.2 ± 38.6 | 75.8 ± 26.3 |
| Vitamin C (mg) | |||||
| Week 0 | 59.9 ± 40.6 | 61.5 ± 42.1 | 52.3 ± 47.2 | 56.2 ± 41.7 | 104.0 ± 86.5 |
| Week 4 | 73.5 ± 43.3 | 54.3 ± 31.0 | 45.9 ± 29.0 | 68.0 ± 79.5 | 71.9 ± 56.1 |
| Week 8 | 58.3 ± 31.3 | 60.7 ± 24.6 | 57.9 ± 46.9 | 65.0 ± 52.0 | 127.8 ± 122.8 |
| Vitamin E (mg) | |||||
| Week 0 | 6.1 ± 6.7 | 3.7 ± 2.6 | 2.9 ± 2.8 | 6.7 ± 5.7 | 6.2 ± 3.4 |
| Week 4 | 5.8 ± 6.2 | 3.6 ± 2.2 | 2.5 ± 1.2 | 5.3 ± 5.7 | 5.4 ± 4.7 |
| Week 8 | 5.8 ± 5.3 | 4.6 ± 2.6 | 2.7 ± 1.7 | 10.6 ± 12.9 | 7.2 ± 4.5 |
| Vitamin A (RE) | |||||
| Week 0 | 346.8 ± 212.9 | 368.4 ± 334.6 | 248.6 ± 307.8 | 437.4 ± 422.8 | 576.7 ± 618.7 |
| Week 4 | 321.2 ± 210.8 | 386.8 ± 286.3 | 288.4 ± 252.2 | 578.3 ± 599.6 | 444.9 ± 302.5 |
| Week 8 | 463.2 ± 345.2 | 413.3 ± 256.8 | 346.4 ± 236.7 | 778.6 ± 1187.8 | 540.0 ± 395.5 |
| Variable | Ambrotose Advanced 4 g (n = 15) | Ambrotose Advanced 2 g (n = 15) | Placebo (n = 15) | Ambrotose LIFE 4 g (n = 15) | Ambrotose LIFE 2 g (n = 15) |
|---|---|---|---|---|---|
| Heart rate (bpm) | |||||
| Week 0 | 69.3 ± 13.3 | 65.7 ± 9.2 | 73.0 ± 13.9 * | 69.8 ± 9.9 | 67.3 ± 14.3 |
| Week 4 | 68.2 ± 11.4 | 66.8 ± 9.6 | 74.3 ± 13.3 | 70.9 ± 8.6 | 66.1 ± 15.9 |
| Week 8 | 72.3 ± 14.8 | 73.0 ± 9.5 | 74.7 ± 11.6 | 77.7 ± 11.1 | 69.4 ± 15.4 |
| Systolic Blood Pressure (mm Hg) | |||||
| Week 0 | 119.9 ± 15.2 | 119.7 ± 8.6 | 122.2 ± 10.8 | 118.6 ± 11.4 | 117.7 ± 15.4 |
| Week 4 | 119.9 ± 13.7 | 122.7 ± 11.2 | 121.7 ± 14.2 | 123.1 ± 13.0 | 116.3 ± 15.0 |
| Week 8 | 122.5 ± 11.5 | 118.4 ± 6.7 | 119.5 ± 9.2 | 122.3 ± 11.0 | 116.9 ± 14.2 |
| Diastolic Blood Pressure (mm Hg) | |||||
| Week 0 | 72.1 ± 6.8 | 69.5 ± 8.9 | 78.1 ± 9.3 * | 74.8 ± 9.9 * | 71.3 ± 13.8 |
| Week 4 | 72.7 ± 5.2 | 73.9 ± 8.7 | 78.5 ± 9.3 | 77.9 ± 10.3 | 72.3 ± 9.8 |
| Week 8 | 72.7 ± 6.9 | 71.9 ± 5.9 | 77.7 ± 8.1 | 79.1 ± 11.8 | 74.5 ± 11.1 |
| Values are Mean ± SD | |||||
| Variable | Ambrotose Advanced 4 g (n = 15) | Ambrotose Advanced 2 g (n = 15) | Placebo (n = 15) | Ambrotose LIFE 4 g (n = 15) | Ambrotose LIFE 2 g (n = 15) |
|---|---|---|---|---|---|
| Attentive | |||||
| Week 0 | 69.7 ± 12.7 | 70.6 ± 16.5 | 76.5 ± 16.3 | 66.2 ± 17.7 | 76.1 ± 18.6 |
| Week 4 | 67.3 ± 16.3 | 68.0 ± 10.2 | 68.3 ± 16.2 | 66.1 ± 17.1 | 67.4 ± 19.4 |
| Week 8 | 66.1 ± 18.1 | 72.2 ± 14.3 | 72.2 ± 17.6 | 69.2 ± 18.0 | 71.3 ± 19.8 |
| Tired | |||||
| Week 0 | 49.8 ± 26.7 | 35.4 ± 22.2 | 36.6 ± 26.3 | 47.1 ± 23.3 | 33.2 ± 22.2 |
| Week 4 | 47.4 ± 27.4 | 42.5 ± 19.3 | 48.3 ± 31.4 | 43.7 ± 24.9 | 34.6 ± 25.3 |
| Week 8 | 43.6 ± 29.0 | 30.0 ± 20.9 | 43.4 ± 27.0 | 34.8 ± 20.9 | 30.1 ± 21.4 |
| Alert | |||||
| Week 0 | 70.3 ± 17.2 | 67.6 ± 18.5 | 74.7 ± 16.6 | 68.1 ± 17.5 | 72.4 ± 19.8 |
| Week 4 | 58.4 ± 17.6 | 64.9 ± 10.4 | 67.5 ± 16.5 | 65.9 ± 16.9 | 65.7 ± 21.8 |
| Week 8 | 64.6 ± 14.6 | 68.1 ± 17.1 | 69.2 ± 18.3 | 68.3 ± 18.5 | 71.7 ± 17.6 |
| Groggy | |||||
| Week 0 | 32.7 ± 29.9 | 22.4 ± 24.3 | 29.4 ± 27.2 | 36.5 ± 27.6 | 21.9 ± 21.9 |
| Week 4 | 40.9 ± 26.6 | 32.4 ± 23.8 | 34.3 ± 26.0 | 27.5 ± 24.1 | 23.7 ± 24.2 |
| Week 8 | 32.4 ± 28.4 | 29.4 ± 23.6 | 27.3 ± 15.3 | 16.9 ± 16.2 | 23.9 ± 20.1 |
| Focused | |||||
| Week 0 | 66.2 ± 15.1 | 63.3 ± 18.1 | 69.7 ± 19.0 | 63.5 ± 19.7 | 72.7 ± 18.9 |
| Week 4 | 63.9 ± 16.9 | 72.7 ± 10.3 | 71.7 ± 15.3 | 68.3 ± 19.6 | 65.4 ± 19.2 |
| Week 8 | 64.4 ± 19.5 | 69.7 ± 16.1 | 68.0 ± 18.1 | 69.6 ± 17.8 | 73.9 ± 16.6 |
| Sluggish | |||||
| Week 0 | 31.2 ± 29.2 | 22.1 ± 25.9 | 24.1 ± 21.7 | 36.7 ± 27.5 | 21.3 ± 20.1 |
| Week 4 | 39.1 ± 27.9 | 29.4 ± 23.3 | 29.1 ± 22.2 | 25.0 ± 23.9 | 25.1 ± 22.5 |
| Week 8 | 33.6 ± 29.5 | 27.0 ± 24.9 | 28.2 ± 22.0 | 20.3 ± 17.2 | 27.5 ± 23.1 |
| Energetic | |||||
| Week 0 | 43.9 ± 21.2 | 56.7 ± 15.9 | 59.8 ± 21.6 | 53.6 ± 19.6 | 66.7 ± 22.4 |
| Week 4 | 53.6 ± 19.0 | 60.1 ± 19.5 | 51.1 ± 29.1 | 60.8 ± 19.3 | 67.1 ± 18.5 |
| Week 8 | 59.9 ± 15.8 | 65.3 ± 18.4 | 62.7 ± 25.2 | 65.8 ± 16.9 | 65.3 ± 17.7 |
| Lethargic | |||||
| Week 0 | 36.2 ± 27.3 | 14.2 ± 20.9 | 20.2 ± 16.1 | 31.4 ± 24.5 | 21.9 ± 20.6 |
| Week 4 | 35.6 ± 23.0 | 17.3 ± 18.5 | 27.7 ± 24.0 | 17.3 ± 15.0 | 24.9 ± 25.6 |
| Week 8 | 27.4 ± 23.7 | 26.0 ± 31.0 | 19.6 ± 17.9 | 12.7 ± 12.0 | 24.1 ± 20.8 |
| Enthusiastic | |||||
| Week 0 | 53.8 ± 24.4 | 56.7 ± 17.7 | 49.8 ± 24.9 | 58.9 ± 24.4 | 70.3 ± 20.9 |
| Week 4 | 59.6 ± 17.5 | 53.1 ± 22.9 | 52.8 ± 27.6 | 61.5 ± 25.2 | 66.7 ± 22.0 |
| Week 8 | 61.4 ± 17.8 | 69.1 ± 11.7 | 62.7 ± 22.4 | 68.8 ± 20.6 | 66.8 ± 20.0 |
| Sore | |||||
| Week 0 | 25.6 ± 29.9 | 27.9 ± 23.6 | 20.8 ± 26.3 | 24.2 ± 24.2 | 19.3 ± 22.0 |
| Week 4 | 34.4 ± 30.3 | 30.7 ± 29.9 | 21.3 ± 24.2 | 21.8 ± 26.1 | 21.7 ± 25.3 |
| Week 8 | 29.0 ± 29.3 | 29.7 ± 29.8 | 14.9 ± 21.8 | 22.1 ± 20.0 | 17.9 ± 20.7 |
| Well-Rested | |||||
| Week 0 | 49.4 ± 24.1 | 54.3 ± 21.5 | 50.5 ± 27.7 | 56.1 ± 27.9 | 55.7 ± 26.0 |
| Week 4 | 45.0 ± 19.9 | 58.3 ± 20.4 | 44.6 ± 27.2 | 58.7 ± 30.3 | 56.6 ± 26.5 |
| Week 8 | 56.3 ± 27.2 | 62.5 ± 21.5 | 58.1 ± 25.8 | 63.9 ± 22.7 | 66.9 ± 24.4 |
| Fatigued | |||||
| Week 0 | 30.9 ± 29.9 | 29.3 ± 20.5 | 33.4 ± 25.4 | 24.7 ± 24.5 | 26.1 ± 25.1 |
| Week 4 | 33.4 ± 24.7 | 22.5 ± 21.7 | 30.4 ± 27.4 | 24.3 ± 20.7 | 31.1 ± 24.0 |
| Week 8 | 33.7 ± 28.9 | 28.7 ± 20.5 | 34.0 ± 26.7 | 15.3 ± 17.8 | 24.3 ± 19.8 |
| Sickly | |||||
| Week 0 | 17.4 ± 22.4 | 17.1 ± 24.8 | 5.1 ± 9.8 | 7.7 ± 11.5 | 17.4 ± 28.8 |
| Week 4 | 11.6 ± 14.8 | 12.6 ± 14.5 | 15.5 ± 21.5 | 5.1 ± 5.3 | 12.1 ± 22.5 |
| Week 8 | 16.9 ± 22.7 | 19.5 ± 25.1 | 14.8 ± 24.7 | 15.8 ± 19.3 | 22.1 ± 28.8 |
| Memory and Cognition | |||||
| Week 0 | 61.4 ± 23.3 | 71.8 ± 15.4 | 64.1 ± 30.3 | 67.7 ± 21.5 | 71.4 ± 21.5 |
| Week 4 | 66.1 ± 16.7 | 62.9 ± 21.0 | 67.2 ± 27.9 | 69.1 ± 20.6 | 65.0 ± 19.5 |
| Week 8 | 64.6 ± 17.4 | 64.3 ± 23.7 | 71.2 ± 15.2 | 74.1 ± 14.7 | 71.1 ± 15.2 |
| Mental Stress | |||||
| Week 0 | 39.1 ± 28.2 | 33.4 ± 28.5 | 30.9 ± 30.6 | 36.3 ± 21.4 | 29.8 ± 25.3 |
| Week 4 | 33.4 ± 26.2 | 27.5 ± 22.0 | 53.7 ± 26.3 | 35.9 ± 24.5 | 42.7 ± 26.3 |
| Week 8 | 43.5 ± 28.0 | 30.7 ± 25.5 | 45.3 ± 29.2 | 35.3 ± 26.1 | 42.5 ± 27.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bloomer, R.J.; Butawan, M.; van der Merwe, M.; Keating, F.H. An Assessment of the Glyconutrient Ambrotose™ on Immunity, Gut Health, and Safety in Men and Women: A Placebo-Controlled, Double-Blind, Randomized Clinical Trial. Nutrients 2020, 12, 1751. https://doi.org/10.3390/nu12061751
Bloomer RJ, Butawan M, van der Merwe M, Keating FH. An Assessment of the Glyconutrient Ambrotose™ on Immunity, Gut Health, and Safety in Men and Women: A Placebo-Controlled, Double-Blind, Randomized Clinical Trial. Nutrients. 2020; 12(6):1751. https://doi.org/10.3390/nu12061751
Chicago/Turabian StyleBloomer, Richard J., Matthew Butawan, Marie van der Merwe, and Faith H. Keating. 2020. "An Assessment of the Glyconutrient Ambrotose™ on Immunity, Gut Health, and Safety in Men and Women: A Placebo-Controlled, Double-Blind, Randomized Clinical Trial" Nutrients 12, no. 6: 1751. https://doi.org/10.3390/nu12061751
APA StyleBloomer, R. J., Butawan, M., van der Merwe, M., & Keating, F. H. (2020). An Assessment of the Glyconutrient Ambrotose™ on Immunity, Gut Health, and Safety in Men and Women: A Placebo-Controlled, Double-Blind, Randomized Clinical Trial. Nutrients, 12(6), 1751. https://doi.org/10.3390/nu12061751





