Effect of Exclusive Enteral Nutrition and Corticosteroid Induction Therapy on the Gut Microbiota of Pediatric Patients with Inflammatory Bowel Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Blinding
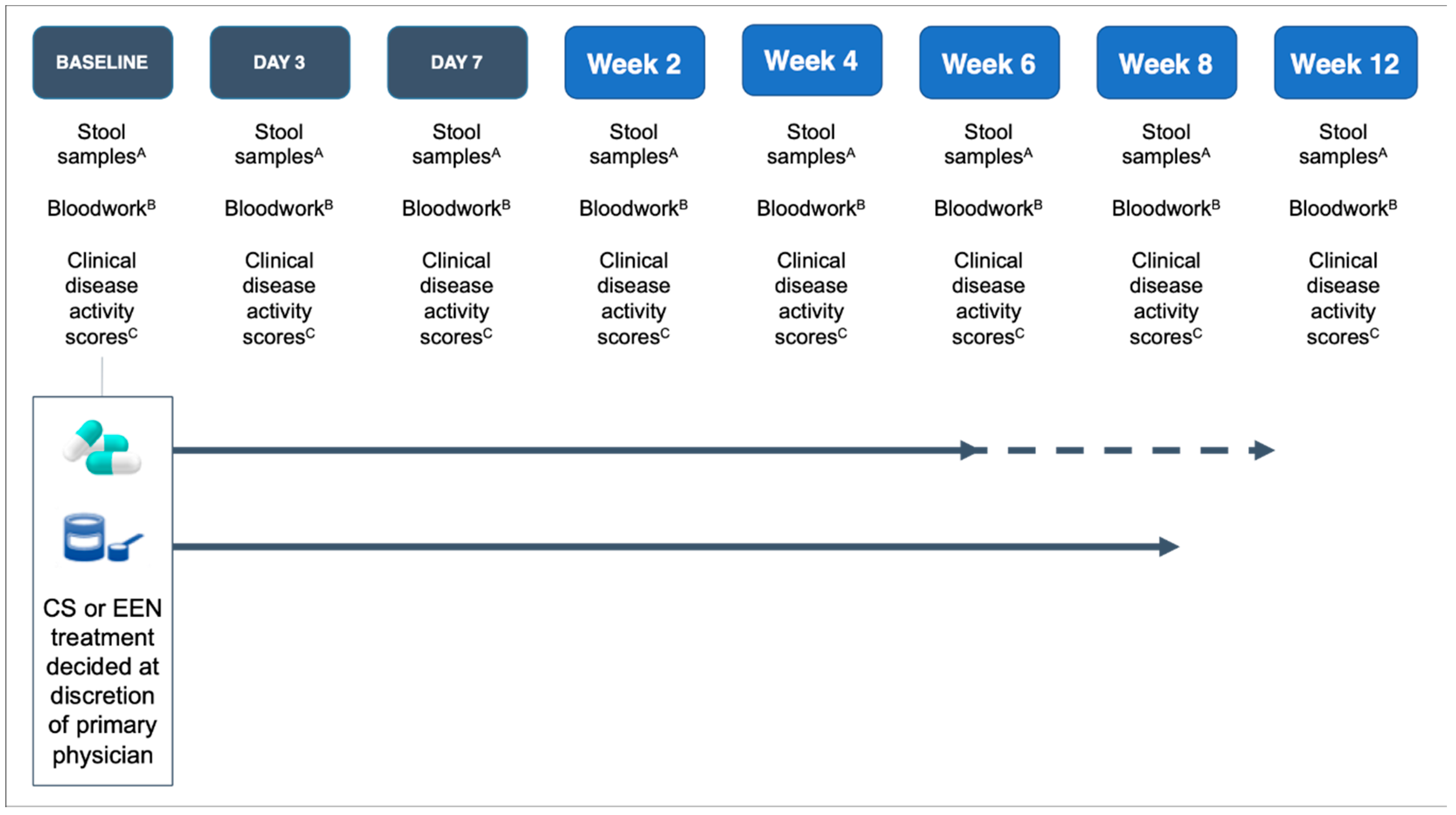
2.4. Intervention
2.5. Sample Collection
2.6. Clinical Assessment
2.7. Microbial Community Structure Analysis
2.8. Statistical Analysis
3. Results
3.1. Participants
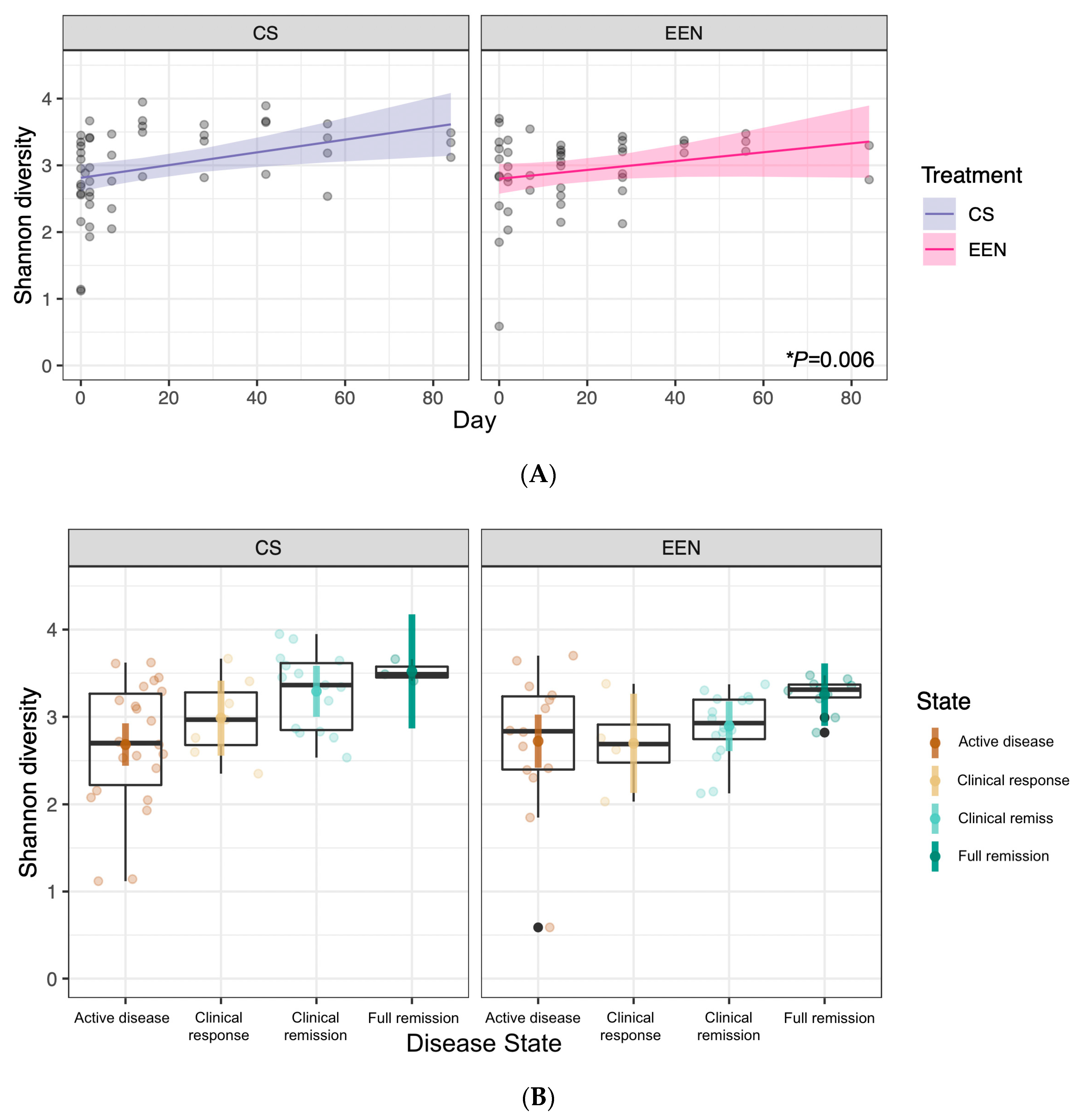
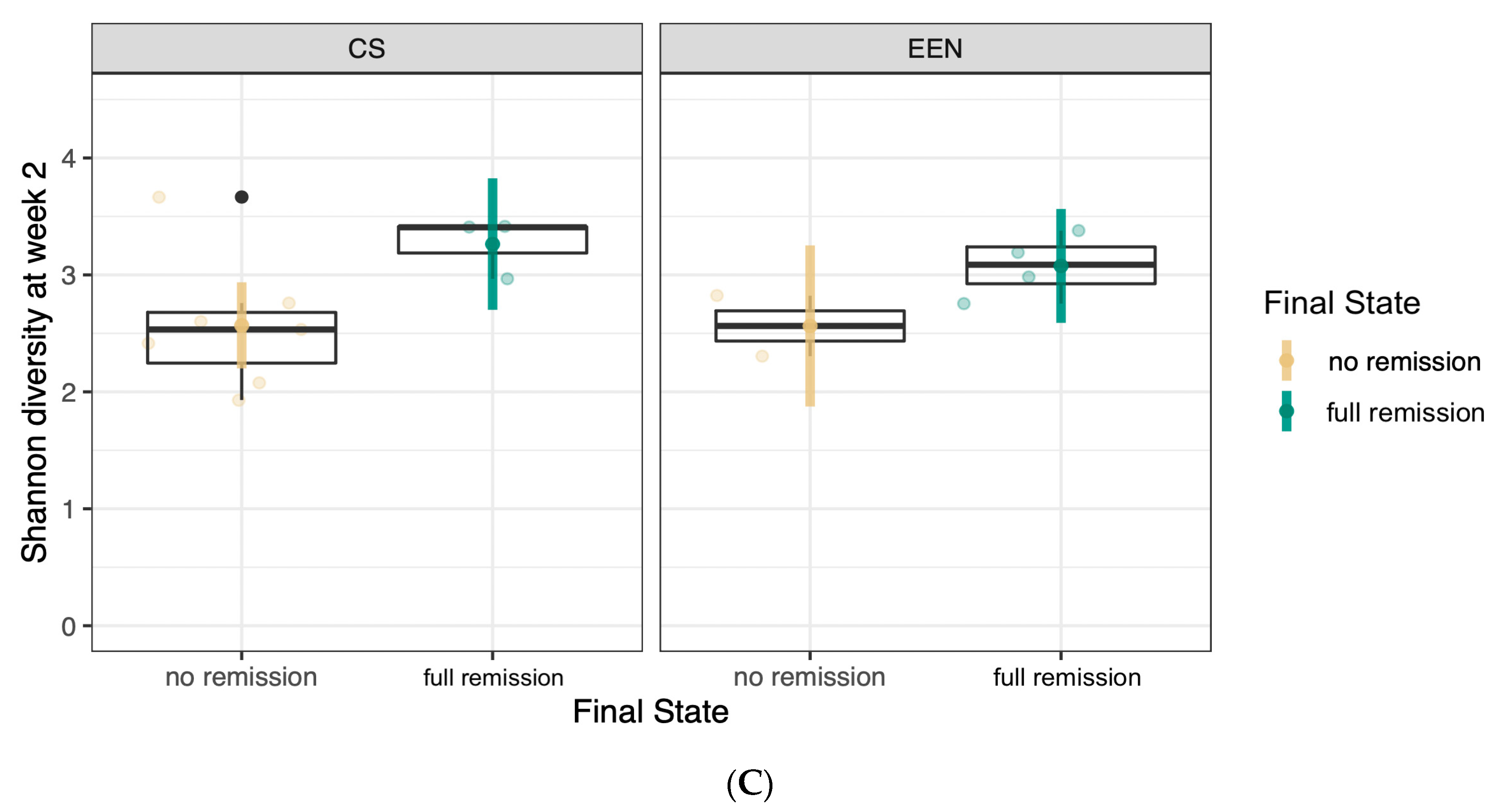
3.2. Response to EEN or CS
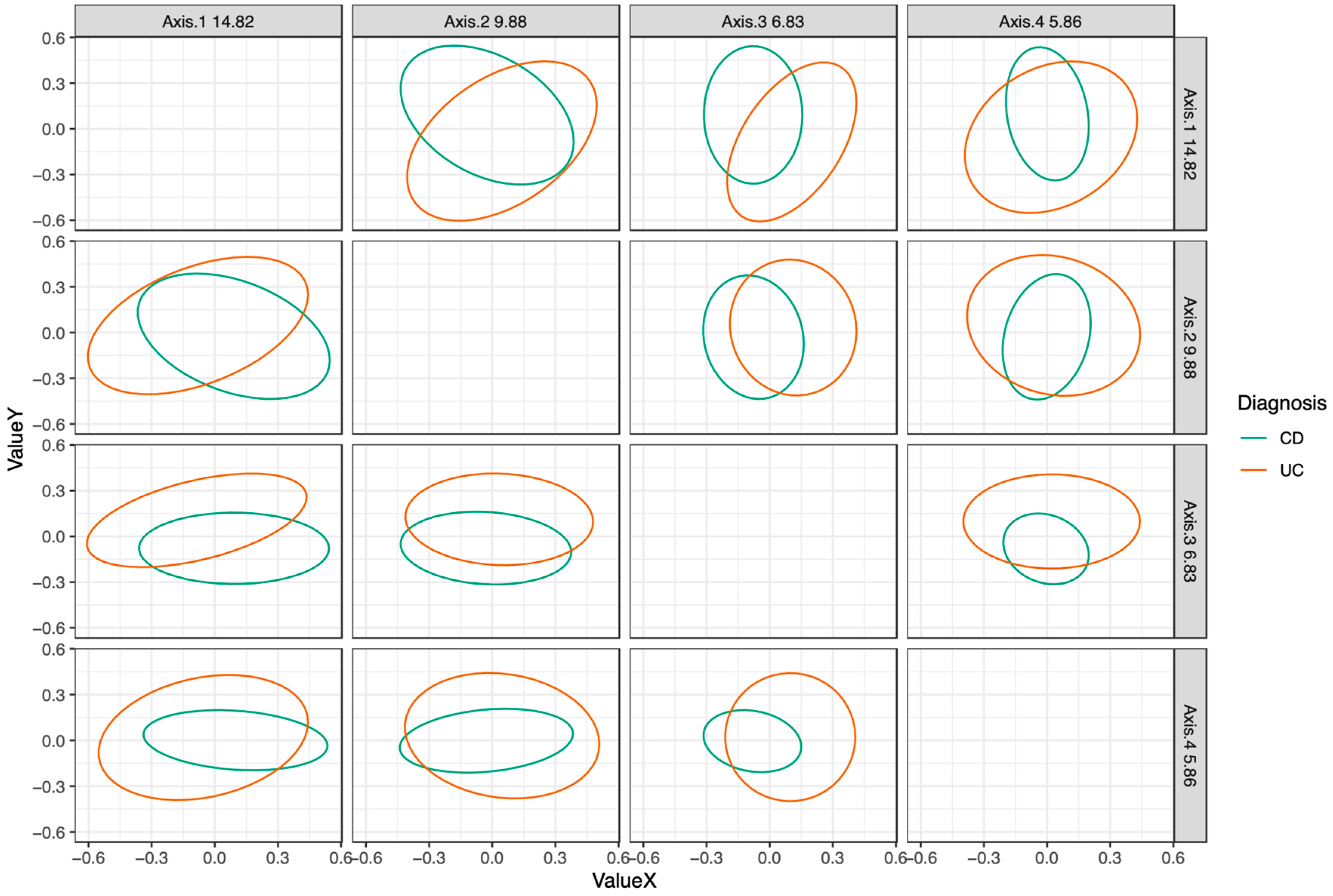
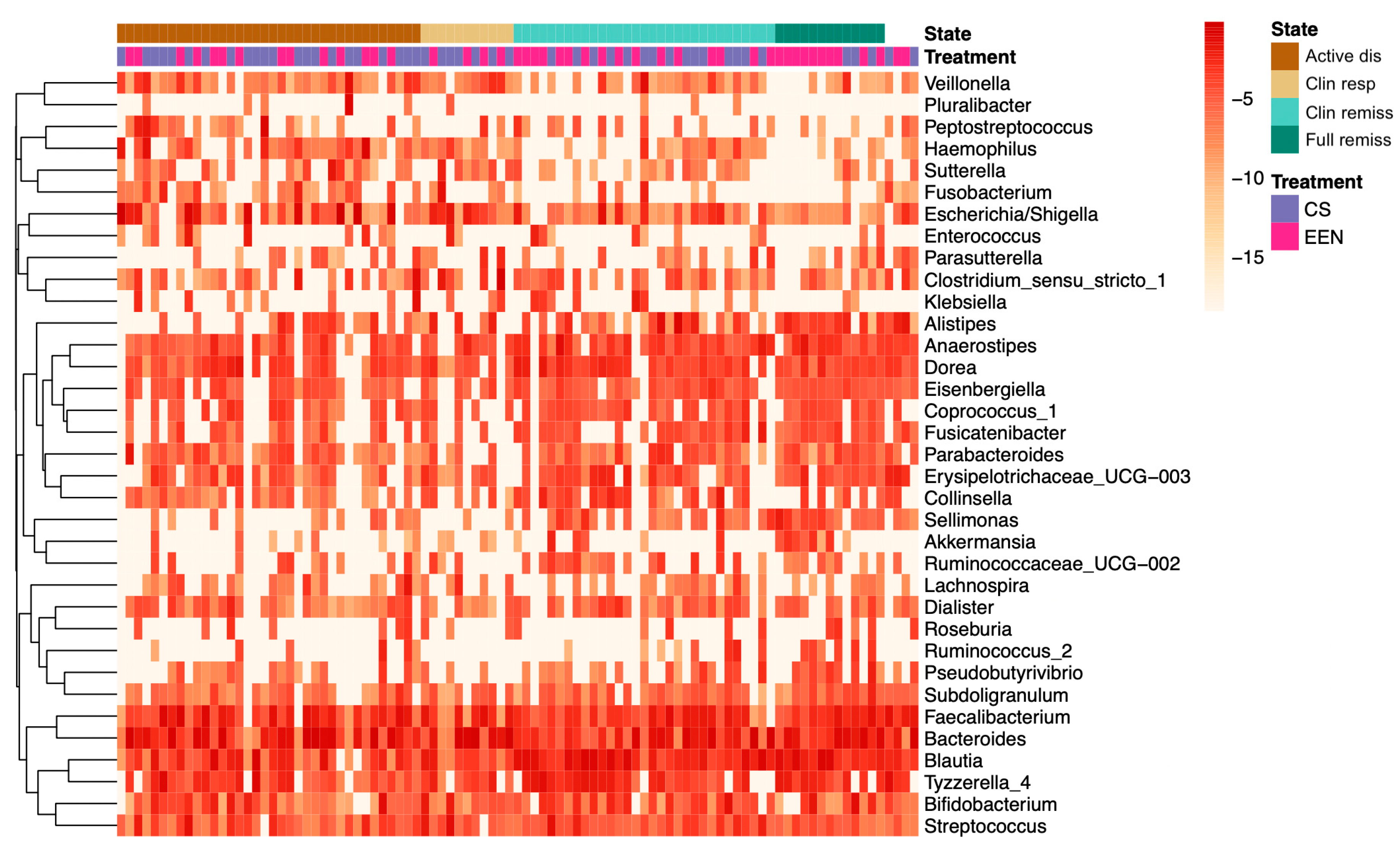
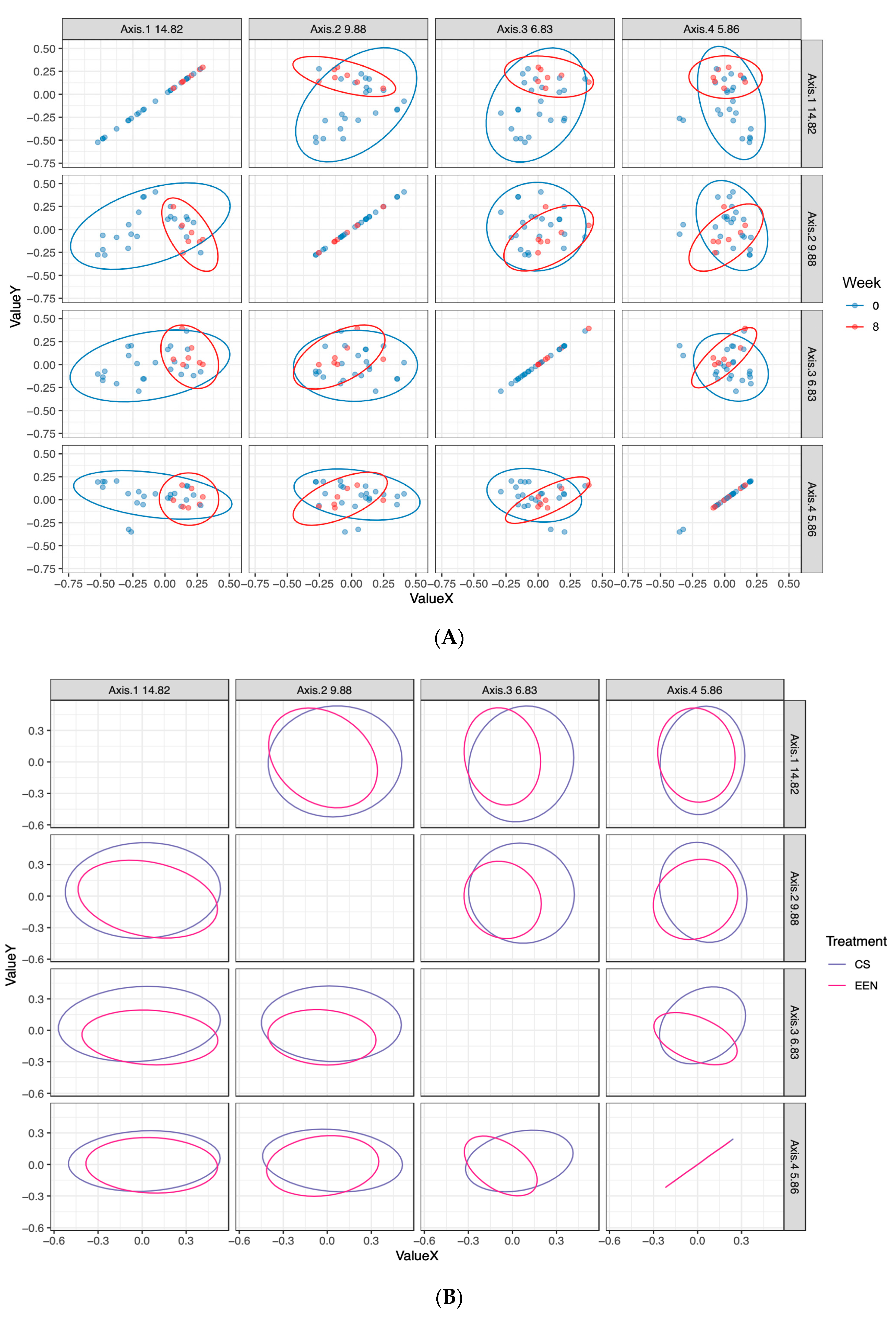
3.3. Microbiota Composition in EEN vs. CS Treated Patients
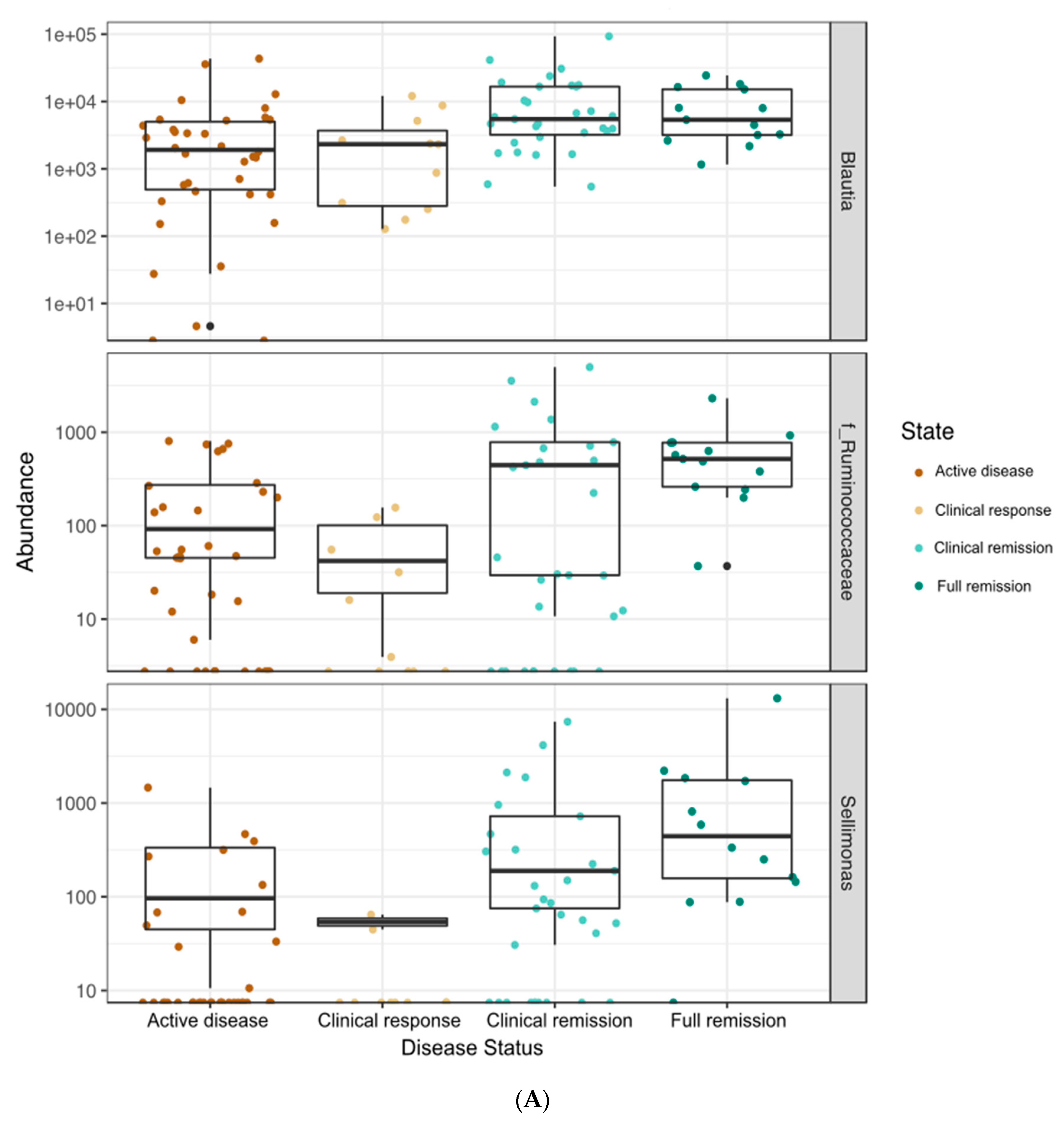
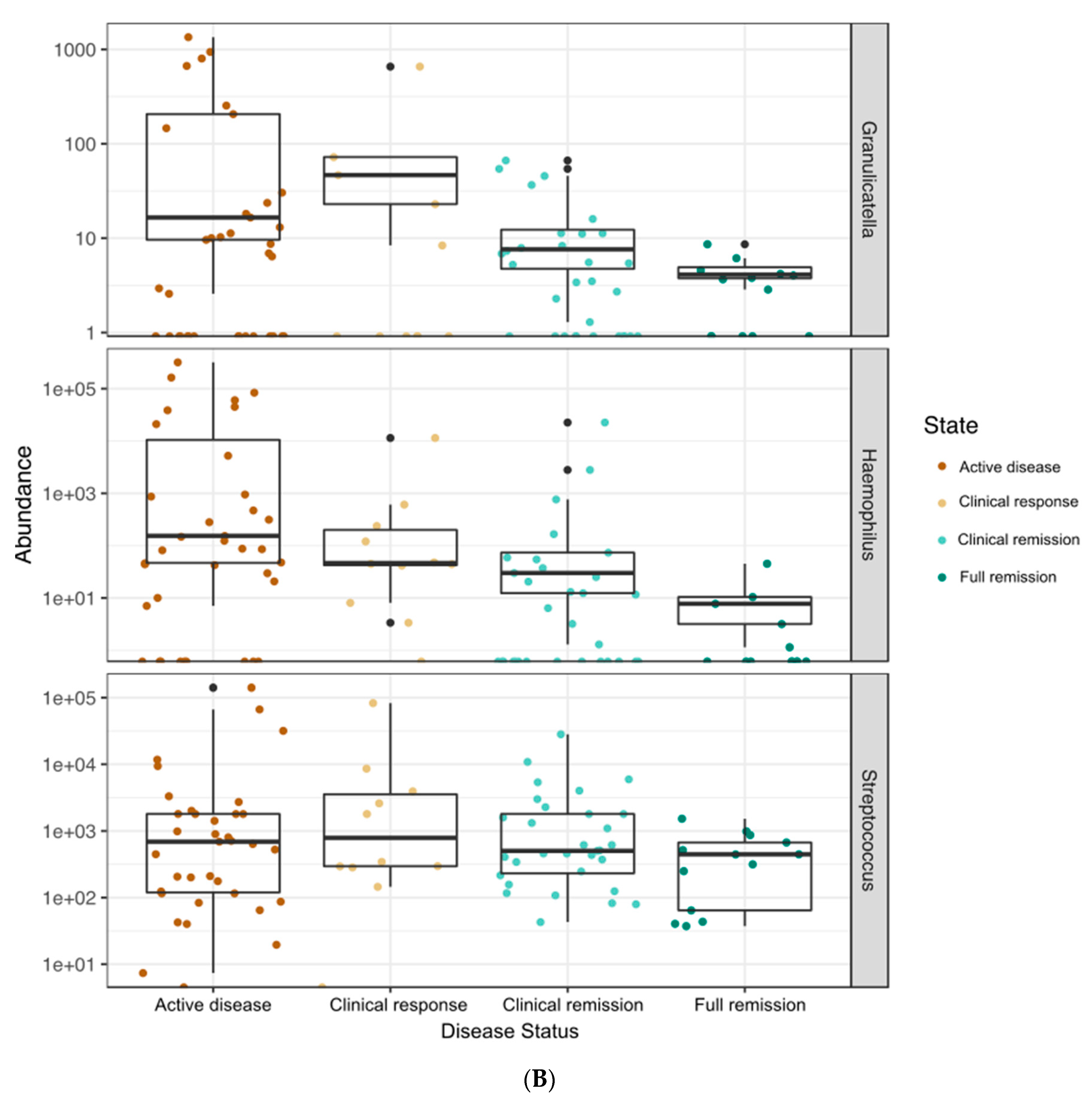
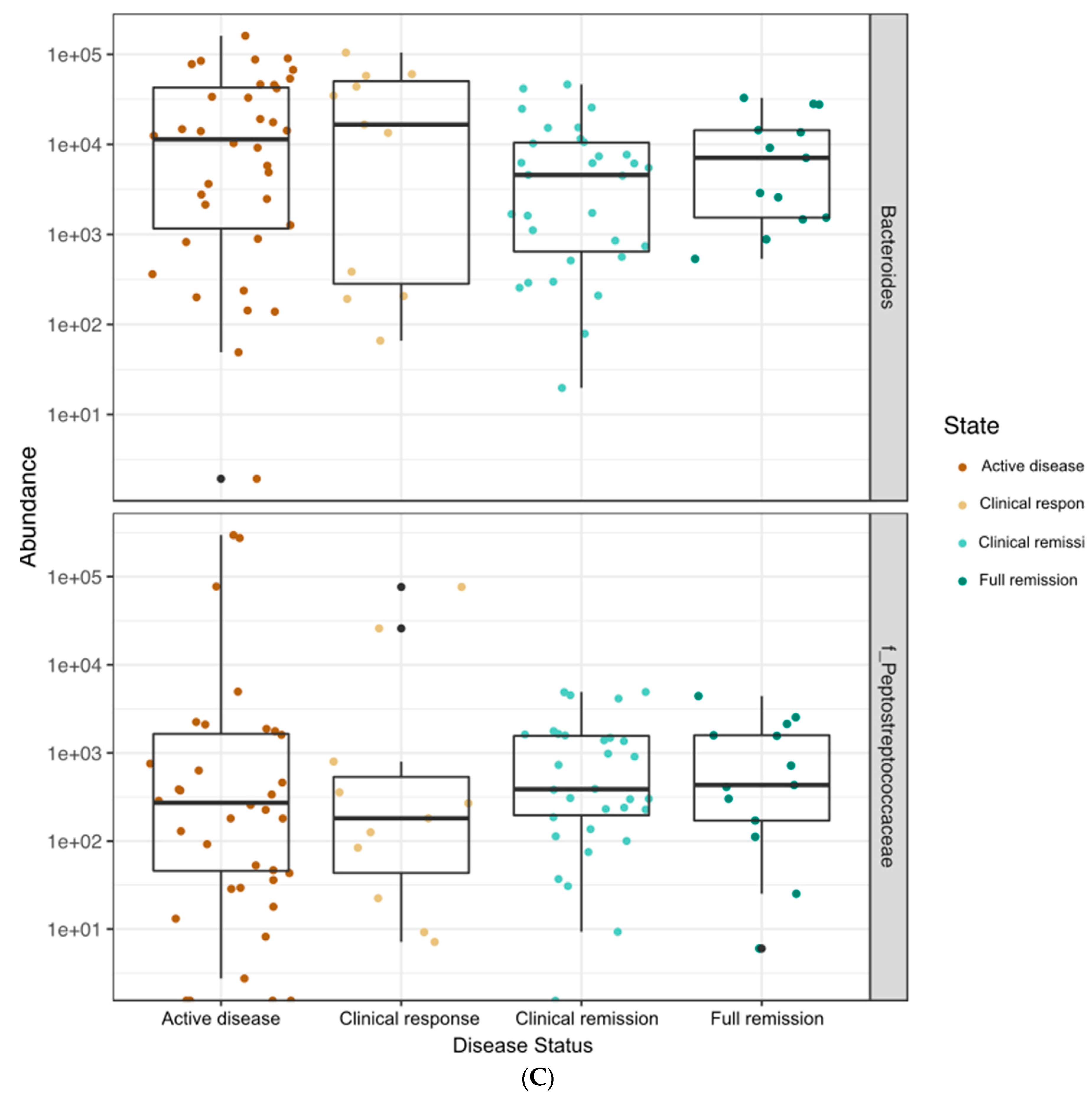
3.4. Correlations between Bacterial Genera and Clinical, Fecal, and Serum Markers of Disease Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sauer, C.G.; Kugathasan, S. Pediatric Inflammatory Bowel Disease: Highlighting Pediatric Differences in IBD. Med. Clin. N. Am. 2010, 94, 35–52. [Google Scholar] [CrossRef]
- Ruemmele, F.M.; Veres, G.; Kolho, K.L.; Griffiths, A.; Levine, A.; Escher, J.C.; Dias, A.J.; Barabino, A.; Braegger, C.P.; Bronsky, J.; et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J. Crohns. Colitis 2014, 8, 1179–1207. [Google Scholar] [CrossRef] [PubMed]
- Zachos, M.; Tondeur, M.; Griffiths, A.M. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2007, 4, CD000542. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, O.; Cordischi, L.; Cirulli, M.; Paganelli, M.; Labalestra, V.; Uccini, S.; Russo, M.P.; Cucchiara, S. Polymeric Diet Alone Versus Corticosteroids in the Treatment of Active Pediatric Crohn’s Disease: A Randomized Controlled Open-Label Trial. Clin. Gastroenterol. Hepatol. 2006, 4, 744–753. [Google Scholar] [CrossRef]
- Gerasimidis, K.; Bertz, M.; Hanske, L.; Junick, J.; Biskou, O.; Aguilera, M.; Garrick, V.; Russell, K.R.; Blaut, P.; McGrogan, P.; et al. Decline in Presumptively Protective Gut Bacterial Species and Metabolites Are Paradoxically Associated with Disease Improvement in Pediatric Crohn’s Disease During Enteral Nutrition. Inflamm. Bowel Dis. 2014, 20, 861–871. [Google Scholar] [CrossRef]
- Buchanan, E.; Gaunt, W.W.; Cardigan, T.; Garrick, V.; McGrogan, P.; Russel, R.K. The use of exclusive enteral nutrition for induction of remission in children with Crohn’s disease demonstrates that disease phenotype does not influence clinical remission. Aliment. Pharmacol. Ther. 2009, 30, 501–507. [Google Scholar] [CrossRef]
- Critch, J.; Day, A.S.; Otley, A.; King-Moore, C.; Teitelbaum, J.E.; Shashidhar, H. Use of Enteral Nutrition for the Control of Intestinal Inflammation in Pediatric Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Levine, A.; Escher, J.C.; Griffiths, A.M.; Russell, R.K.; Dignass, A.; Dias, J.A.; Bronsky, J.; Braegger, C.P.; Cucchiara, S.; et al. Management of Pediatric Ulcerative Colitis. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 340–361. [Google Scholar] [CrossRef] [PubMed]
- Leach, S.T.; Mitchell, H.M.; Eng, W.R.; Zhang, L.; Day, A.S. Sustained modulation of intestinal bacteria by exclusive enteral nutrition used to treat children with Crohn’s disease. Aliment. Pharmacol. Ther. 2008, 28, 724–733. [Google Scholar] [CrossRef]
- Chen, W.-X. Enteric microbiota leads to new therapeutic strategies for ulcerative colitis. World J. Gastroenterol. 2014, 20, 15657. [Google Scholar] [CrossRef]
- Fite, A.; Macfarlane, S.; Furrie, E.; Bahrami, B.; Cummings, H.J.; Steinke, T.D.; Macfarlane, T.G. Longitudinal Analyses of Gut Mucosal Microbiotas in Ulcerative Colitis in Relation to Patient Age and Disease Severity and Duration. J. Clin. Microbiol. 2013, 51, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Wedrychowicz, A.; Kowalska-Duplaga, K.; Jedynak-Wasowicz, U.; Pieczarkowski, S.; Sladek, M.; Tomasik, P.; Fyderek, K. Serum Concentrations of VEGF and TGF-β1 During Exclusive Enteral Nutrition in IBD. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 150–155. [Google Scholar] [CrossRef]
- Turner, D.; Ruemmele, F.M.; Orlanski-Meyer, E.; Griffiths, A.M.; de Carpi, J.M.; Bronsky, J.; Veres, G.; Aloi, M.; Strisciuglio, C.; Braegger, C.P.; et al. Management of Paediatric Ulcerative Colitis, Part 1. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 292–310. [Google Scholar] [CrossRef] [PubMed]
- Pigneur, B.; Lepage, P.; Mondot, S.; Schmitz, J.; Goulet, O.; Doré, J.; Ruemmele, F.M. Mucosal Healing and Bacterial Composition in Response to Enteral Nutrition Vs Steroid-based Induction Therapy—A Randomised Prospective Clinical Trial in Children With Crohn’s Disease. J. Crohn’s Colitis 2018, 13, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Quince, C.; Ijaz, U.Z.; Loman, N.; Eren, A.M.; Saulnier, D.; Russell, J.; Haig, S.J.; Calus, S.T.; Quick, J.; Barclay, A.; et al. Extensive Modulation of the Fecal Metagenome in Children with Crohn’s Disease During Exclusive Enteral Nutrition. Am. J. Gastroenterol. 2015, 110, 1718–1729. [Google Scholar] [CrossRef] [PubMed]
- Svolos, V.; Hansen, R.; Nichols, B.; Quince, C.; Ijaz, U.Z.; Papadopoulou, R.T.; Edwards, C.A.; Watson, D.; Alghamdi, A.; Brejnrod, A.; et al. Treatment of Active Crohn’s Disease with an Ordinary Food-based Diet That Replicates Exclusive Enteral Nutrition. Gastroenterology 2019, 156, 1354–1367. [Google Scholar] [CrossRef]
- Turner, D.; Otley, A.R.; Mack, D.; Hyams, J.; de Bruijne, J.; Uusoue, K.; Walters, T.D.; Zachos, M.; Mamula, P.; Beaton, D.E.; et al. Development, Validation, and Evaluation of a Pediatric Ulcerative Colitis Activity Index: A Prospective Multicenter Study. Gastroenterology 2007, 133, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Hyams, J.S.; Ferry, G.D.; Mandel, F.S.; Gryboski, J.D.; Kibort, P.M.; Kirschner, B.S.; Griffiths, A.M.; Katz, A.J.; Grand, R.J.; Boyle, J.T.; et al. Development and validation of a pediatric Crohn’s disease activity index. J. Pediatr. Gastroenterol. Nutr. 1991, 12, 439–447. Available online: http://www.ncbi.nlm.nih.gov/pubmed/1678008 (accessed on 1 January 2016). [CrossRef]
- Whelan, F.J.; Verschoor, C.P.; Stearns, J.C.; Rossi, L.; Luinstra, K.; Loeb, M.; Smieja, M.; Johnstone, J.; Surette, M.G.; Bowdish, D.M.E. The Loss of Topography in the Microbial Communities of the Upper Respiratory Tract in the Elderly. Ann. Am. Thorac. Soc. 2014, 11, 513–521. [Google Scholar] [CrossRef]
- Bartram, A.K.; Lynch, M.D.J.; Stearns, J.C.; Moreno-Hagelsieb, G.; Neufeld, J.D. Generation of Multimillion-Sequence 16S rRNA Gene Libraries from Complex Microbial Communities by Assembling Paired-End Illumina Reads. Appl. Environ. Microbiol. 2011, 77, 3846–3852. [Google Scholar] [CrossRef]
- Cleynen, I.; Boucher, G.; Jostins, L.; Schumm, L.P.; Zeissig, S.; Ahmad, T.; Andersen, V.; Andrews, J.M.; Annese, V.; Brand, S.; et al. Inherited determinants of Crohn’s disease and ulcerative colitis phenotypes: A genetic association study. Lancet 2016, 387, 156–167. [Google Scholar] [CrossRef]
- Aschard, H.; Laville, V.; Tchetgen, E.T.; Knights, D.; Imhann, F.; Seksik, P.; Zaitlen, N.; Silverberg, M.S.; Cosnes, J.; Weersma, R.K.; et al. Genetic effects on the commensal microbiota in inflammatory bowel disease patients. PLoS Genet. 2019, 15, e1008018. [Google Scholar] [CrossRef] [PubMed]
- Knights, D.; Lassen, K.G.; Xavier, R.J. Advances in inflammatory bowel disease pathogenesis: Linking host genetics and the microbiome. Gut 2013, 62, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Schwerd, T.; Frivolt, K.; Clavel, T.; Lagkouvardos, I.; Katona, G.; Mayr, D.; Uhlig, H.H.; Haller, D.; Koletzko, S.; Bufler, P. Exclusive enteral nutrition in active pediatric Crohn disease: Effects on intestinal microbiota and immune regulation. J. Allergy Clin. Immunol. 2016, 138, 592–596. [Google Scholar] [CrossRef] [PubMed]
- D’Argenio, V.; Precone, V.; Casaburi, G.; Miele, E.; Martinelli, M.; Staiano, A.; Salvatore, F.; Sacchetti, L. An Altered Gut Microbiome Profile in a Child Affected by Crohn’s Disease Normalized After Nutritional Therapy. Am. J. Gastroenterol. 2013, 108, 851–852. [Google Scholar] [CrossRef]
- Kaakoush, N.O.; Day, A.S.; Leach, S.T.; Lemberg, D.A.; Nielsen, S.; Mitchell, H.M. Effect of Exclusive Enteral Nutrition on the Microbiota of Children with Newly Diagnosed Crohnʼs Disease. Clin. Transl. Gastroenterol. 2015, 6, e71. [Google Scholar] [CrossRef]
- Yu, T.; Yu, Q.; Chen, X.; Zhou, L.; Wang, Y.; Yu, C. Exclusive enteral nutrition protects against inflammatory bowel disease by inhibiting NF-κB activation through regulation of the p38/MSK1 pathway. Int. J. Mol. Med. 2018, 42, 1305–1316. [Google Scholar] [CrossRef]


| Variable | Category | EEN (n = 16) | CS (n = 14) | p | TOTAL (n = 30) |
|---|---|---|---|---|---|
| Gender, n (%) | Male | 11 (68.8) | 7 (50.0) | 0.80 | 18 |
| Female | 5 (31.3) | 7 (50.0) | 12 | ||
| Age, n (%) | ≤12 years | 5 (31.3) | 7 (50.0) | 0.80 | 12 |
| ≥13 years | 11 (68.8) | 7 (50.0) | 18 | ||
| Diagnosis, n (%) | UC | 0 | 10 (71.4) | 0.94 | 10 |
| CD | 16 (100) | 4 (28.6) | 20 | ||
| Disease Activity Score, mean ± SD | PUCAI | -- | 54.5 ± 13.8 | NA | 10 |
| PCDAI | 21.9 ± 10.8 | 31.25 ± 10.3 | 0.135 | 20 | |
| Stage, n (%) | New diagnosis | 10 (62.5) | 8 (57.1) | 0.50 | 18 |
| Established disease | 6 (37.5) | 6 (37.5) | 12 | ||
| EEN Episode, n (%) | First-time EEN | 13 (81.3) | -- | -- | |
| Repeat EEN | 3 (18.8) | -- | -- | ||
| Investigations, median (IQR) | CRP (mg/L) | 21 (7.8–46.2) | 21.75 (10.3–35.8) | 0.83 | 21.75 |
| Hgb (g/dL) | 108.5 (97.5–188.8) | 103 (93.0–117.0) | 0.54 | 108.6 | |
| Alb (g/L) | 28 (25.2–32.2) | 30.5 (28.2–33.5) | 0.26 | 30 | |
| FC (mcg/g) | 3079 (1104–4305) | 3069 (1105–4307) | 0.95 | 2803 |
| Variable | Category | EEN (n = 16) | CS (n = 14) | p |
|---|---|---|---|---|
| Disease Activity Score, mean ± SD | PUCAI | -- | 17.5 ± 27.5 | -- |
| PCDAI | 2.5 ± 3.8 | 8.3 ± 10.4 | 0.18 | |
| Investigations, median (IQR) | CRP (mg/L) | 1.45 (0.3–14.8) | 0.7 (0–5.7) | 0.67 |
| Hgb (g/dL) | 119.5 (115.8–130.0) | 113 (100.3–128.0) | 0.39 | |
| Alb (g/L) | 36 (31.5–39.0) | 35.5 (33.5–40.5) | 0.78 | |
| FC (mcg/g) | 274 (104.7–1010.0) | 546 (110.6–1093.0) | 0.91 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hart, L.; Farbod, Y.; Szamosi, J.C.; Yamamoto, M.; Britz-McKibbin, P.; Halgren, C.; Zachos, M.; Pai, N. Effect of Exclusive Enteral Nutrition and Corticosteroid Induction Therapy on the Gut Microbiota of Pediatric Patients with Inflammatory Bowel Disease. Nutrients 2020, 12, 1691. https://doi.org/10.3390/nu12061691
Hart L, Farbod Y, Szamosi JC, Yamamoto M, Britz-McKibbin P, Halgren C, Zachos M, Pai N. Effect of Exclusive Enteral Nutrition and Corticosteroid Induction Therapy on the Gut Microbiota of Pediatric Patients with Inflammatory Bowel Disease. Nutrients. 2020; 12(6):1691. https://doi.org/10.3390/nu12061691
Chicago/Turabian StyleHart, Lara, Yasamin Farbod, Jake C. Szamosi, Mai Yamamoto, Philip Britz-McKibbin, Camilla Halgren, Mary Zachos, and Nikhil Pai. 2020. "Effect of Exclusive Enteral Nutrition and Corticosteroid Induction Therapy on the Gut Microbiota of Pediatric Patients with Inflammatory Bowel Disease" Nutrients 12, no. 6: 1691. https://doi.org/10.3390/nu12061691
APA StyleHart, L., Farbod, Y., Szamosi, J. C., Yamamoto, M., Britz-McKibbin, P., Halgren, C., Zachos, M., & Pai, N. (2020). Effect of Exclusive Enteral Nutrition and Corticosteroid Induction Therapy on the Gut Microbiota of Pediatric Patients with Inflammatory Bowel Disease. Nutrients, 12(6), 1691. https://doi.org/10.3390/nu12061691






