Association Between Serum Retinol and α-Tocopherol Levels and Metabolic Syndrome in Korean General Population: Analysis of Population-Based Nationally Representative Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Ethical Approval
2.3. Data Collection and Measurements
2.4. Statistical Analysis
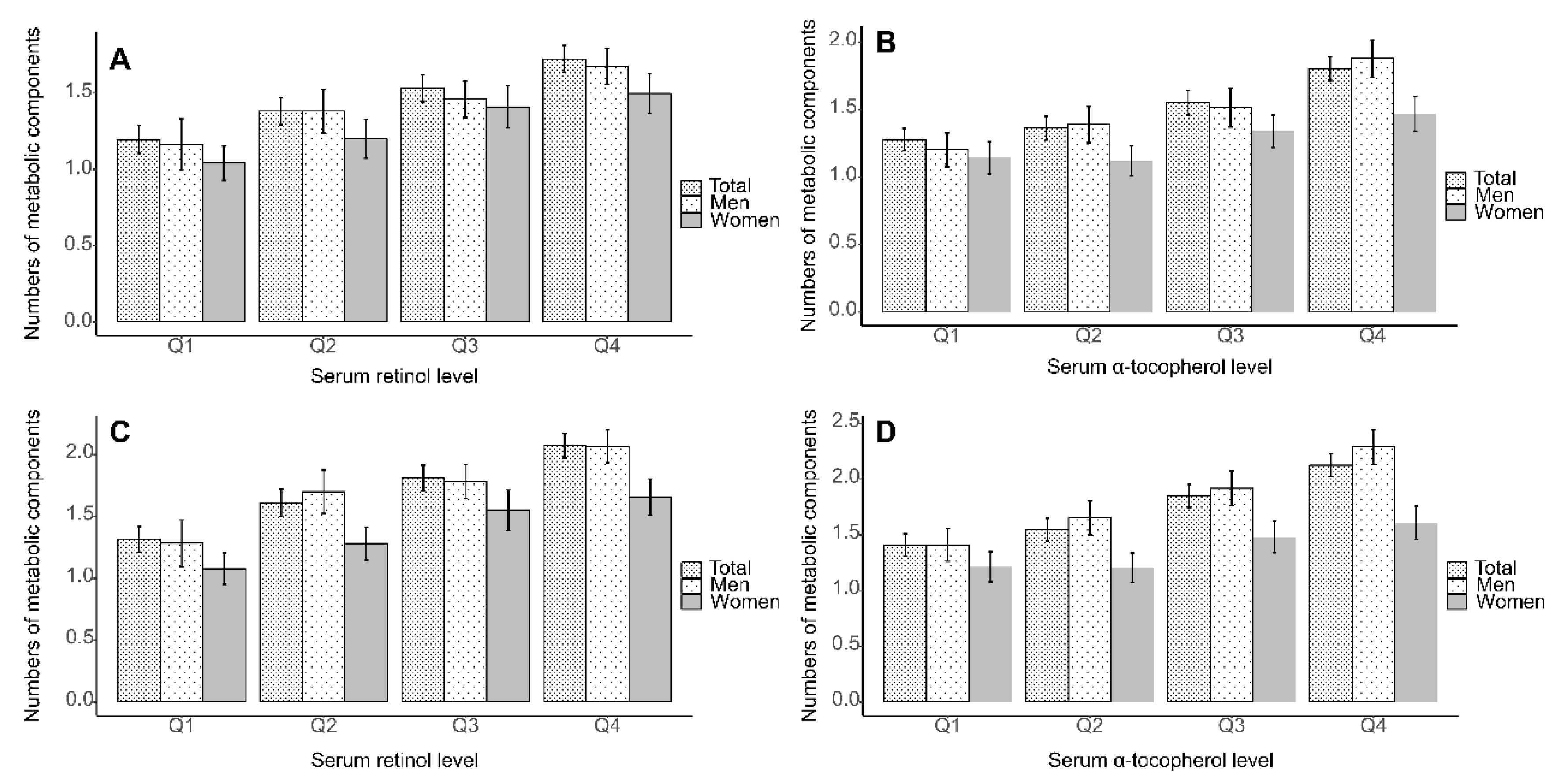
3. Results
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kaur, J. A comprehensive review on metabolic syndrome. Cardiol Res. Pract. 2014, 2014, 943162. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Chiodini, P.; Colao, A.; Lenzi, A.; Giugliano, D. Metabolic syndrome and risk of cancer: A systematic review and meta-analysis. Diabetes Care 2012, 35, 2402–2411. [Google Scholar] [CrossRef]
- Galassi, A.; Reynolds, K.; He, J. Metabolic syndrome and risk of cardiovascular disease: A meta-analysis. Am. J. Med. 2006, 119, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, C.; Okoloise, M.; Williams, K.; Stern, M.P.; Haffner, S.M. The metabolic syndrome as predictor of type 2 diabetes: The San Antonio heart study. Diabetes Care 2003, 26, 3153–3159. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, P.; Mathangasinghe, Y.; Jayawardena, R.; Hills, A.; Misra, A. Prevalence and trends of metabolic syndrome among adults in the asia-pacific region: A systematic review. BMC Public Health 2017, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Shin, H.; Song, J.H.; Kwak, S.H.; Kang, S.M.; Won Yoon, J.; Choi, S.H.; Cho, S.I.; Park, K.S.; Lee, H.K.; et al. Increasing prevalence of metabolic syndrome in Korea: The Korean National Health and Nutrition Examination Survey for 1998–2007. Diabetes Care 2011, 34, 1323–1328. [Google Scholar] [CrossRef]
- Lee, S.E.; Han, K.; Kang, Y.M.; Kim, S.O.; Cho, Y.K.; Ko, K.S.; Park, J.Y.; Lee, K.U.; Koh, E.H.; Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Trends in the prevalence of metabolic syndrome and its components in South Korea: Findings from the Korean National Health Insurance Service Database (2009–2013). PLoS ONE 2018, 13, e0194490. [Google Scholar] [CrossRef]
- Romeo, G.R.; Lee, J.; Shoelson, S.E. Metabolic syndrome, insulin resistance, and roles of inflammation–mechanisms and therapeutic targets. Arterioscler. Thromb Vasc. Biol. 2012, 32, 1771–1776. [Google Scholar] [CrossRef]
- Kuk, J.L.; Ardern, C.I. Age and sex differences in the clustering of metabolic syndrome factors: Association with mortality risk. Diabetes Care 2010, 33, 2457–2461. [Google Scholar] [CrossRef]
- Lakka, T.A.; Laaksonen, D.E. Physical activity in prevention and treatment of the metabolic syndrome. Appl. Physiol. Nutr. Metab. 2007, 32, 76–88. [Google Scholar] [CrossRef]
- Sun, K.; Liu, J.; Ning, G. Active smoking and risk of metabolic syndrome: A meta-analysis of prospective studies. PLoS ONE 2012, 7, e47791. [Google Scholar] [CrossRef] [PubMed]
- Baik, I.; Shin, C. Prospective study of alcohol consumption and metabolic syndrome. Am. J. Clin. Nutr. 2008, 87, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D. The role of vitamin A and related retinoids in immune function. Nutr. Rev. 1998, 56, S38–S48. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Graham, T.E.; Mody, N.; Preitner, F.; Peroni, O.D.; Zabolotny, J.M.; Kotani, K.; Quadro, L.; Kahn, B.B. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005, 436, 356–362. [Google Scholar] [CrossRef]
- Graham, T.E.; Yang, Q.; Blüher, M.; Hammarstedt, A.; Ciaraldi, T.P.; Henry, R.R.; Wason, C.J.; Oberbach, A.; Jansson, P.A.; Smith, U. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N. Engl. J. Med. 2006, 354, 2552–2563. [Google Scholar] [CrossRef]
- Wu, Y.; Li, H.; Loos, R.J.; Qi, Q.; Hu, F.B.; Liu, Y.; Lin, X. RBP4 variants are significantly associated with plasma RBP4 levels and hypertriglyceridemia risk in Chinese Hans. J. Lipid Res. 2009, 50, 1479–1486. [Google Scholar] [CrossRef]
- Waniek, S.; Di Giuseppe, R.; Plachta-Danielzik, S.; Ratjen, I.; Jacobs, G.; Koch, M.; Borggrefe, J.; Both, M.; Müller, H.P.; Kassubek, J. Association of vitamin E levels with metabolic syndrome, and MRI-derived body fat volumes and liver fat content. Nutrients 2017, 9, 1143. [Google Scholar] [CrossRef]
- Manning, P.J.; Sutherland, W.H.; Walker, R.J.; Williams, S.M.; De Jong, S.A.; Ryalls, A.R.; Berry, E.A. Effect of high-dose vitamin E on insulin resistance and associated parameters in overweight subjects. Diabetes Care 2004, 27, 2166–2171. [Google Scholar] [CrossRef]
- Kweon, S.; Kim, Y.; Jang, M.J.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.H.; Oh, K. Data resource profile: The Korea national health and nutrition examination survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Seo, M.H.; Lee, W.Y.; Kim, S.S.; Kang, J.H.; Kang, J.H.; Kim, K.K.; Kim, B.Y.; Kim, Y.H.; Kim, W.J.; Kim, E.M.; et al. 2018 Korean society for the study of obesity guideline for the management of obesity in Korea. J. Obese. Metab. Syndr. 2019, 28, 40. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, R.; Babb, S.; O’Halloran, A.; Asman, K.; Bishop, E.; Tynan, M.; Caraballo, R.; Pechacek, T.; Bernert, J.; Blount, B. Vital signs: Nonsmokers’ exposure to secondhand smoke-United States, 1999–2008. Morb. Mortal. Wkly. Rep. 2010, 59, 1141–1146. [Google Scholar]
- Gaziano, J.M.; Buring, J.E.; Breslow, J.L.; Goldhaber, S.Z.; Rosner, B.; VanDenburgh, M.; Willett, W.; Hennekens, C.H. Moderate alcohol intake, increased levels of high-density lipoprotein and its subfractions, and decreased risk of myocardial infarction. N. Engl. J. Med. 1993, 329, 1829–1834. [Google Scholar] [CrossRef] [PubMed]
- Chinedu, S.N.; Ogunlana, O.O.; Azuh, D.E.; Iweala, E.E.J.; Afolabi, I.S.; Uhuegbu, C.C.; Idachaba, M.E.; Osamor, V.C. Correlation between body mass index and waist circumference in nigerian adults: Implication as indicators of health status. J. Public Health Res. 2013, 2, e16. [Google Scholar] [CrossRef] [PubMed]
- Gamble, M.V.; Ramakrishnan, R.; Palafox, N.A.; Briand, K.; Berglund, L.; Blaner, W.S. Retinol binding protein as a surrogate measure for serum retinol: Studies in vitamin A-deficient children from the Republic of the Marshall Islands. Am. J. Clin. Nutr. 2001, 73, 594–601. [Google Scholar] [CrossRef]
- Majerczyk, M.; Kocelak, P.; Choreza, P.; Arabzada, H.; Owczarek, A.J.; Bozentowicz-Wikarek, M.; Brzozowska, A.; Szybalska, A.; Puzianowska-Kuznicka, M.; Grodzicki, T.; et al. Components of metabolic syndrome in relation to plasma levels of retinol binding protein 4 (RBP4) in a cohort of people aged 65 years and older. J. Endocrinol. Investig. 2018, 41, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Wessel, H.; Saeed, A.; Heegsma, J.; Connelly, M.A.; Faber, K.N.; Dullaart, R.P.F. Plasma Levels of Retinol Binding Protein 4 Relate to Large VLDL and Small LDL Particles in Subjects with and without Type 2 Diabetes. J. Clin. Med. 2019, 8, 1792. [Google Scholar] [CrossRef] [PubMed]
- Wallström, P.; Wirfält, E.; Lahmann, P.H.; Gullberg, B.; Janzon, L.; Berglund, G. Serum concentrations of β-carotene and α-tocopherol are associated with diet, smoking, and general and central adiposity. Am. J. Clin. Nutr. 2001, 73, 777–785. [Google Scholar] [CrossRef]
- Park, S.; Ham, J.O.; Lee, B.K. Effects of total vitamin A, vitamin C, and fruit intake on risk for metabolic syndrome in Korean women and men. Nutrition 2015, 31, 111–118. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, H.S.; Park, H.J.; Kim, W.Y. Risk factors associated with metabolic syndrome in Korean elderly. Ann. Nutr. Metab. 2007, 51, 533–540. [Google Scholar] [CrossRef]
- Ahn, S.; Jun, S.; Shin, J.; Ham, D.; Choi, E.; Joung, H. Association Between Intake of Antioxidant Vitamins and Metabolic Syndrome Prevalence Among Korean Adults (P24-001-19). Curr. Dev. Nutr. 2019, 3, nzz044.P024-001-19. [Google Scholar] [CrossRef]
- Cho, S.W.; Paek, Y.M.; Kang, J.Y.; Park, Y.K.; Choi, T.I. The relationship between plasma antioxidant levels and metabolic syndrome risk factors in male workers. Korean J. Food Nutr. 2009, 22, 357–366. [Google Scholar]
- Palmer, A.C.; West, K.P., Jr.; Dalmiya, N.; Schultink, W. The use and interpretation of serum retinol distributions in evaluating the public health impact of vitamin A programmes. Public Health Nutr. 2012, 15, 1201–1215. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.; Moser, U.; Pilz, S.; Eggersdorfer, M.; Weber, P. The challenge of setting appropriate intake recommendations for vitamin E: Considerations on status and functionality to define nutrient requirements. Int. J. Vitam. Nutr. Res. 2013, 83, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, Y.N.; Cho, Y.O. Vitamin A status of 20- to 59-year-old adults living in Seoul and the metropolitan area, Korea. Nutr. Res. Pract. 2012, 6, 45–50. [Google Scholar] [CrossRef][Green Version]
- Tangney, C.C.; Bienias, J.L.; Evans, D.A.; Morris, M.C. Reasonable estimates of serum vitamin E, vitamin C, and beta-cryptoxanthin are obtained with a food frequency questionnaire in older black and white adults. J. Nutr. 2004, 134, 927–934. [Google Scholar] [CrossRef]
- Tangney, C.C.; Shekelle, R.B.; Raynor, W.; Gale, M.; Betz, E.P. Intra- and interindividual variation in measurements of beta-carotene, retinol, and tocopherols in diet and plasma. Am. J. Clin. Nutr. 1987, 45, 764–769. [Google Scholar] [CrossRef]
- Dal-Pizzol, F.; Klamt, F.; Benfato, M.S.; Bernard, E.A.; Moreira, J.C.F. Retinol supplementation induces oxidative stress and modulates antioxidant enzyme activities in rat Sertoli cells. Free Radic. Res. 2001, 34, 395–404. [Google Scholar] [CrossRef]
- Wakil, S.J.; Abu-Elheiga, L.A. Fatty acid metabolism: Target for metabolic syndrome. J. Lipid Res. 2009, 50, S138–S143. [Google Scholar] [CrossRef]
- Yanagitani, A.; Yamada, S.; Yasui, S.; Shimomura, T.; Murai, R.; Murawaki, Y.; Hashiguchi, K.; Kanbe, T.; Saeki, T.; Ichiba, M. Retinoic acid receptor α dominant negative form causes steatohepatitis and liver tumors in transgenic mice. Hepatology 2004, 40, 366–375. [Google Scholar] [CrossRef]
- Amengual, J.; Petrov, P.; Bonet, M.L.; Ribot, J.; Palou, A. Induction of carnitine palmitoyl transferase 1 and fatty acid oxidation by retinoic acid in HepG2 cells. Int. J. Biochem. Cell Biol. 2012, 44, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Mah, E.; Leonard, S.W.; Bobe, G.; Bruno, R.S. Metabolic syndrome increases dietary α-tocopherol requirements as assessed using urinary and plasma vitamin E catabolites: A double-blind, crossover clinical trial. Am. J. Clin. Nutr. 2017, 105, 571–579. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 5885) | Men (n = 2672) | Women (n = 3213) | |
|---|---|---|---|
| Age, years | 47.1 (0.3) | 46.2 (0.4) | 48.1 (0.4) |
| Residence | |||
| Rural | 87.1 (1.3) | 87.1 (1.4) | 87.1 (1.4) |
| Urban | 12.9 (1.3) | 12.9 (1.4) | 12.9 (1,4) |
| Household income | |||
| lowest | 13.1 (0.6) | 11.9 (0.8) | 14.4 (0.8) |
| lower middle | 23.5 (0.8) | 22.3 (1.0) | 24.7 (1.0) |
| higher middle | 29.9 (0.8) | 29.9 (1.1) | 29.9 (1.0) |
| highest | 33.5 (1.1) | 35.9 (1.4) | 31.0 (1.3) |
| Educational level | |||
| Middle school or lower | 19.8 (0.7) | 14.2 (0.8) | 25.5 (1.0) |
| High school | 26.6 (0.8) | 26.1 (1.2) | 27.0 (1.0) |
| College or more | 53.6 (1.0) | 59.6 (1.3) | 47.5 (1.1) |
| High risk alcohol consumption | |||
| Non-consumer | 22.4 (0.7) | 14.3 (0.9) | 30.6 (1.0) |
| <1/week | 55.9 (0.9) | 52.3 (1.3) | 59.5 (1.1) |
| ≥1/week | 21.7 (0.7) | 33.4 (1.2) | 9.8 (0.7) |
| Smoking status | |||
| Never | 60.7 (0.8) | 31.6 (1.2) | 90.2 (0.7) |
| Former | 19.7 (0.7) | 34.4 (1.2) | 4.9 (0.5) |
| Current | 19.5 (0.7) | 34.0 (1.2) | 4.9 (0.5) |
| Physical activity§ | |||
| Yes | 52.1 (0.9) | 48.6 (1.3) | 55.6 (1.1) |
| No | 47.9 (0.9) | 51.4 (1.3) | 44.4 (1.1) |
| Body mass index, kg/m2 | |||
| <18.5 | 3.5 (0.3) | 2.5 (0.4) | 4.6 (0.5) |
| 18.5–22.9 | 39.0 (0.8) | 30.1 (1.2) | 48.1 (1.2) |
| 23–24.9 | 22.2 (0.7) | 25.5 (1.1) | 18.9 (0.8) |
| ≥25 | 35.2 (0.8) | 42.0 (1.2) | 28.3 (0.8) |
| Hs-CRP, mg/dL | |||
| Mean (SE) | 1.13 (0.0) | 1.19 (0.0) | 1.07 (0.0) |
| Median (IQR) | 0.56 (0.35–1.10) | 0.62 (0.40–1.20) | 0.50 (0.31–1.00) |
| Metabolic variables | |||
| WC, cm | 82.3 (0.2) | 86.2 (0.2) | 78.4 (0.2) |
| Triglyceride, mg/dL | 137.4 (2.0) | 159.6 (3.4) | 114.8 (0.4) |
| Fasting glucose, mg/dL | 100.1 (0.4) | 102.5 (0.6) | 97.8 (0.5) |
| HDL-cholesterol, mg/dL | 51.1 (0.2) | 47.4 (0.3) | 54.8 (0.3) |
| SBP, mmHg | 117.6 (0.3) | 119.9 (0.4) | 115.1 (0.4) |
| DBP, mmHg | 75.9 (0.2) | 78.3 (0.3) | 73.4 (0.2) |
| Retinol, mg/L | |||
| Median (IQR) | 0.49 (0.39–0.61) | 0.55 (0.45–0.68) | 0.44 (0.36–0.55) |
| α-tocopherol, mg/L | |||
| Median (IQR) | 12.58 (10.36–15.67) | 12.38 (10.07–15.42) | 12.76 (10.51–15.84) |
| Prevalence % (SE) | Unadjusted | Adjusted for Age and Sex | Multivariate-Adjusted | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BMI Unadjusted | Adjusted for BMI | ||||||||
| OR (95% CI) | Ptrend | OR (95% CI) | Ptrend | OR (95% CI) | Ptrend | OR (95% CI) | Ptrend | ||
| Retinol | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| Q1 | 12.1 (1.1) | 1 | 1 | 1 | 1 | ||||
| Q2 | 20.9 (1.4) | 1.912 (1.468–2.491) | 1.672 (1.280–2.184) | 1.802 (1.368–2.374) | 1.703 (1.254–2.313) | ||||
| Q3 | 25.9 (1.5) | 2.526 (1.962–3.253) | 2.032 (1.561–2.645) | 2.243 (1.717–2.931) | 2.027 (1.526–2.691) | ||||
| Q4 | 33.5 (1.5) | 3.649 (2.868–4.642) | 2.651 (2.054–3.423) | 2.795 (2.129–3.670) | 2.351 (1.748–3.163) | ||||
| α-tocopherol | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| Q1 | 14.5 (1.3) | 1 | 1 | 1 | 1 | ||||
| Q2 | 18.6 (1.2) | 1.338 (1.039–1.723) | 1.260 (0.969–1.639) | 1.280 (0.977–1.678) | 1.302 (0.973–1.743) | ||||
| Q3 | 26.5 (1.5) | 2.113 (1.650–2.706) | 1.848 (1.436–2.377) | 1.910 (1.479–2.466) | 1.713 (1.303–2.252) | ||||
| Q4 | 34.9 (1.6) | 3.154 (2.503–3.974) | 2.643 (2.074–3.368) | 2.690 (2.098–3.449) | 2.559 (1.953–3.353) | ||||
| Retinol | α-Tocopherol | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prev. % (SE) | Multivariate-Adjusted | Prev. % (SE) | Multivariate-Adjusted | |||||||
| BMI Unadjusted | Adjusted for BMI | BMI Unadjusted | Adjusted for BMI | |||||||
| OR (95% CI) | Ptrend | OR (95% CI) | Ptrend | OR (95% CI) | Ptrend | OR (95% CI) | Ptrend | |||
| Abdominal obesity | <0.001 | 0.545 | 0.001 | 0.547 | ||||||
| Q1 | 19.9 (1.4) | 1 | 1 | 24.1 (1.6) | 1 | 1 | ||||
| Q2 | 26.3 (1.5) | 1.376 (1.085–1.746) | 1.111 (0.799–1.545) | 25.3 (1.5) | 1.048 (0.821–1.339) | 0.993 (0.711–1.386) | ||||
| Q3 | 29.5 (1.7) | 1.550 (1.223–1.963) | 1.161 (0.842–1.603) | 29.5 (1.5) | 1.253 (0.998–1.573) | 0.822 (0.600–1.125) | ||||
| Q4 | 34.2 (1.5) | 1.759 (1.386–2.233) | 1.125 (0.806–1.570) | 32.8 (1.5) | 1.384 (1.108–1.730) | 0.952 (0.701–1.291) | ||||
| High triglycerides | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| Q1 | 10.8 (1.1) | 1 | 1 | 13.4 (1.2) | 1 | 1 | ||||
| Q2 | 23.4 (1.6) | 2.290 (1.718–3.052) | 2.149 (1.604–2.878) | 21.0 (1.5) | 1.948 (1.487–2.551) | 1.897 (1.444–2.492) | ||||
| Q3 | 31.7 (1.6) | 3.276 (2.495–4.302) | 2.980 (2.260–3.929) | 33.0 (1.6) | 3.941 (3.033–5.122) | 3.622 (2.780–4.717) | ||||
| Q4 | 47.3 (1.6) | 5.376 (4.054–7.131) | 4.718 (3.538–6.293) | 49.3 (1.6) | 8.149 (6.225–10.668) | 7.633 (5.841–9.975) | ||||
| Low HDL-cholesterol | 0.494 | 0.465 | 0.896 | 0.213 | ||||||
| Q1 | 31.7 (1.6) | 1 | 1 | 31.3 (1.7) | 1 | 1 | ||||
| Q2 | 32.2 (1.7) | 1.074 (0.867–1.330) | 1.010 (0.811–1.258) | 28.2 (1.5) | 0.782 (0.629–0.972) | 0.748 (0.596–0.938) | ||||
| Q3 | 34.0 (1.5) | 1.232 (0.990–1.533) | 1.102 (0.878–1.383) | 31.7 (1.6) | 0.841 (0.683–1.037) | 0.762 (0.614–0.944) | ||||
| Q4 | 28.8 (1.5) | 1.053 (0.846–1.310) | 0.898 (0.715–1.126) | 34.5 (1.6) | 0.935 (0.755–1.158) | 0.832 (0.668–1.037) | ||||
| High fasting glucose | <0.001 | <0.001 | <0.001 | 0.006 | ||||||
| Q1 | 19.5 (1.3) | 1 | 1 | 26.0 (1.6) | 1 | 1 | ||||
| Q2 | 28.7 (1.5) | 1.438 (1.143–1.809) | 1.350 (1.067–1.708) | 29.0 (1.4) | 1.064 (0.838–1.352) | 1.031 (0.805–1.320) | ||||
| Q3 | 35.2 (1.7) | 1.755 (1.374–2.240) | 1.569 (1.221–2.016) | 35.8 (1.7) | 1.312 (1.028–1.673) | 1.168 (0.911–1.497) | ||||
| Q4 | 45.0 (1.7) | 2.233 (1.758–2.836) | 1.906 (1.489–2.439) | 41.2 (1.6) | 1.521 (1.198–1.930) | 1.353 (1.061–1.726) | ||||
| High blood pressure | <0.001 | 0.007 | <0.001 | <0.001 | ||||||
| Q1 | 18.4 (1.4) | 1 | 1 | 21.9 (1.5) | 1 | 1 | ||||
| Q2 | 25.8 (1.4) | 1.227 (0.952–1.583) | 1.162 (0.895–1.508) | 25.9 (1.4) | 1.149 (0.901–1.466) | 1.137 (0.892–1.450) | ||||
| Q3 | 30.8 (1.6) | 1.381 (1.067–1.787) | 1.272 (0.977–1.655) | 32.9 (1.6) | 1.468 (1.156–1.863) | 1.368 (1.076–1.739) | ||||
| Q4 | 39.5 (1.6) | 1.594 (1.233–2.061) | 1.420 (1.092–1.847) | 36.6 (.1.6) | 1.568 (1.244–1.976) | 1.460 (1.154–1.846) | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.; Kang, J. Association Between Serum Retinol and α-Tocopherol Levels and Metabolic Syndrome in Korean General Population: Analysis of Population-Based Nationally Representative Data. Nutrients 2020, 12, 1689. https://doi.org/10.3390/nu12061689
Kim T, Kang J. Association Between Serum Retinol and α-Tocopherol Levels and Metabolic Syndrome in Korean General Population: Analysis of Population-Based Nationally Representative Data. Nutrients. 2020; 12(6):1689. https://doi.org/10.3390/nu12061689
Chicago/Turabian StyleKim, Taeyun, and Jihun Kang. 2020. "Association Between Serum Retinol and α-Tocopherol Levels and Metabolic Syndrome in Korean General Population: Analysis of Population-Based Nationally Representative Data" Nutrients 12, no. 6: 1689. https://doi.org/10.3390/nu12061689
APA StyleKim, T., & Kang, J. (2020). Association Between Serum Retinol and α-Tocopherol Levels and Metabolic Syndrome in Korean General Population: Analysis of Population-Based Nationally Representative Data. Nutrients, 12(6), 1689. https://doi.org/10.3390/nu12061689





