Risk of Iodine Deficiency in Extremely Low Gestational Age Newborns on Parenteral Nutrition
Abstract
1. Introduction
2. Objectives
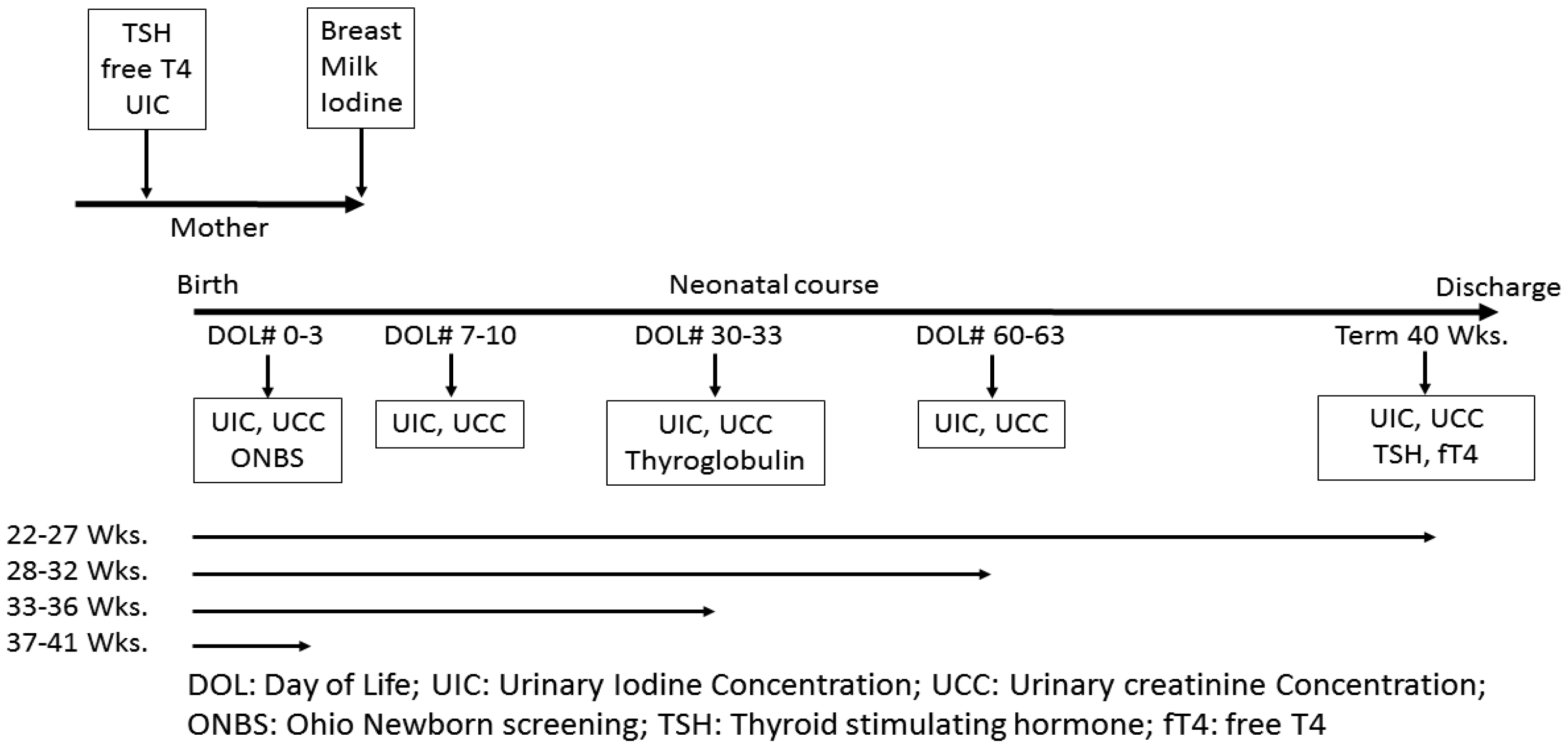
3. Study Design/Methods
4. Statistical Methods
5. Results
6. Discussion
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Trumpff, C.; De Schepper, J.; Tafforeau, J.; Van Oyen, H.; Vanderfaeillie, J.; Vandevijvere, S. Mild iodine deficiency in pregnancy in Europe and its consequences for cognitive and psychomotor development of children: A review. J. Trace Elem. Med. Boil. 2013, 27, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M. The role of iodine in human growth and development. Semin. Cell Dev. Boil. 2011, 22, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M. Iodine Deficiency. Endocr. Rev. 2009, 30, 376–408. [Google Scholar] [CrossRef] [PubMed]
- Reuss, M.L.; Paneth, N.; Pinto-Martin, J.; Lorenz, J.M.; Susser, M. The Relation of Transient Hypothyroxinemia in Preterm Infants to Neurologic Development at Two Years of Age. N. Engl. J. Med. 1996, 334, 821–827. [Google Scholar] [CrossRef]
- Williams, F.L.; Watson, J.; Ogston, S.; Hume, R.; Willatts, P.; Visser, T.; the Scottish Preterm Thyroid Group. Mild Maternal Thyroid Dysfunction at Delivery of Infants Born ≤34 Weeks and Neurodevelopmental Outcome at 5.5 Years. J. Clin. Endocrinol. Metab. 2012, 97, 1977–1985. [Google Scholar] [CrossRef]
- Bath, S.; Steer, C.; Golding, J.; Emmett, P.; Rayman, M.P. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: Results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 2013, 382, 331–337. [Google Scholar] [CrossRef]
- Vermiglio, F.; Presti, V.P.L.; Moleti, M.; Sidoti, M.; Tortorella, G.; Scaffidi, G.; Castagna, M.G.; Mattina, F.; Violi, M.A.; Crisà, A.; et al. Attention Deficit and Hyperactivity Disorders in the Offspring of Mothers Exposed to Mild-Moderate Iodine Deficiency: A Possible Novel Iodine Deficiency Disorder in Developed Countries. J. Clin. Endocrinol. Metab. 2004, 89, 6054–6060. [Google Scholar] [CrossRef]
- Bath, S.; Furmidge-Owen, V.L.; Redman, C.W.; Rayman, M.P. Gestational changes in iodine status in a cohort study of pregnant women from the United Kingdom: Season as an effect modifier. Am. J. Clin. Nutr. 2015, 101, 1180–1187. [Google Scholar] [CrossRef]
- Davis, K.; Li, X.; Adams-Huet, B.; Sandon, L. Infant feeding practices and dietary consumption of US infants and toddlers: National Health and Nutrition Examination Survey (NHANES) 2003–2012. Public Health Nutr. 2017, 21, 711–720. [Google Scholar] [CrossRef]
- Caldwell, K.L.; Pan, Y.; Mortensen, M.E.; Makhmudov, A.; Merrill, L.; Moye, J. Iodine Status in Pregnant Women in the National Children’s Study and in U.S. Women (15–44 Years), National Health and Nutrition Examination Survey 2005–2010. Thyroid 2013, 23, 927–937. [Google Scholar] [CrossRef]
- Murray, C.W.; Egan, S.K.; Kim, H.; Beru, N.; Bolger, P.M. US Food and Drug Administration’s Total Diet Study: Dietary intake of perchlorate and iodine. J. Expo. Sci. Environ. Epidemiol. 2008, 18, 571–580. [Google Scholar] [CrossRef]
- Dasgupta, P.K.; Liu, Y.; Dyke, J.V. Iodine Nutrition: Iodine Content of Iodized Salt in the United States. Environ. Sci. Technol. 2008, 42, 1315–1323. [Google Scholar] [CrossRef]
- Ibrahim, M.; De Escobar, G.M.; Visser, T.J.; Duran, S.; Van Toor, H.; Strachan, J.; Williams, F.L.; Hume, R. Iodine deficiency associated with parenteral nutrition in extreme preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2003, 88, F56–F57. [Google Scholar] [CrossRef]
- Zimmermann, M.; Crill, C.M. Iodine in Enteral and Parenteral Nutrition. Best Pr. Res. Clin. Endocrinol. Metab. 2010, 24, 143–158. [Google Scholar] [CrossRef]
- Uhrmann, S.; Marks, K.H.; Maisels, M.J.; Friedman, Z.; Murray, F.; Kulin, H.E.; Kaplan, M.; Utiger, R. Thyroid function in the preterm infant: A longitudinal assessment. J. Pediatr. 1978, 92, 968–973. [Google Scholar] [CrossRef]
- Bodamer, O.A.; Leonard, J.V.; Halliday, D. The relation between neonatal thyroxine levels and neurodevelopmental outcome at age 5 and 9 years in a national cohort of very preterm and/or very low birth weight infants. Pediatr. Res. 1996, 39, 142. [Google Scholar] [CrossRef]
- Ares, S.; Escobar-Morreale, H.F.; Quero, J.; Durán, S.; Presas, M.J.; Herruzo, R.; De Escobar, G.M. Neonatal Hypothyroxinemia: Effects of Iodine Intake and Premature Birth1. J. Clin. Endocrinol. Metab. 1997, 82, 1704–1712. [Google Scholar] [CrossRef]
- Nath, S.K.; Moinier, B.; Thuillier, F.; Rongier, M.; Desjeux, J.F. Urinary excretion of iodide and fluoride from supplemented food grade salt. Int. J. Vitam. Nutr. Res. 1992, 62, 66–72. [Google Scholar]
- Finch, C.W. Review of Trace Mineral Requirements for Preterm Infants. Nutr. Clin. Pr. 2014, 30, 44–58. [Google Scholar] [CrossRef]
- Agostoni, C.; Buonocore, G.; Carnielli, V.; De Curtis, M.; Darmaun, D.; Decsi, T.; Domellöf, M.; Embleton, N.; Fusch, C.; Genzel-Boroviczeny, O.; et al. Enteral Nutrient Supply for Preterm Infants: Commentary From the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef]
- Belfort, M.B.; Pearce, E.N.; Braverman, L.E.; He, X.; Brown, R.S. Low iodine content in the diets of hospitalized preterm infants. J. Clin. Endocrinol. Metab. 2012, 97, E632–E636. [Google Scholar] [CrossRef] [PubMed]
- Ghirri, P.; Lunardi, S.; Boldrini, A. Iodine Supplementation in the Newborn. Nutrients 2014, 6, 382–390. [Google Scholar] [CrossRef]
- Domellöf, M.; Szitányi, P.; Simchowitz, V.; Franz, A.; Mimouni, F.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Iron and trace minerals. Clin. Nutr. 2018, 37, 2354–2359. [Google Scholar] [CrossRef] [PubMed]
- Cicalese, M.P.; Bruzzese, E.; Guarino, A.; Spagnuolo, M.I. Requesting iodine supplementation in children on parenteral nutrition. Clin. Nutr. 2009, 28, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Clarridge, K.E.; Conway, E.E.; Bucuvalas, J. Hypothyroidism and Iodine Deficiency in an Infant Requiring Total Parenteral Nutrition. J. Parenter. Enter. Nutr. 2013, 38, 901–904. [Google Scholar] [CrossRef]
- Passos, A.C.V.; Barros, F.; Damiani, D.; Semer, B.; Cespedes, W.C.J.; Sannicola, B.; Tannuri, A.C.A.; Tannuri, U. Hypothyroidism associated with short bowel syndrome in children: A report of six cases. Arch. Endocrinol. Metab. 2018, 62, 655–660. [Google Scholar] [CrossRef]
- Ittermann, T.; Johner, S.; Below, H.; Leiterer, M.; Thamm, M.; Remer, T.; Völzke, H. Interlaboratory variability of urinary iodine measurements. Clin. Chem. Lab. Med. 2018, 56, 441–447. [Google Scholar] [CrossRef]
- Buyukgebiz, A. Newborn screening for congenital hypothyroidism. J. Clin. Res. Pediatr. Endocrinol. 2013, 5 (Suppl. 1), 8–12. [Google Scholar] [CrossRef]
- Zimmermann, M.; Aeberli, I.; Andersson, M.; Assey, V.; Yorg, J.A.J.; Jooste, P.; Jukic, T.; Kartono, D.; Kusić, Z.; Pretell, E.; et al. Thyroglobulin Is a Sensitive Measure of Both Deficient and Excess Iodine Intakes in Children and Indicates No Adverse Effects on Thyroid Function in the UIC Range of 100–299 μg/L: A UNICEF/ICCIDD Study Group Report. J. Clin. Endocrinol. Metab. 2013, 98, 1271–1280. [Google Scholar] [CrossRef]
- WHO. e-Library of Evidence for Nutrition Actions (eLENA). Iodine in pregnancy and lactation, Biological, behavioral and contextual rationale. Available online: https://www.who.int/elena/titles/bbc/iodine_pregnancy/en/ (accessed on 27 May 2020).
- Greenwald, I. Observations on the History of Goiter in Ohio and in West Virginia. J. Hist. Med. Allied Sci. 1955, 10, 277–289. [Google Scholar] [CrossRef]
- Trumbo, P.; A Yates, A.; Schlicker, S.; Poos, M. Dietary Reference Intakes. J. Am. Diet. Assoc. 2001, 101, 294–301. [Google Scholar] [CrossRef]
- Berghout, A.; Wiersinga, W. Thyroid size and thyroid function during pregnancy: An analysis. Eur. J. Endocrinol. 1998, 138, 536–542. [Google Scholar] [CrossRef]
- Becker, D.V.; Braverman, L.E.; Delange, F.; Dunn, J.T.; Franklyn, J.A.; Hollowell, J.G.; Lamm, S.H.; Mitchell, M.L.; Pearce, E.; Robbins, J.; et al. Iodine Supplementation for Pregnancy and Lactation—United States and Canada: Recommendations of the American Thyroid Association. Thyroid 2006, 16, 949–951. [Google Scholar] [CrossRef]
- Stagnaro-Green, A.; Abalovich, M.; Alexander, E.; Azizi, F.; Mestman, J.; Negro, R.; Nixon, A.; Pearce, E.N.; Soldin, O.P.; Sullivan, S.; et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011, 21, 1081–1125. [Google Scholar] [CrossRef]
- Patel, A.; Lee, S.Y.; Stagnaro-Green, A.; Mackay, D.; Wong, A.W.; Pearce, E.N. Iodine Content of the Best-Selling United States Adult and Prenatal Multivitamin Preparations. Thyroid 2019, 29, 124–127. [Google Scholar] [CrossRef]
- Leung, A.M.; Pearce, E.N.; Braverman, L.E. Iodine Content of Prenatal Multivitamins in the United States. N. Engl. J. Med. 2009, 360, 939–940. [Google Scholar] [CrossRef]
- Teas, J.; Pino, S.; Critchley, A.T.; Braverman, L.E. Variability of Iodine Content in Common Commercially Available Edible Seaweeds. Thyroid 2004, 14, 836–841. [Google Scholar] [CrossRef]
- Taylor, P.; E Okosieme, O.; Dayan, C.M.; Lazarus, J.H. THERAPY OF ENDOCRINE DISEASE: Impact of iodine supplementation in mild-to-moderate iodine deficiency: Systematic review and meta-analysis. Eur. J. Endocrinol. 2014, 170, R1–R15. [Google Scholar] [CrossRef]
- Ares, S.; Quero, J.; De Escobar, G.M. Iodine Balance, Iatrogenic Excess, and Thyroid Dysfunction in Premature Newborns. Semin. Perinatol. 2008, 32, 407–412. [Google Scholar] [CrossRef]
- Leung, A.M.; Pearce, E.N.; Hamilton, T.; He, X.; Pino, S.; Merewood, A.; Braverman, L.E. Colostrum iodine and perchlorate concentrations in Boston-area women: A cross-sectional study. Clin. Endocrinol. 2009, 70, 326–330. [Google Scholar] [CrossRef]
- Ares, S.; Quero, J.; Duran, S.; Presas, M.J.; Herruzo, R.; De Escobar, G.M. Iodine content of infant formulas and iodine intake of premature babies: High risk of iodine deficiency. Arch. Dis. Child. Fetal Neonatal Ed. 1994, 71, F184–F191. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wolff, J.; Chaikoff, I.L. The inhibitory action of excessive iodide upon the synthesis of diiodotyrosine and of thyroxine in the thyroid gland of the normal rat. Endocrinology 1948, 43, 174–179. [Google Scholar] [CrossRef] [PubMed]

| Gestational Age (weeks) | Number (%) |
|---|---|
| 22–27 28–32 33–36 37–40 | 13(26) 13(26) 14(28) 10(20) |
| Birth weight (grams), Median (Q1–Q3) | 1690 (880–2551) |
| Male Gender | 25(50) |
| Race Caucasian African American Hispanic Others | 19(38) 25(50) 3(6) 3(6) |
| Delivery Vaginal Caesarian section | 22(44) 28(56) |
| Umbilical lines (Iodine prep) | 31(62) |
| PICC lines PICC line with Iodine PICC line with Chlorhexidine | 17(34) 13(26) 4(8) |
| Maternal Iodine Status & Thyroid Functions | Median (Q1, Q3) |
|---|---|
| Maternal Urine Iodine Concentration (mcg/L) Maternal iodine deficiency, N (%) Maternal Urine Iodine/Creatinine Ratio (mcg/g) Maternal Urine Creatinine Concentration (mg/dL) Maternal Free T4, (ng/dL) Maternal TSH, (µIU/mL) | 98 (56, 177) 32 (64) 220 (117, 308) 47(27, 86) 0.484 (0.411, 0.540) 1.504 (0.860, 2.309) |
| Prenatal Vitamin usage (%) | 44 (88) |
| Breast Milk Iodine Concentration (mcg/L) | 71 (31, 281) |
| Infant Iodine Status & Thyroid Functions: TSH on ONBS (µIU/mL) Final TSH (40 weeks PMA or PTD) (µIU/mL) Final free T4 (40 weeks PMA or PTD) (ng/dL) Thyroglobulin (ng/mL) at 30–33 #DOL Urine Iodine Concentration (mcg/L) at 0–3 days (N = 48) Urine Iodine Concentration (mcg/L) at 7–10 days (N = 33) Urine Iodine Concentration (mcg/L) at 30–33 days (N = 25) Urine Iodine Concentration (mcg/L) at 60–63 days (N = 16) Urine Iodine Concentration (mcg/L) at 40 wks. PMA (N = 8) Urine Iodine Concentration (mcg/L) at PTD (N = 4) | 7.2 (3.2, 11.5) 3.5 (1.9, 3.84) 1.26 (1.11, 1.48) 187.7 (156.5, 271.6) 151 (58, 2317) 103 (52, 213) 167 (54, 299) 213 (76, 440) 296 (193, 378) 315 (229, 525) |
| Total days on Parenteral nutrition | 8 (0, 34) |
| 22–27 Weeks N = 13 | 28–32 Weeks N = 13 | 33–36 Weeks N = 14 | 37–40 Weeks N = 10 | p-Value | |
|---|---|---|---|---|---|
| Birth Weight (g) | 630 (600, 720) | 1360 (1195, 1580) | 2280 (1990, 2551) | 3275 (3020, 3520) | |
| Sex, No. (%) | |||||
| Male | 9(69) | 8(62) | 4(29) | 4(40) | |
| Female | 4(31) | 5(38) | 10(71) | 6(60) | |
| Race, No. (%) | |||||
| Caucasian | 2(15) | 5(38) | 5(36) | 7(70) | |
| AA | 10(77) | 7(54) | 8(57) | 0(0) | |
| Hispanic | 1(8) | 0(0) | 0(0) | 2(20) | |
| Other | 0(0) | 1(8) | 1(7) | 1(10) | |
| Maternal UIC (mcg/L) | 99 (56, 168) | 63 (35, 154) | 123 (81, 161) | 146 (79, 277) | 0.38 * |
| Maternal Iodine deficiency, | |||||
| No (N(%)) | 4(31) | 4(31) | 5(36) | 5(50) | |
| Yes (N(%)) | 9(69) | 9(69) | 9(64) | 5(50) | |
| UIC (0–3 days) (mcg/L) a | 2954 (2632, 3007) 2,3,4 | 362 (69, 985) 1 | 45 (25, 341) 1 | 96 (49, 105) 1 | <0.001 * |
| UIC (7–10 days) (mcg/L) a | 56 (39, 98) | 132 (67, 215) | 119 (94, 294) | ---- | 0.19 * |
| UIC (30–33 days) (mcg/L) a | 73 (34, 133) 2 | 299 (189, 457) 1 | 189 (113, 265) | ---- | 0.017 * |
| UIC (60–63 days) (mcg/L) a | 146 (65, 336) | 380 (230, 718) | ---- | ---- | 0.090 * |
| UIC (40 weeks PMA) (mcg/L) a | 296 (193, 378) | ---- | ---- | ---- | |
| UIC (PTD) (mcg/L) a | 315 (229, 525) | ---- | ---- | ---- |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanike, N.; Groh-Wargo, S.; Thomas, M.; Chien, E.K.; Mhanna, M.; Kumar, D.; Worley, S.; Singh, R.J.; Shekhawat, P.S. Risk of Iodine Deficiency in Extremely Low Gestational Age Newborns on Parenteral Nutrition. Nutrients 2020, 12, 1636. https://doi.org/10.3390/nu12061636
Kanike N, Groh-Wargo S, Thomas M, Chien EK, Mhanna M, Kumar D, Worley S, Singh RJ, Shekhawat PS. Risk of Iodine Deficiency in Extremely Low Gestational Age Newborns on Parenteral Nutrition. Nutrients. 2020; 12(6):1636. https://doi.org/10.3390/nu12061636
Chicago/Turabian StyleKanike, Neelakanta, Sharon Groh-Wargo, Megan Thomas, Edward K. Chien, Maroun Mhanna, Deepak Kumar, Sarah Worley, Ravinder J. Singh, and Prem S. Shekhawat. 2020. "Risk of Iodine Deficiency in Extremely Low Gestational Age Newborns on Parenteral Nutrition" Nutrients 12, no. 6: 1636. https://doi.org/10.3390/nu12061636
APA StyleKanike, N., Groh-Wargo, S., Thomas, M., Chien, E. K., Mhanna, M., Kumar, D., Worley, S., Singh, R. J., & Shekhawat, P. S. (2020). Risk of Iodine Deficiency in Extremely Low Gestational Age Newborns on Parenteral Nutrition. Nutrients, 12(6), 1636. https://doi.org/10.3390/nu12061636





