Intake of Non-Nutritive Sweeteners in Chilean Children after Enforcement of a New Food Labeling Law that Regulates Added Sugar Content in Processed Foods
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Registry of Foods Containing NNS
2.3. Assessment of NNS Intake
2.4. Anthropometric Evaluation
2.5. Ethical Approval
2.6. Statistical Analysis
3. Results
3.1. Overall NNS Intake
3.2. NNS Intake According to Sociodemographic Variables and Their Main Food Sources
3.2.1. Sucralose
3.2.2. Acesulfame-K
3.2.3. Stevia
3.2.4. Aspartame
3.2.5. Cyclamate and Saccharin
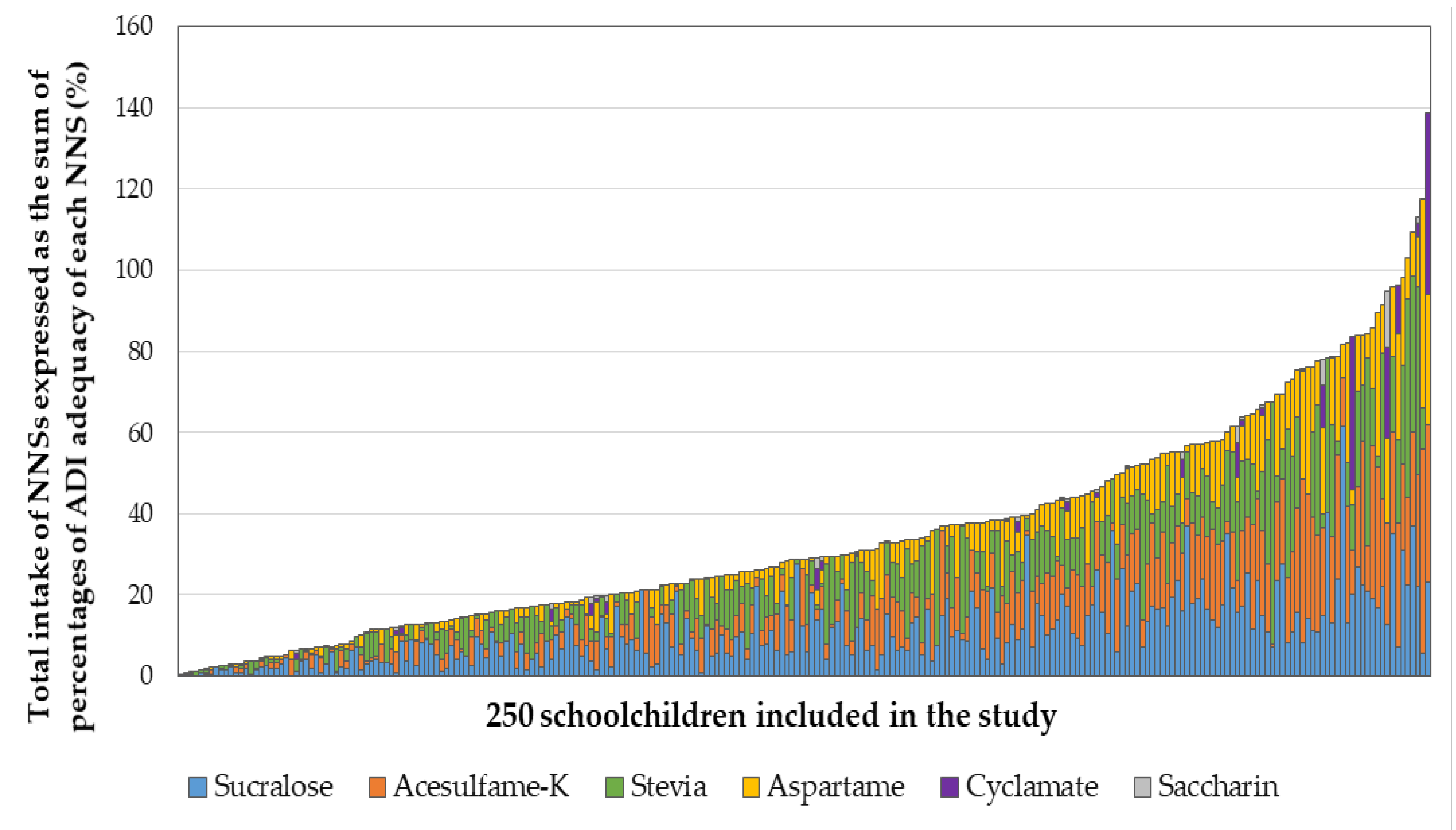
3.3. Combined NNS Intake
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ministerio de Salud, Gobierno de Chile. Encuesta Nacional de Salud Chile 2016–2017. Primeros Resultados. Available online: http://www.minsal.cl/wp-content/uploads/2017/11/ENS-2016-17_PRIMEROS-RESULTADOS.pdf (accessed on 3 March 2020).
- Junta Nacional de Auxilio Escolar y Becas, Gobierno de Chile. Informe Mapa Nutricional 2019. Available online: https://www.junaeb.cl/wp-content/uploads/2013/03/Mapa-Nutricional-2019-1.pdf (accessed on 6 April 2020).
- Hawkes, C.; Smith, T.G.; Jewell, J.; Wardle, J.; Hammond, R.A.; Friel, S.; Thow, A.M.; Kain, J. Smart food policies for obesity prevention. Lancet 2015, 385, 2410–2421. [Google Scholar] [CrossRef]
- World Health Organization. Information Note about Intake of Sugars Recommended in the WHO Guideline for Adults and Children. Available online: https://apps.who.int/iris/bitstream/handle/10665/325335/WHO-NMH-NHD-15.3-eng.pdf?sequence=1&isAllowed=y (accessed on 15 January 2020).
- Stanhope, K.L. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit. Rev. Clin. Lab Sci. 2016, 53, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition therapy for adults with diabetes or prediabetes: A consensus report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef]
- Cediel, G.; Reyes, M.; da Costa, M.L.; Martínez, E.; Monteiro, C.A.; Corvalán, C.; Uauy, R. Ultra-processed foods and added sugars in the Chilean diet (2010). Public Health Nutr. 2018, 21, 125–133. [Google Scholar] [CrossRef]
- Corvalán, C.; Reyes, M.; Garmendia, M.L.; Uauy, R. Structural responses to the obesity and non-communicable diseases epidemic: The Chilean Law of Food Labeling and Advertising. Obes. Rev. 2013, 14, 79–87. [Google Scholar] [CrossRef]
- Correa, T.; Fierro, C.; Reyes, M.; Carpentier, F.R.D.; Taillie, L.S.; Corvalan, C. Responses to the Chilean law of food labeling and advertising: Exploring knowledge, perceptions and behaviors of mothers of young children. Int. J. Behav. Nutr. Phys. Act 2019, 16, 21. [Google Scholar] [CrossRef]
- Rodríguez, L.; Pizarro, T. Ley de Etiquetado y Publicidad de Alimentos: Chile innovando en nutrición pública una vez más. Rev. Chil. Pediatr. 2018, 89, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Durán, S.; Angarita, L.; Escobar, M.C.; Rojas, D.; de Assis, J. Noncaloric sweeteners in children: A controversial theme. Biomed Res. Int. 2018, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ashwell, M.; Gibson, S.; Bellisle, F.; Buttriss, J.; Drewnowski, A.; Fantino, M.; Gallagher, A.M.; de Graaf, K.; Goscinny, S.; Hardman, C.; et al. Expert consensus on low-calorie sweeteners: Facts, research gaps and suggested actions. Nutr. Res. Rev. 2020, 13, 1–10. [Google Scholar] [CrossRef]
- Pepino, M.Y.; Tiemann, C.D.; Patterson, B.W.; Wice, B.M.; Klein, S. Sucralose affects glycemic and hormonal responses to an oral glucose load. Diabetes Care 2013, 36, 2530–2535. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- De Ruyter, J.C.; Olthof, M.R.; Seidell, J.C.; Katan, M.B. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N. Engl. J. Med. 2012, 367, 1397–1406. [Google Scholar] [PubMed]
- Katan, M.B.; de Ruyter, J.C.; Kuijper, L.D.J.; Chow, C.C.; Hall, K.D.; Olthof, M.R. Impact of masked replacement of sugar-sweetened with sugar-free beverages on body weight increases with initial BMI: Secondary analysis of data from an 18 month double–blind trial in children. PLoS ONE 2016, 11, e0159771. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Abou-Setta, A.M.; Chauhan, B.F.; Rabbani, R.; Lys, J.; Copstein, L.; Mann, A.; Jeyaraman, M.M.; Reid, A.E.; Fiander, M.; et al. Nonnutritive sweeteners and cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ 2017, 189, E929–E939. [Google Scholar] [CrossRef] [PubMed]
- García-Almeida, J.M.; Casado Fdez, G.; García Alemán, J. A current and global review of sweeteners; regulatory aspects. Nutr. Hosp. 2013, 28, 17–31. [Google Scholar] [PubMed]
- Sylvetsky, A.C.; Gardner, A.L.; Bauman, V.; Blau, J.E.; Garraffo, H.M.; Walter, P.J.; Rother, K.I. Nonnutritive sweeteners in breast milk. J. Toxicol. Environ. Health A 2015, 78, 1029–1032. [Google Scholar] [CrossRef]
- Mennella, J.A. Ontogeny of taste preferences: Basic biology and implications for health. Am. J. Clin. Nutr. 2014, 99, 704S–711S. [Google Scholar]
- Fitch, C.; Keim, K. Position of the Academy of Nutrition and Dietetics: Use of nutritive and nonnutritive sweeteners. J. Acad. Nutr. Diet 2012, 112, 739–758. [Google Scholar] [CrossRef]
- Ministerio de Salud, Gobierno de Chile. Reglamento Sanitario de los Alimentos Chile. Available online: http://www.minsal.cl/wp-content/uploads/2017/04/DECRETO_977_96_actualizado_a-octubre-2016.pdf (accessed on 15 January 2020).
- Serra-Majem, L.; Raposo, A.; Aranceta-Bartrina, J.; Varela-Moreiras, G.; Logue, C.; Laviada, H.; Socolovsky, S.; Pérez-Rodrigo, C.; Aldrete-Velasco, J.; Meneses, S.E.; et al. Ibero–American consensus on low- and no-calorie sweeteners: Safety, nutritional aspects and benefits in food and beverages. Nutrients 2018, 10, 818. [Google Scholar] [CrossRef]
- Renwick, A.G. Incidence and severity in relation to magnitude of intake above the ADI or TDI: Use of critical effect data. Regul. Toxicol. Pharmacol. 1999, 30, S79–S86. [Google Scholar] [CrossRef]
- Durán, A.S.; Quijada, M.M.; Silva, V.L.; Almonacid, M.N.; Berlanga, Z.M.; Rodríguez, N.M. Niveles de ingesta diaria de edulcorantes no nutritivos en escolares de la Región de Valparaíso. Rev. Chil. Nutr. 2011, 38, 444–449. [Google Scholar] [CrossRef]
- Ministerio de Salud, Gobierno de Chile. Ley de Alimentos: Manual de Etiquetado Nutricional—Ministerio de Salud—Gobierno de Chile. Available online: https://www.minsal.cl/ley-de-alimentos-manual-etiquetado-nutricional/ (accessed on 15 January 2020).
- Hamilton, V.V.; Guzmán, E.; Golusda, C.; Lera, L.; Cornejo, E.V. Edulcorantes no nutritivos e ingesta diaria admisible en adultos y niños de peso normal y obesos de tres niveles socioeconómicos, y un grupo de diabéticos de la Región Metropolitana. Rev. Chil. Nutr. 2013, 40, 123–128. [Google Scholar] [CrossRef]
- Duran, S.; Oñate, G.; Haro, P. Consumption of non-nutritive sweeteners and nutritional status in 10–16 year old students. Arch. Argent. Pediatr. 2014, 112, 207–214. [Google Scholar]
- Myers, E.; Passaro, E.; Hedrick, V.; Myers, E.A.; Passaro, E.M.; Hedrick, V.E. The reproducibility and comparative validity of a non-nutritive sweetener food frequency questionnaire. Nutrients 2018, 10, 334. [Google Scholar] [CrossRef]
- de Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 9, 660–667. [Google Scholar] [CrossRef]
- Wilson, T.; Murray, B.; Price, T.; Atherton, D.; Hooks, T. Non-nutritive (artificial) sweetener knowledge among university students. Nutrients 2019, 11, E2201. [Google Scholar] [CrossRef]
- Swithers, S.E. Artificial sweeteners are not the answer to childhood obesity. Appetite 2015, 93, 85–90. [Google Scholar] [CrossRef]
- Sylvetsky, A.; Rother, K.I.; Brown, R. Artificial sweetener use among children: Epidemiology, recommendations, metabolic outcomes, and future directions. Pediatr. Clin. North Am. 2011, 58, 1467–1480. [Google Scholar] [CrossRef]
- Ma, J.; Bellon, M.; Wishart, J.M.; Young, R.; Blackshaw, L.A.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am. J. Physiol. Gastrointest Liver Physiol. 2009, 296, G735–G739. [Google Scholar] [CrossRef]
- Anton, S.D.; Martin, C.K.; Han, H.; Coulon, S.; Cefalu, W.T.; Geiselman, P.; Williamson, D.A. Effects of stevia, aspartame, and sucrose on food intake, satiety, and postprandial glucose and insulin levels. Appetite 2010, 55, 37–43. [Google Scholar] [CrossRef]
- Bellisle, F. Intense sweeteners, appetite for the sweet taste, and relationship to weight management. Curr. Obes. Rep. 2015, 4, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.J. The role of low-calorie sweeteners in the prevention and management of overweight and obesity: Evidence vs. conjecture. Proc. Nutr. Soc. 2018, 77, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Martyn, D.; Darch, M.; Roberts, A.; Lee, H.Y.; Tian, T.Y.; Kaburagi, N.; Belmar, P. Low-/no-calorie sweeteners: A review of global intakes. Nutrients 2018, 10, 357. [Google Scholar] [CrossRef] [PubMed]
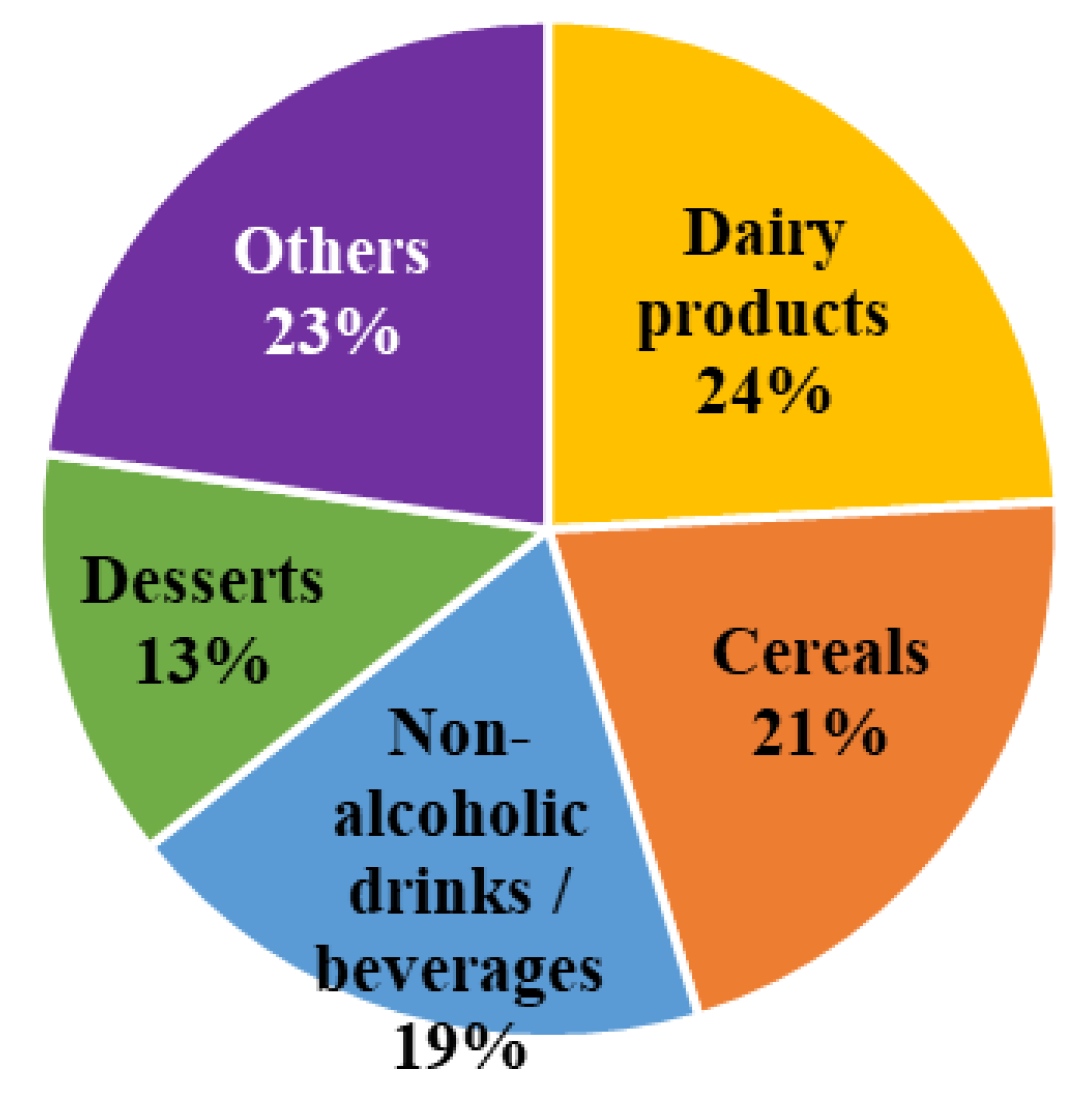

| Parameter | Number of Children (n) | Frequency (%) |
|---|---|---|
| Sex | ||
| Girls | 116 | 46.4 |
| Boys | 134 | 53.6 |
| Type of school system | ||
| Private | 90 | 36.0 |
| Public | 160 | 64.0 |
| Age | ||
| 6 to 9 years old | 147 | 58.8 |
| 10 to 12 years old | 103 | 41.2 |
| Nutritional status | ||
| Underweight | 26 | 10.4 |
| Normal weight | 127 | 50.8 |
| Overweight | 59 | 23.6 |
| Obese | 38 | 15.2 |
| NNS | Number (%) of Products Containing each NNS | Intake Frequency (%) | Median (Interquartile Range) Intake (mg/kg·Day) | Maximal Intake (mg/kg·Day) | ADI (mg/kg·Day) | Maximal Adequacy to ADI (%) |
|---|---|---|---|---|---|---|
| Sucralose | 291 (73%) | 99.2 | 1.32 (0.67–2.32) | 9.24 | 15.0 | 61.63 |
| Acesulfame-K | 70 (18%) | 92.8 | 0.88 (0.25–2.10) | 7.60 | 15.0 | 50.69 |
| Stevia (mg steviol eq.) | 139 (35%) | 86.0 | 0.24 (0.04–0.47) | 2.39 | 4.0 | 59.64 |
| Aspartame | 51 (13%) | 85.2 | 1.42 (0.26–3.55) | 20.62 | 40.0 | 51.55 |
| Cyclamate | 7 (2%) | 12.0 | 0.00 (0.00–0.00) | 3.13 | 7.0 | 44.75 |
| Saccharin | 7 (2%) | 10.8 | 0.00 (0.00–0.00) | 0.68 | 5.0 | 13.62 |
| NNS Intake (mg/kg·Day) Median (Interquartile Range) | Sucralose | Acesulfame-K | Stevia (mg Steviol eq.) | Aspartame |
|---|---|---|---|---|
| By sex: | ||||
| Girls | 1.16 (0.66–2.17) | 0.88 (0.40–2.17) | 0.28 (0.04–0.54) | 1.43 (0.44–3.70) |
| Boys | 1.46 (0.68–2.38) | 0.88 (0.17–2.04) | 0.21 (0.04–0.42) | 1.28 (0.12–3.22) |
| p-value | 0.423 | 0.226 | 0.190 | 0.134 |
| By type of educational system: | ||||
| Private | 1.25 (0.55–2.20) | 0.72 (0.22–1.92) | 0.30 (0.07–0.58) | 1.14 (0.24–3.17) |
| Public | 1.36 (0.80–2.38) | 0.99 (0.28–2.18) | 0.20 (0.04–0.39) | 1.56 (0.26–4.20) |
| p-value | 0.285 | 0.313 | 0.029 | 0.177 |
| By age group: | ||||
| 6-9 years old | 1.69 (0.89–2.38) | 0.98 (0.33–2.34) | 0.31 (0.10–0.57) | 1.74 (0.34–4.41) |
| 10-12 years old | 0.97 (0.35–1.66) | 0.72 (0.17–1.60) | 0.13 (0.02–0.31) | 0.93 (0.23–2.51) |
| p-value | <0.001 | 0.036 | <0.001 | 0.012 |
| By nutritional status: | ||||
| Under and normal weight | 1.37 (0.71–2.36) | 0.72 (0.17–2.07) | 0.28 (0.04–0.49) | 1.30 (0.18–3.39) |
| Overweight and obesity | 1.20 (0.61–2.31) | 1.07 (0.40–2.11) | 0.19 (0.04–0.39) | 1.61 (0.41–3.63) |
| p-value | 0.380 | 0.211 | 0.250 | 0.221 |
| Sociodemographic Variables | ß | p-Value | |
|---|---|---|---|
| Adequacy of sucralose consumption to ADI (%) | |||
| Sex | Girls vs. Boys | −0.826 | 0.439 |
| Type of educational system | Private vs. Public | −1.390 | 0.241 |
| Age (years) | 6-9 vs. 10-12 | 5.676 | 0.000 |
| Nutritional status | Under and normal weight vs. Overweight and obesity | 1.602 | 0.172 |
| Adequacy of acesulfame-K consumption to ADI (%) | |||
| Sex | Girls vs. Boys | 0.986 | 0.386 |
| Type of educational system | Private vs. Public | −1.786 | 0.075 |
| Age (years) | 6–9 vs. 10–12 | 2.525 | 0.030 |
| Nutritional status | Under and normal weight vs. Overweight and obesity | 0.704 | 0.572 |
| Adequacy of stevia consumption to ADI (%) | |||
| Sex | Girls vs. Boys | 1.268 | 0.287 |
| Type of educational system | Private vs. Public | 2.042 | 0.123 |
| Age (years) | 6–9 vs. 10–12 | 4.898 | 0.000 |
| Nutritional status | Under and normal weight vs. Overweight and obesity | 0.660 | 0.613 |
| Adequacy of aspartame consumption to ADI (%) | |||
| Sex | Girls vs. Boys | 0.741 | 0.410 |
| Type of educational system | Private vs. Public | −2.670 | 0.008 |
| Age (years) | 6–9 vs. 10–12 | 2.235 | 0.015 |
| Nutritional status | Under and normal weight vs. Overweight and obesity | 0.466 | 0.637 |
| Adequacy of cyclamate consumption to ADI (%) | |||
| Sex | Girls vs. Boys | −0.039 | 0.940 |
| Type of educational system | Private vs. Public | −1.291 | 0.027 |
| Age (years) | 6–9 vs. 10–12 | −0.265 | 0.617 |
| Nutritional status | Under and normal weight vs. Overweight and obesity | 1.403 | 0.015 |
| Adequacy of saccharin consumption to ADI (%) | |||
| Sex | Girls vs. Boys | −0.116 | 0.373 |
| Type of educational system | Private vs. Public | −0.206 | 0.153 |
| Age (years) | 6–9 vs. 10–12 | −0.138 | 0.296 |
| Nutritional status | Under and normal weight vs. Overweight and obesity | 0.259 | 0.069 |
| Variables | ß (95% Confidence Interval) | p-Value |
|---|---|---|
| Sex | ||
| Girls | 21% (−41% a 84%) | 0.500 |
| Boys | Reference | |
| Type of educational system | ||
| Private | −7% (−14% a 0%) | 0.049 |
| Public | Reference | |
| Age | ||
| 6 to 9 years old | 15% (8% a 21%) | <0.001 |
| 10 to 12 years old | Reference | |
| Nutritional status | ||
| Under and normal weight | 13% (3% a 22%) | 0.010 |
| Overweight | 12% (1% a 22%) | 0.027 |
| Obesity | Reference |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, X.; Zapata, Y.; Pinto, V.; Cornejo, C.; Elbers, M.; van der Graaf, M.; Villarroel, L.; Hodgson, M.I.; Rigotti, A.; Echeverría, G. Intake of Non-Nutritive Sweeteners in Chilean Children after Enforcement of a New Food Labeling Law that Regulates Added Sugar Content in Processed Foods. Nutrients 2020, 12, 1594. https://doi.org/10.3390/nu12061594
Martínez X, Zapata Y, Pinto V, Cornejo C, Elbers M, van der Graaf M, Villarroel L, Hodgson MI, Rigotti A, Echeverría G. Intake of Non-Nutritive Sweeteners in Chilean Children after Enforcement of a New Food Labeling Law that Regulates Added Sugar Content in Processed Foods. Nutrients. 2020; 12(6):1594. https://doi.org/10.3390/nu12061594
Chicago/Turabian StyleMartínez, Ximena, Yazmín Zapata, Victoria Pinto, Camila Cornejo, Martje Elbers, Maaike van der Graaf, Luis Villarroel, María Isabel Hodgson, Attilio Rigotti, and Guadalupe Echeverría. 2020. "Intake of Non-Nutritive Sweeteners in Chilean Children after Enforcement of a New Food Labeling Law that Regulates Added Sugar Content in Processed Foods" Nutrients 12, no. 6: 1594. https://doi.org/10.3390/nu12061594
APA StyleMartínez, X., Zapata, Y., Pinto, V., Cornejo, C., Elbers, M., van der Graaf, M., Villarroel, L., Hodgson, M. I., Rigotti, A., & Echeverría, G. (2020). Intake of Non-Nutritive Sweeteners in Chilean Children after Enforcement of a New Food Labeling Law that Regulates Added Sugar Content in Processed Foods. Nutrients, 12(6), 1594. https://doi.org/10.3390/nu12061594





