Meat Consumption, Cognitive Function and Disorders: A Systematic Review with Narrative Synthesis and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria and Screening Process
2.3. Information Extraction and Quality Assessment
2.4. Narrative Synthesis and Meta-Analysis
3. Results
3.1. Characteristics of Studies and Quality Assessment
3.2. Observational Evidence
3.3. Intervention Study Evidence
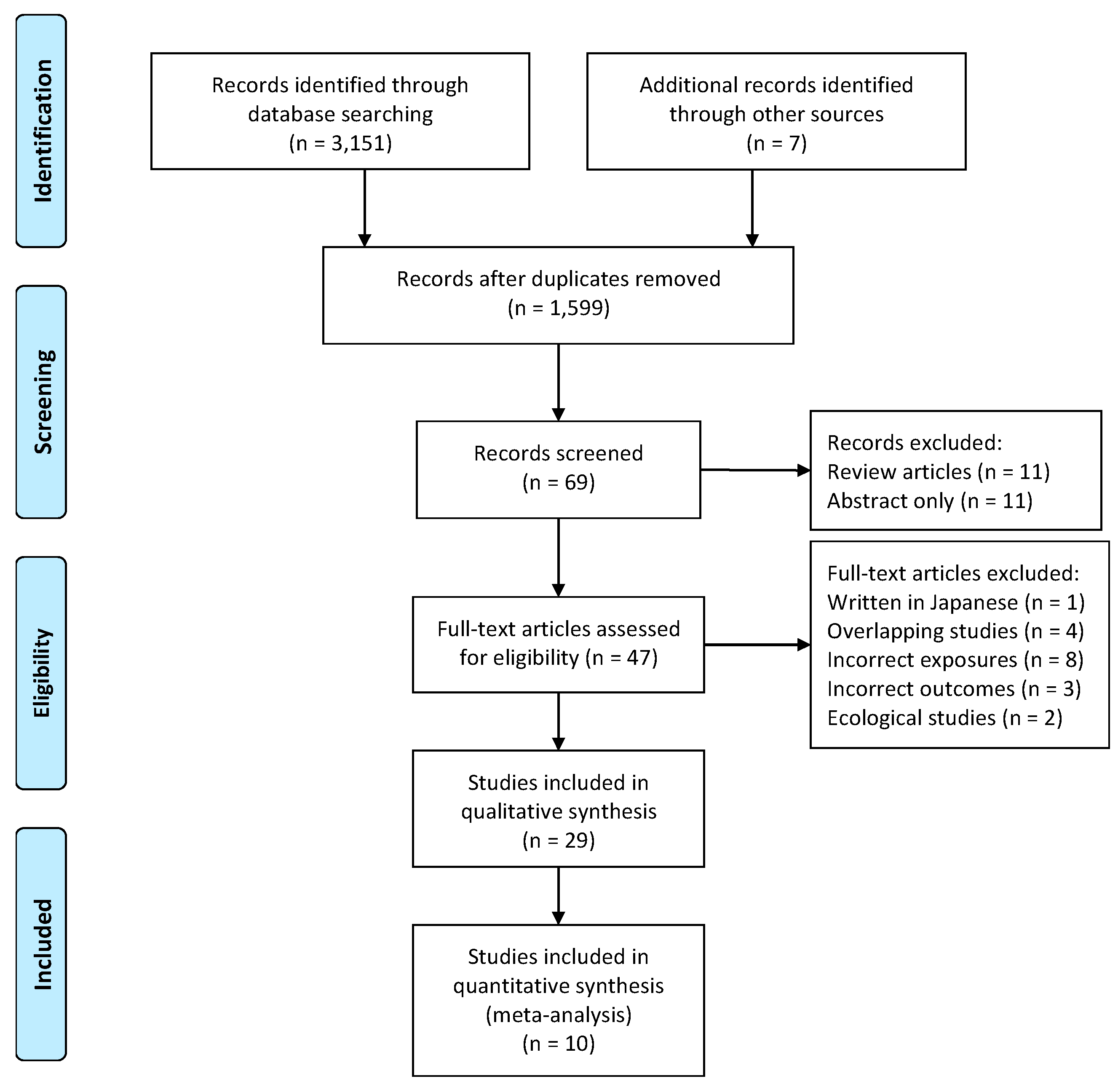
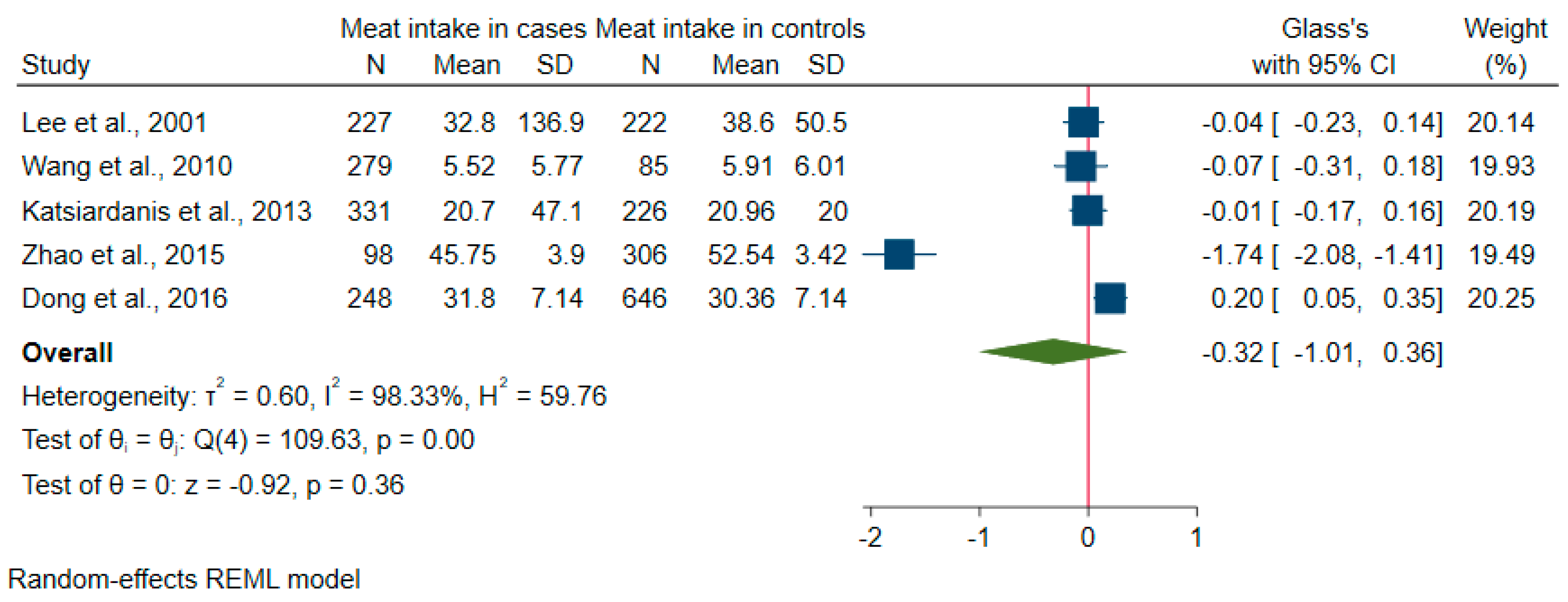
3.4. Meta-Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SD | standard deviation |
| PAQUID | Personnes Agées QUID epidemiological study of cognitive and functional ageing |
| MMSE | Mini-mental state examination |
| AD | Alzheimer’s disease |
| DSM-III-R | Diagnostic and Statistical Manual of Mental Disorders, third edition, revised |
| HR | Hazard ratio |
| CI | confidence interval |
| FFQ | food frequency questionnaire |
| DSM-IV | Diagnostic and Statistical Manual of Mental Disorders, fourth edition |
| NINCDS-ADRDA | National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s disease and related disorders association criteria |
| aOR | adjusted OR |
| cMMSE | change of mini-mental state examination |
| ICD | international classification of disease |
| CERAD | the Consortiumto Establish a Registry for Alzheimer’s Disease |
| MCI | mild cognitive impairment |
| PR | prevalence ratio |
References
- Dementia Fact Sheet. Available online: http://www.who.int/mediacentre/factsheets/fs362/en/ (accessed on 10 October 2019).
- Burns, A.; Iliffe, S. Alzheimer’s disease. BMJ 2009, 338, b158. [Google Scholar] [CrossRef] [PubMed]
- Yusufov, M.; Weyandt, L.L.; Piryatinsky, I. Alzheimer’s disease and diet: A systematic review. Int. J. Neurosci. 2017, 127, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Kouvari, M.; Tyrovolas, S.; Panagiotakos, D.B. Red meat consumption and healthy ageing: A review. Maturitas 2016, 84, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. Trends in diet and Alzheimer’s disease during the nutrition transition in Japan and developing countries. J. Alzheimers Dis. 2014, 38, 611–620. [Google Scholar] [CrossRef]
- Grant, W.B. Using Multicountry Ecological and Observational Studies to Determine Dietary Risk Factors for Alzheimer’s Disease. J. Am. Coll. Nutr. 2016, 35, 476–489. [Google Scholar] [CrossRef]
- Wang, X.; Lin, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G.; Pan, A.; Hu, F.B. Red and processed meat consumption and mortality: Dose–response meta-analysis of prospective cohort studies. Public Health Nutr. 2015, 19, 893–905. [Google Scholar] [CrossRef]
- Granic, A.; Davies, K.; Adamson, A.J.; Kirkwood, T.; Hill, T.R.; Siervo, M.; Mathers, J.C.; Jagger, C. Dietary Patterns High in Red Meat, Potato, Gravy, and Butter Are Associated with Poor Cognitive Functioning but Not with Rate of Cognitive Decline in Very Old Adults. J. Nutr. 2016, 146, 265–274. [Google Scholar] [CrossRef]
- Titova, O.E.; Ax, E.; Brooks, S.; Sjögren, P.; Cederholm, T.; Kilander, L.; Kullberg, J.; Larsson, E.-M.; Johansson, L.; Ahlström, H.; et al. Mediterranean diet habits in older individuals: Associations with cognitive functioning and brain volumes. Exp. Gerontol. 2013, 48, 1443–1448. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Y.; Cheng, H.G. Lower intake of vegetables and legumes associated with cognitive decline among illiterate elderly Chinese: A 3-year cohort study. J. Nutr. Health Aging 2012, 16, 549–552. [Google Scholar] [CrossRef]
- Crichton, G.; Elias, M.; Davey, A.; Alkerwi, A.; Dore, G. Higher Cognitive Performance Is Prospectively Associated with Healthy Dietary Choices: The Maine Syracuse Longitudinal Study. J. Prev. Alzheimer Dis. 2015, 2, 24–32. [Google Scholar]
- Key Facts of Healthy Diet. Available online: https://www.who.int/en/news-room/fact-sheets/detail/healthy-diet (accessed on 15 October 2019).
- 2015–2020 Dietary Guidelines for Americans. Available online: https://health.gov/dietaryguidelines/2015/guidelines/ (accessed on 15 October 2019).
- SACN Iron and Health Report: The Scientific Advisory Committee on Nutrition Recommendations on Iron and Health, and Consumption of Red and Processed Meat. Available online: https://www.gov.uk/government/publications/sacn-iron-and-health-report (accessed on 15 October 2019).
- Healthy Diet Recommendations. Available online: https://www.nutrition.org.uk/healthyliving/healthydiet.html (accessed on 15 October 2019).
- Food-Based Dietary Guidelines—China: Dietary Guidelines for Chinese Residents. Available online: http://www.fao.org/nutrition/education/food-dietary-guidelines/regions/countries/china/en/ (accessed on 20 October 2019).
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, U.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B.; et al. Meta-analysis of Observational Studies in EpidemiologyA Proposal for Reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 15 October 2019).
- Campbell, M.; E McKenzie, J.; Sowden, A.; Katikireddi, S.V.; E Brennan, S.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.E.; Schmidt, F.L. Methods of Meta-Analysis Correcting Error and Bias in Research Findings, 3rd ed.; SAGE: Thousand Oaks, CA, USA, 2015; pp. 453–482. ISBN 978-145-228-689-1. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, U.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Heys, M.; Jiang, C.; Schooling, C.M.; Zhang, W.; Cheng, K.K.; Lam, T.H.; Leung, G.M. Is childhood meat eating associated with better later adulthood cognition in a developing population? Eur. J. Epidemiol. 2010, 25, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Barberger-Gateau, P.; Letenneur, L.; Deschamps, V.; Pérès, K.; Dartigues, J.-F.; Renaud, S. Fish, meat, and risk of dementia: Cohort study. BMJ 2002, 325, 932–933. [Google Scholar] [CrossRef]
- Barberger-Gateau, P.; Raffaitin, C.; Letenneur, L.; Berr, C.; Tzourio, C.; Dartigues, J.F.; Alpérovitch, A. Dietary patterns and risk of dementia: The Three-City cohort study. Neurology 2007, 69, 1921–1930. [Google Scholar] [CrossRef]
- Vercambre, M.-N.; Boutron-Ruault, M.-C.; Ritchie, C.W.; Clavel-Chapelon, F.; Berr, C. Long-term association of food and nutrient intakes with cognitive and functional decline: A 13-year follow-up study of elderly French women. Br. J. Nutr. 2009, 102, 419–427. [Google Scholar] [CrossRef]
- Samieri, C.; Grodstein, F.; Rosner, B.A.; Kang, J.H.; Cook, N.R.; Manson, J.E.; Buring, J.E.; Willett, W.C.; Okereke, O.I. Mediterranean diet and cognitive function in older age. Epidemiology 2013, 24, 490–499. [Google Scholar] [CrossRef]
- Wengreen, H.; Munger, R.G.; Cutler, A.; Quach, A.; Bowles, A.; Corcoran, C.; Tschanz, J.T.; Norton, M.C.; A Welsh-Bohmer, K. Prospective study of Dietary Approaches to Stop Hypertension–and Mediterranean-style dietary patterns and age-related cognitive change: The Cache County Study on Memory, Health and Aging123. Am. J. Clin. Nutr. 2013, 98, 1263–1271. [Google Scholar] [CrossRef]
- Ashby-Mitchell, K.; Peeters, A.; Anstey, K. Role of Dietary Pattern Analysis in Determining Cognitive Status in Elderly Australian Adults. Nutrients 2015, 7, 1052–1067. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Kyrozis, A.; Rossi, M.; Katsoulis, M.; Trichopoulos, D.; La Vecchia, C.; Lagiou, P. Mediterranean diet and cognitive decline over time in an elderly Mediterranean population. Eur. J. Nutr. 2014, 54, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Van Lent, D.M.; Wolfsgruber, S.; Weinhold, L.; Kleineidam, L.; Bickel, H.; Scherer, M.; Eisele, M.; Bussche, H.V.D.; Wiese, B.; et al. Prospective Associations between Single Foods, Alzheimer’s Dementia and Memory Decline in the Elderly. Nutrients 2018, 10, 852. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xiang, Y.-B.; Cai, H.; Li, H.; Gao, Y.-T.; Zheng, W.; Shu, X.-O. A Prospective Investigation of Dietary Intake and Functional Impairments Among the Elderly. Am. J. Epidemiol. 2018, 187, 2372–2386. [Google Scholar] [CrossRef]
- Baker, F.M.; Jordan, B.; Barclay, L.; Schoenberg, B.S. Risk factors for clinically diagnosed alzheimer’s disease. Int. J. Geriatr. Psychiatry 1993, 8, 379–385. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, L.; Feng, L.; Xi, Y.; Yu, H.; Ma, W.; Zhang, D.; Xiao, R. Association of dietary intake and lifestyle pattern with mild cognitive impairment in the elderly. J. Nutr. Health Aging 2014, 19, 164–168. [Google Scholar] [CrossRef]
- Dong, L.; Xiao, R.; Cai, C.; Xu, Z.; Wang, S.; Pan, L.; Yuan, L. Diet, lifestyle and cognitive function in old Chinese adults. Arch. Gerontol. Geriatr. 2016, 63, 36–42. [Google Scholar] [CrossRef]
- Lee, L.; Kang, S.A.; Lee, H.O.; Lee, B.H.; Park, J.S.; Kim, J.H.; Jung, I.K.; Park, Y.J.; Lee, J.E. Relationships between dietary intake and cognitive function level in Korean elderly people. Public Health 2001, 115, 133–138. [Google Scholar] [CrossRef]
- Requejo, A.M.; Ortega, R.M.; Robles, F.; Navia, B.; Faci, M.; Aparicio, A. Influence of nutrition on cognitive function in a group of elderly, independently living people. Eur. J. Clin. Nutr. 2003, 57, S54–S57. [Google Scholar] [CrossRef]
- Rahman, A.; Baker, P.S.; Allman, R.M.; Zamrini, E. Dietary factors and cognitive impairment in community-dwelling elderly. J. Nutr. Health Aging 2007, 11, 49–54. [Google Scholar]
- Albanese, E.; Dangour, A.D.; Uauy, R.; Acosta, D.; Guerra, M.; Guerra, S.S.G.; Huang, Y.; Jacob, K.S.; Rodriguez, J.J.L.; Noriega, L.H.; et al. Dietary fish and meat intake and dementia in Latin America, China, and India: A 10/66 Dementia Research Group population-based study. Am. J. Clin. Nutr. 2009, 90, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Vizuete, A.A.; Robles, F.; Rodríguez-Rodríguez, E.; López-Sobaler, A.M.; Ortega, R.M. Association between food and nutrient intakes and cognitive capacity in a group of institutionalized elderly people. Eur. J. Nutr. 2009, 49, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dong, B.; Zeng, G.; Li, J.; Wang, W.; Wang, B.; Yuan, Q. Is there an association between mild cognitive impairment and dietary pattern in chinese elderly? Results from a cross-sectional population study. BMC Public Health 2010, 10, 595. [Google Scholar] [CrossRef] [PubMed]
- Katsiardanis, K.; Diamantaras, A.-A.; Dessypris, N.; Michelakos, T.; Anastasiou, A.; Katsiardani, K.-P.; Kanavidis, P.; Papadopoulos, F.C.; Stefanadis, C.; Panagiotakos, D.B.; et al. Cognitive Impairment and Dietary Habits Among Elders: The Velestino Study. J. Med. Food 2013, 16, 343–350. [Google Scholar] [CrossRef]
- Crichton, G.E.; Bryan, J.; Hodgson, J.M.; Murphy, K.J. Mediterranean diet adherence and self-reported psychological functioning in an Australian sample. Appetite 2013, 70, 53–59. [Google Scholar] [CrossRef]
- Bajerska, J.; Woźniewicz, M.; Suwalska, A.; Jeszka, J. Eating patterns are associated with cognitive function in the elderly at risk of metabolic syndrome from rural areas. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3234–3245. [Google Scholar]
- França, V.F.; Barbosa, A.R.; D’Orsi, E. Cognition and Indicators of Dietary Habits in Older Adults from Southern Brazil. PLoS ONE 2016, 11, e0147820. [Google Scholar] [CrossRef][Green Version]
- Brouwer-Brolsma, E.M.; Benati, A.; Van De Wiel, A.; Van Lee, L.; De Vries, J.H.; Feskens, E.J.; Van De Rest, O. Higher Mediterranean Diet scores are not cross-sectionally associated with better cognitive scores in 20- to 70-year-old Dutch adults: The NQplus study. Nutr. Res. 2018, 59, 80–89. [Google Scholar] [CrossRef]
- Rocaspana-García, M.; Blanco-Blanco, J.; Arias-Pastor, A.; Gea-Sánchez, M.; Piñol-Ripoll, G. Study of community-living Alzheimer’s patients’ adherence to the Mediterranean diet and risks of malnutrition at different disease stages. PeerJ 2018, 6, e5150. [Google Scholar] [CrossRef]
- França, V.F.; Azzolini, T.; Pissaia, E.; Bortoloti, D.S.; Signorini, T.; Costa, L.D.; Souza, M.M.Q.; Lívero, F.A.D.R.; Lovato, E.C.W. Diet, Epidemiological Factors and Cognitive Impairment: A Cross-Sectional Study in the Elderly Population. Braz. Arch. Boil. Technol. 2018, 61. [Google Scholar] [CrossRef]
- Charlton, K.; Walton, K.; Batterham, M.; Brock, E.; Langford, K.; McMahon, A.; Roodenrys, S.; Koh, F.; Host, A.; Crowe, R.; et al. Pork and Chicken Meals Similarly Impact on Cognitive Function and Strength in Community-Living Older Adults: A Pilot Study. J. Nutr. Gerontol. Geriatr. 2016, 35, 124–145. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E. Diet and Dementia, is there a Link? A Systematic Review. Nutr. Neurosci. 1999, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzi, V.; Custodero, C.; Lozupone, M.; Imbimbo, B.P.; Valiani, V.; Agosti, P.; Schilardi, A.; D’Introno, A.; La Montagna, M.; Calvani, M.; et al. Relationships of Dietary Patterns, Foods, and Micro- and Macronutrients with Alzheimer’s Disease and Late-Life Cognitive Disorders: A Systematic Review. J. Alzheimers Dis. 2017, 59, 815–849. [Google Scholar] [CrossRef] [PubMed]
- Custodero, C.; Valiani, V.; Agosti, P.; Schilardi, A.; D’Introno, A.; Lozupone, M.; La Montagna, M.; Panza, F.; Solfrizzi, V.; Sabbà, C. Dietary patterns, foods, and food groups: Relation to late-life cognitive disorders. J. Gerontol. Geriatr. 2018, 66, 75–86. [Google Scholar]
- Van De Rest, O.; Van Der Zwaluw, N.L.; De Groot, L.C.P.G.M. Literature review on the role of dietary protein and amino acids in cognitive functioning and cognitive decline. Amino Acids 2013, 45, 1035–1045. [Google Scholar] [CrossRef]
- Ballard, C.; Gauthier, S.; Corbett, A.; Brayne, C.; Aarsland, D.; Jones, E. Alzheimer’s disease. Lancet 2011, 377, 1019–1031. [Google Scholar] [CrossRef]
- Hooda, J.; Shah, A.; Zhang, L. Heme, an Essential Nutrient from Dietary Proteins, Critically Impacts Diverse Physiological and Pathological Processes. Nutrients 2014, 6, 1080–1102. [Google Scholar] [CrossRef]
- Ritchie, S.J.; Dickie, D.A.; Cox, S.R.; Hernández, M.D.C.V.; Corley, J.; Royle, N.A.; Pattie, A.; Aribisala, B.S.; Redmond, P.; Maniega, S.M.; et al. Brain volumetric changes and cognitive ageing during the eighth decade of life. Hum. Brain Mapp. 2015, 36, 4910–4925. [Google Scholar] [CrossRef]
- Fletcher, E.; Gavett, B.E.; Harvey, D.; Farias, S.T.; Olichney, J.; Beckett, L.; DeCarli, C.; Mungas, D. Brain volume change and cognitive trajectories in aging. Neuropsychology 2018, 32, 436–449. [Google Scholar] [CrossRef]
- Polvikoski, T.; Kainulainen, K.; Vuorio, A.; Verkkoniemi, A.; Niinistö, L.; Halonen, P.; Sulkava, R.; Haltia, M.; Kontula, K. Apolipoprotein E, Dementia, and Cortical Deposition of β-Amyloid Protein. N. Engl. J. Med. 1995, 333, 1242–1248. [Google Scholar] [CrossRef]
- Staubo, S.C.; Aakre, J.A.; Vemuri, P.; Syrjanen, J.A.; Mielke, M.M.; Geda, Y.E.; Kremers, W.K.; Machulda, M.M.; Knopman, D.S.; Petersen, R.C.; et al. Mediterranean diet, micronutrients and macronutrients, and MRI measures of cortical thickness. Alzheimers Dement. 2016, 13, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Brickman, A.M.; Stern, Y.; Habeck, C.G.; Razlighi, Q.R.; Luchsinger, J.; Manly, J.J.; Schupf, N.; Mayeux, R.; Scarmeas, N. Mediterranean diet and brain structure in a multiethnic elderly cohort. Neurology 2015, 85, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Luciano, M.; Corley, J.; Cox, S.R.; Hernández, M.C.V.; Craig, L.C.; Dickie, D.A.; Karama, S.; McNeill, G.; Bastin, M.E.; Wardlaw, J.M.; et al. Mediterranean-type diet and brain structural change from 73 to 76 years in a Scottish cohort. Neurology 2017, 88, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Vassilaki, M.; Aakre, J.A.; Syrjanen, J.A.; Mielke, M.M.; Geda, Y.E.; Kremers, W.K.; Machulda, M.M.; Alhurani, R.E.; Staubo, S.C.; Knopman, D.S.; et al. Mediterranean Diet, Its Components, and Amyloid Imaging Biomarkers. J. Alzheimers Dis. 2018, 64, 281–290. [Google Scholar] [CrossRef]
- Hattemer-Frey, H.A.; Travis, C.C. Benzo-a-Pyrene: Environmental Partitioning and Human Exposure. Toxicol. Ind. Health 1991, 7, 141–157. [Google Scholar] [CrossRef]
- Niu, Q.; Zhang, H.; Li, X.; Li, M. Benzo[a]pyrene-induced neurobehavioral function and neurotransmitter alterations in coke oven workers. Occup. Environ. Med. 2009, 67, 444–448. [Google Scholar] [CrossRef]
- Van De Rest, O.; Berendsen, A.A.; Haveman-Nies, A.; De Groot, L.C. Dietary patterns, cognitive decline, and dementia: A systematic review. Adv. Nutr. 2015, 6, 154–168. [Google Scholar] [CrossRef]
- Petersson, S.D.; Philippou, E. Mediterranean Diet, Cognitive Function, and Dementia: A Systematic Review of the Evidence. Adv. Nutr. 2016, 7, 889–904. [Google Scholar] [CrossRef]
- Aridi, Y.S.; Walker, J.L.; Wright, O.R.L. The Association between the Mediterranean Dietary Pattern and Cognitive Health: A Systematic Review. Nutrients 2017, 9, 674. [Google Scholar] [CrossRef]
- Chen, X.; Maguire, B.; Brodaty, H.; O’Leary, F. Dietary Patterns and Cognitive Health in Older Adults: A Systematic Review. J. Alzheimers Dis. 2019, 67, 583–619. [Google Scholar] [CrossRef]
| Author, Year [Ref] | Country, Study Name | Follow-Up, Year | Sample Size (Female/Male) | Age 1 (Mean ± SD/Range) | Exposure Measures | Outcomes (Measure Methods) | Effects | Quality Scores |
|---|---|---|---|---|---|---|---|---|
| Cohort studies | ||||||||
| Barberger-Gateau et al., 2002 [24] | France, PAQUID | 7 | 1416 (Not Reported) | ≥ 68 | Frequency of consumption of meat | Dementia (MMSE), AD (DSM-III-R) | No significant association between meat consumption and risk of dementia (P-trend = 0.59, adjusted HR = 0.56, 95% CI 0.26–1.20, for weekly consumers). | 6 |
| Barberger-Gateau et al., 2007 [25] | France, The Three-City cohort study (3C) | 4 | 8085 (Not Reported) | ≥ 65 | FFQ including meat | Dementia (neuropsychological tests and DSM-IV), AD (NINCDS-ADRDA) | No association between risk for all cause dementia and meat consumption (p > 0.25) adjusted for age. | 7 |
| Vercambre et al., 2009 [26] | France, Etude Epidemiologique de Femmes de la Mutuelle Generale de Education Nationale (E3N) | 13 | 4809 (4809/0) | 65·5 ± 1·8 | 208-item FFQ including red meat, offal, processed meat, poultry | Recent cognitive decline (Deterioration Cognitive Observee questionnaire (observed cognitive deterioration), DECO) | High intake of poultry reduced risk of recent cognitive decline (>median consumption vs. no consumption: aOR = 0.73, 95% CI, 0.58–0.91, P-trend = 0.004); but offal, red or processed meat did not. | 7 |
| Chen et al., 2012 [10] | China, The Chinese Longitudinal Health Longevity Study (CLHLS) | 3 | 5691 (4302/1389) | 82.94 ± 11.03 | Frequency of meat intake (pork, beef, mutton, and poultry) | Cognitive decline (MMSE) | Always meat intake (around daily) could reduce the risk of cognitive decline in bivariate regression model (unadjusted OR = 0.71, 95% CI 0.56–0.89, P = 0.0029), but no significant associations emerged for meat intake in adjusted models. | 6 |
| Samieri, et al., 2013 [27] | USA, Women’s Health Study | 4 | 6174 (6174/0) | 71.9 ± 4.1 | 131-item FFQ including meat | Global cognitive score (telephone adapted MMSE), verbal memory (the East Boston memory test) | No significant association between red and processed meat consumption and mean score of global cognition (P-trend = 0.16) or verbal memory (P-trend = 0.15). | 6 |
| Titova et al., 2013 [9] | Sweden, Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) | 5 | 194 (93/101) | 70 | 7-day dietary records including amounts of meat | Cognitive score (seven-minute screening, 7MS) | A low consumption of meat and meat products was linked to a better performance on the 7MS test (β coefficient = −0.26, P < 0.001). | 5 |
| Wengreen et al., 2013 [28] | USA, The Cache County Memory Study (CCMS) | 11 | 3580 (Not Reported) | ≥65 | 142-item FFQ over past year including meat | Cognitive score (modified MMSE, 3MS) | No significant association between increasing quintiles of red and processed meat and higher 3MS scores (P-linear trend = 0.2796). | 5 |
| Ashby-Mitchell et al., 2015 [29] | Australia, AusDiab study | 12 | 577 (284/293) | 66.07 ± 4.85 | 101-item FFQ over past year including meat | Cognitive impairment (MMSE) | No association between odds of cognitive impairment and meat consumption (aOR = 1.005, 95% CI 0.964–1.048). | 5 |
| Crichton et al., 2015 [11] | USA, The Maine Syracuse Longitudinal Study (MSLS) | 18 ± 5.3 | 333 (Not Reported) | 60.5 ± 12.8 | 37-item FFQ including meat | Cognitive score (the Wechsler adult intelligence scale, WAIS) | Higher WAIS Scores at baseline were prospectively associated with higher intakes of meats (β coefficient = 0.062, se = 0.012, P < 0.001). | 8 |
| Trichopoulou et al., 2015 [30] | Greece, the European Prospective Investigation into Cancer and Nutrition (EPIC) -Greece cohort | 6.6 | 401 (257/144) | Mean = 74 | FFQ including meat | Improved or unchanged score (cMMSE ≥ 0), mildly lower score (cMMSE −4 to −1), substantially lower score (cMMSE ≤ −5) | No significant odds of having mildly lower score (aOR = 1.14, 95% CI 0.89–1.47) or substantially lower score (aOR = 1.09, 95% CI 0.71–1.69) for an increment of one SD of meat intake. | 5 |
| Fischer et al., 2018 [31] | Germany, The German Study on Ageing, Cognition and Dementia in Primary Care Patients (AgeCoDe) | 4.5 | 2622 (1712/910) | 81.2 ± 3.4 | Single-food-questionnaire on frequency of use of red meat and sausages | AD (DSM-IV and ICD-10), memory decline (CERAD neuropsychological assessment battery) | No significant association was detected between frequency of meat and sausage with incident AD (adjusted HR: 1.09, 95% CI 0.94–1.26, p = 0.236) or memory decline (adjusted β = 0.01, 95% CI −0.11 −0.14, p = 0.845) | 9 |
| Zhu et al. 2018 [32] | China, The Shanghai Women’s Health Study and Shanghai Men’s Health Study (SWHS and SMHS) | 14.4 | 30,484 (18,458/12,026) | 70–86 | FFQ over past year including meat | Questions on memory, and decision-making ability: no, minor, or serious impairments | High red meat intake (fourth quintile: 44.7–64.3 g/d for women, 52.9–75.8 g/d for men) was associated with a lower likelihood of impairments in memory (aOR = 0.86, 95% CI: 0.75, 0.99), and decision-making (aOR = 0.82, 95% CI: 0.72, 0.93). | 6 |
| Case-control studies | ||||||||
| Baker et al., 1993 [33] | USA | _ | 72 (50/22) | 75.4 | Frequency of beef or pork intake | Clinically diagnosed AD cases (McKnann criteria) | No association between the daily or weekly use of beef or pork with a risk for clinically diagnosed AD (aOR = 4.0, CI = 0.30–∞, p = 0.37). | 5 |
| Zhao et al., 2015 [34] | China | _ | 404 (Not Reported) | 60–90 | FFQ including meat | MCI (Montreal cognitive assessment, MoCA) | No difference (P > 0.05) in meat intake (pork, beef and mutton) between MCI cases (45.8 ± 3.9 g/d) and controls (52.5 ± 3.4 g/d). | 4 |
| Dong et al., 2016 [35] | China | _ | 894 (604/290) | 62.9 ± 5.25 | 41-item FFQ including meat and poultry | Cognitive score (Montreal cognitive assessment, MoCA) | No significant association was detected between intake of poultry, red meat with MoCA (P > 0.05). | 5 |
| Cross-sectional studies | ||||||||
| Lee et al., 2001 [36] | Korea | _ | 449 (239/210) | 60–83 | 24 h dietary recall | Cognitive score (MMSE for Korea) | No significant correlations between MMSE score and meat intake (Correlation coefficients: −0.004 for men 0.096 for women) | 6 |
| Requejo et al., 2003 [37] | Spain | _ | 168 (Not Reported) | 65–90 | 7-day food record | Cognitive decline (MMSE) | No significant difference in meat consumption between MMSE ≥ 28 group and MMSE < 28 group with being stratified by age (p > 0.1). | 5 |
| Rahman et al., 2007 [38] | USA | _ | 1056 (708/348) | 69 ± 8.9 | Frequency of consumption of meat | Cognitive decline (mental status questionnaire, MSQ) | No association between risk of cognitive impairment and intakes of meat (aOR = 0.11, 95% CI: 0.67, 1.84). | 9 |
| Albanese et al., 2009 [39] | Latin America, China, and India | _ | 14,960 (Not Reported) | ≥65 | Frequency of average weekly meat intake | Dementia (the 10/66 diagnostic algorithm) | A less-consistent, dose-dependent, direct association between meat consumption and prevalence of dementia (adjusted PR: 1.19; 95% CI: 1.07, 1.31). | 10 |
| Aránzazu et al., 2010 [40] | Spain | _ | 178 (Not Reported) | 65–97 | 7 consecutive days food record | Cognitive score (short portable mental state questionnaire, SPMSQ) | The intake of meat correlated with a greater number of errors incurred (Correlation coefficient: r2 = 0.1086; p < 0.001). | 3 |
| Wang et al., 2010 [41] | China, Project of Longevity and Aging in Dujiangyan (PLAD) | _ | 364 (204/160) | 93.02 ± 3.01 | Frequency of consumption of meat | MCI (MMSE) | No significant association was detected in both unadjusted and adjusted models (aOR = 1.01, 95% CI 0.92–1.10). | 7 |
| Katsiardanis et al., 2013 [42] | Greece | _ | 557 (320/237) | >65 | 157-item FFQ | Cognitive impairment (MMSE) | No association between meat and meat products with the presence of cognitive impairment (aOR = 0.96, 95% CI 0.81–1.16 for women; aOR = 1.03, 95% CI 0.84–1.27 for men). | 6 |
| Crichton et al., 2013 [43] | Australia | _ | 1183 (751/432) | 40–65 | 215-item FFQ | Cognitive failures questionnaire (CFQ); Memory Functioning Questionnaire (MFQ) | No associations between CFQ score and MFQ score with consumption of meat (P > 0.05). | 6 |
| Bajerska, et al., 2014 [44] | Poland | _ | 87 (Not Reported) | ≥60 | Frequency and potion size of meat and meat products intake over the last month | Global cognitive (MMSE), executive function (cognitive test battery) | The consumption of red meat and meat products was negatively related to executive function (β = −0.02, 95% CI: −0.03–−0.007, standardized β = −0.33, p = 0.01) and global cognition (β = −0.02, 95% CI: −0.04–−0.007, standardized β = −0.25, P = 0.01). | 6 |
| Franca et al., 2016 [45] | Brazil, The EpiFloripa Elderly survey | _ | 1197 (778/419) | 73.9 ± 19.3 | Habitual intake of red meat with fat or chicken with skin (yes/no) | Cognition score (MMSE) | No significant association was detected between intake of red meat with fat or chicken with skin and MMSE scores both in women and men (P ≥ 0.057). | 7 |
| Brouwer-Brolsma et al., 2018 [46] | Netherlands, Nutrition Questionnaires plus (NQplus) study | _ | 1607 (770/837) | Mean = 52.9 | 183-item FFQ over past 4 weeks | Semantic memory and language production (letter fluency test, LFT; processing speed (symbol digit modalities test, SDMT); everyday memory (story recall test, SRT) | The meat intake was negatively related to LFT score (β = −0.006, se = 0.002, p = 0.007), SDMT score (β = −0.011, se = 0.005, p = 0.02), and SRT score (β = −0.003, se = 0.002, p = 0.14) in unadjusted model but not in adjusted models. | 6 |
| Rocaspana-García et al., 2018 [47] | Spain | _ | 111 (70/41) | 78.5 ± 6.4 | 45-item FFQ | AD patients diagnosed in hospital | Almost half of the AD patients (46.8%) ate more meat than recommended. | 3 |
| Franca et al., 2018 [48] | Brazil | _ | 400 (288/112) | ≥60 | Habitual intake of red meat with fat or chicken with skin (yes/no) | Cognition deficit (MMSE) | No significant association was detected between cognitive deficit and intake of red meat with fat (aOR = 1.053, 95% CI 0.568–1.952) or chicken with skin (aOR = 0.952, 95% CI 0.505–1.793). | 6 |
| Intervention studies | ||||||||
| Charlton et al., 2016 [49] | Australia | 12 weeks | 31 (Not Reported) | 78.0 ± 6.2 | Intervention: Pork meals; Control: chicken meals | Cognitive score (cognitive test battery) | No significant cognition change in the pork intervention group over the 12 weeks, while the chicken group had improved verbal learning and memory at six weeks (p < 0.001). | 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Hardie, L.; Bawajeeh, A.O.; Cade, J. Meat Consumption, Cognitive Function and Disorders: A Systematic Review with Narrative Synthesis and Meta-Analysis. Nutrients 2020, 12, 1528. https://doi.org/10.3390/nu12051528
Zhang H, Hardie L, Bawajeeh AO, Cade J. Meat Consumption, Cognitive Function and Disorders: A Systematic Review with Narrative Synthesis and Meta-Analysis. Nutrients. 2020; 12(5):1528. https://doi.org/10.3390/nu12051528
Chicago/Turabian StyleZhang, Huifeng, Laura Hardie, Areej O. Bawajeeh, and Janet Cade. 2020. "Meat Consumption, Cognitive Function and Disorders: A Systematic Review with Narrative Synthesis and Meta-Analysis" Nutrients 12, no. 5: 1528. https://doi.org/10.3390/nu12051528
APA StyleZhang, H., Hardie, L., Bawajeeh, A. O., & Cade, J. (2020). Meat Consumption, Cognitive Function and Disorders: A Systematic Review with Narrative Synthesis and Meta-Analysis. Nutrients, 12(5), 1528. https://doi.org/10.3390/nu12051528





