Anxiolytic Activity and Brain Modulation Pattern of the α-Casozepine-Derived Pentapeptide YLGYL in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Studied Compounds and Peptides
2.2. Animals
2.3. Experiment 1: Evaluation of Anxiolytic-Like Properties of YLGYL
2.4. Experiment 2: Evaluation of YLGYL Effects on Neuronal Activation
2.5. Statistical Analysis
3. Results
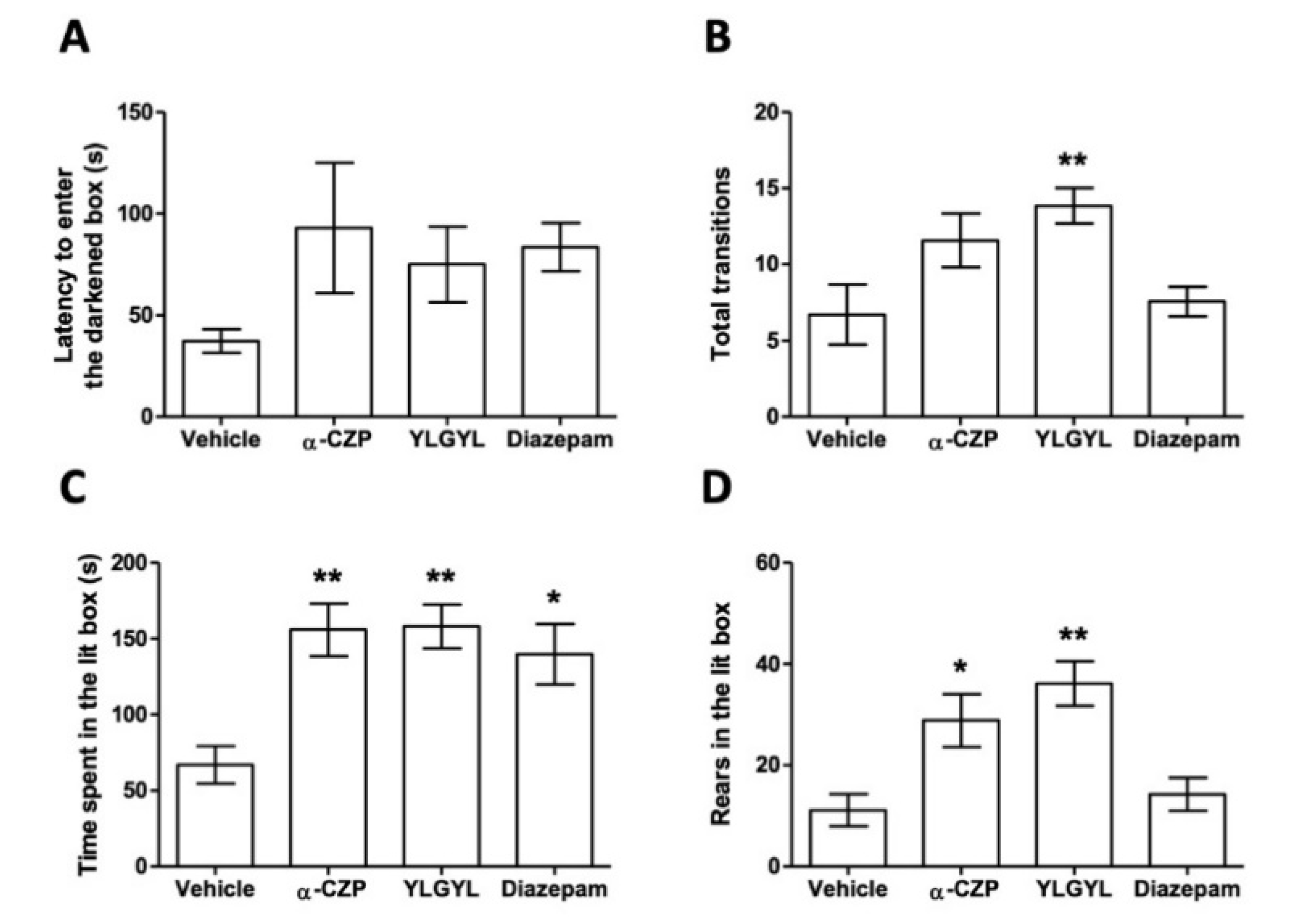
3.1. Anxiolytic-Like Activity of YLGYL in the LDB.
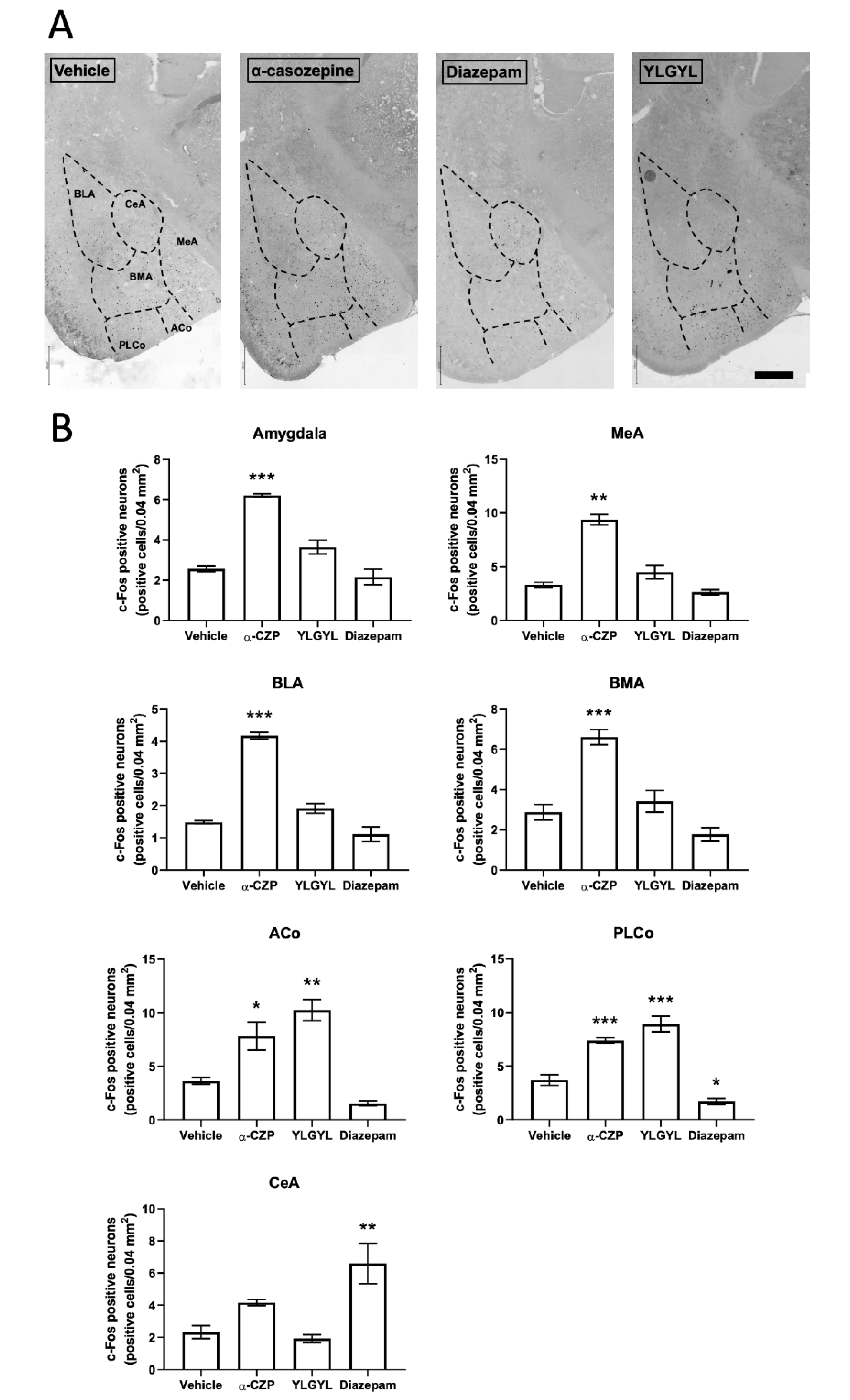
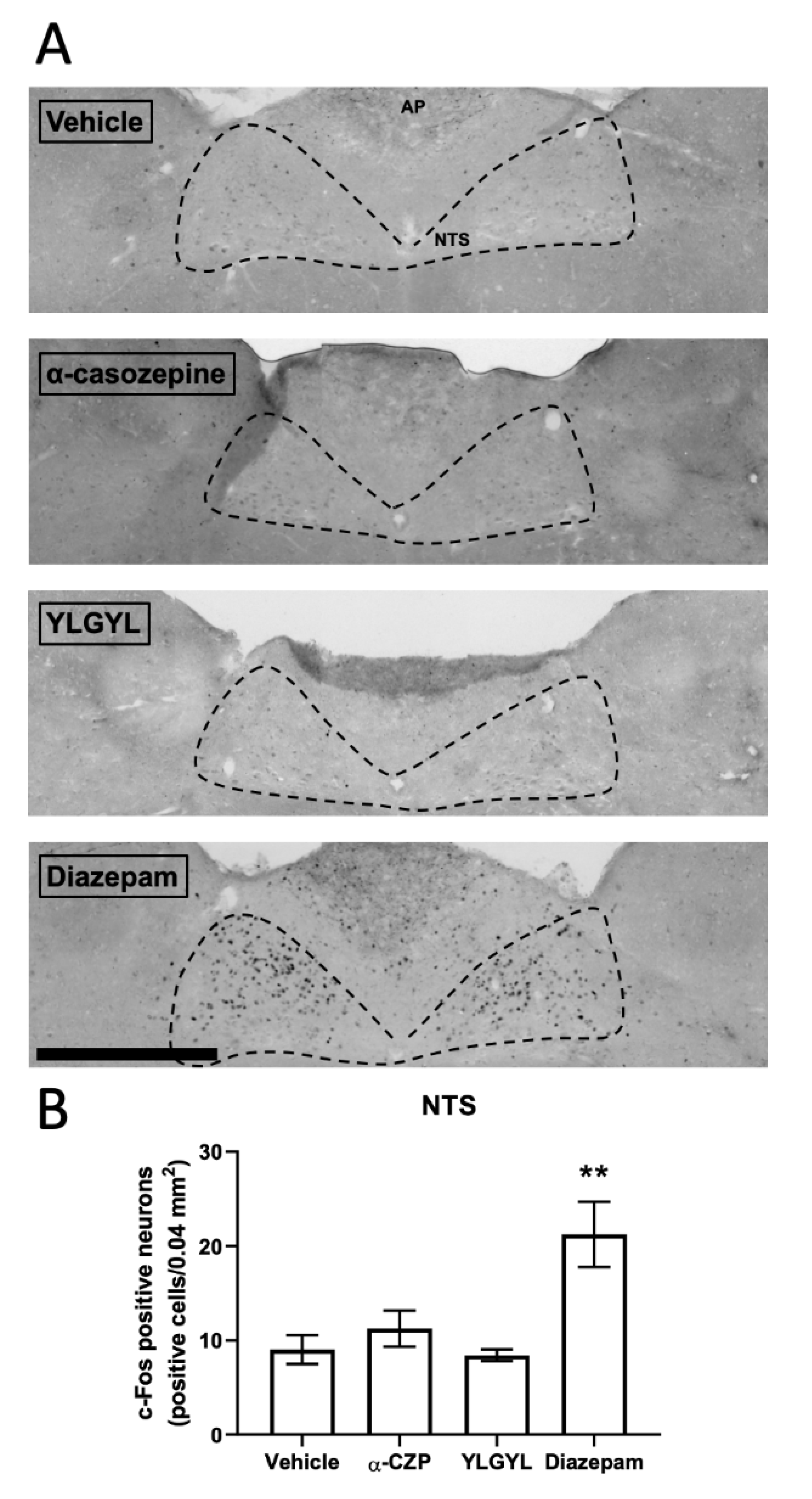
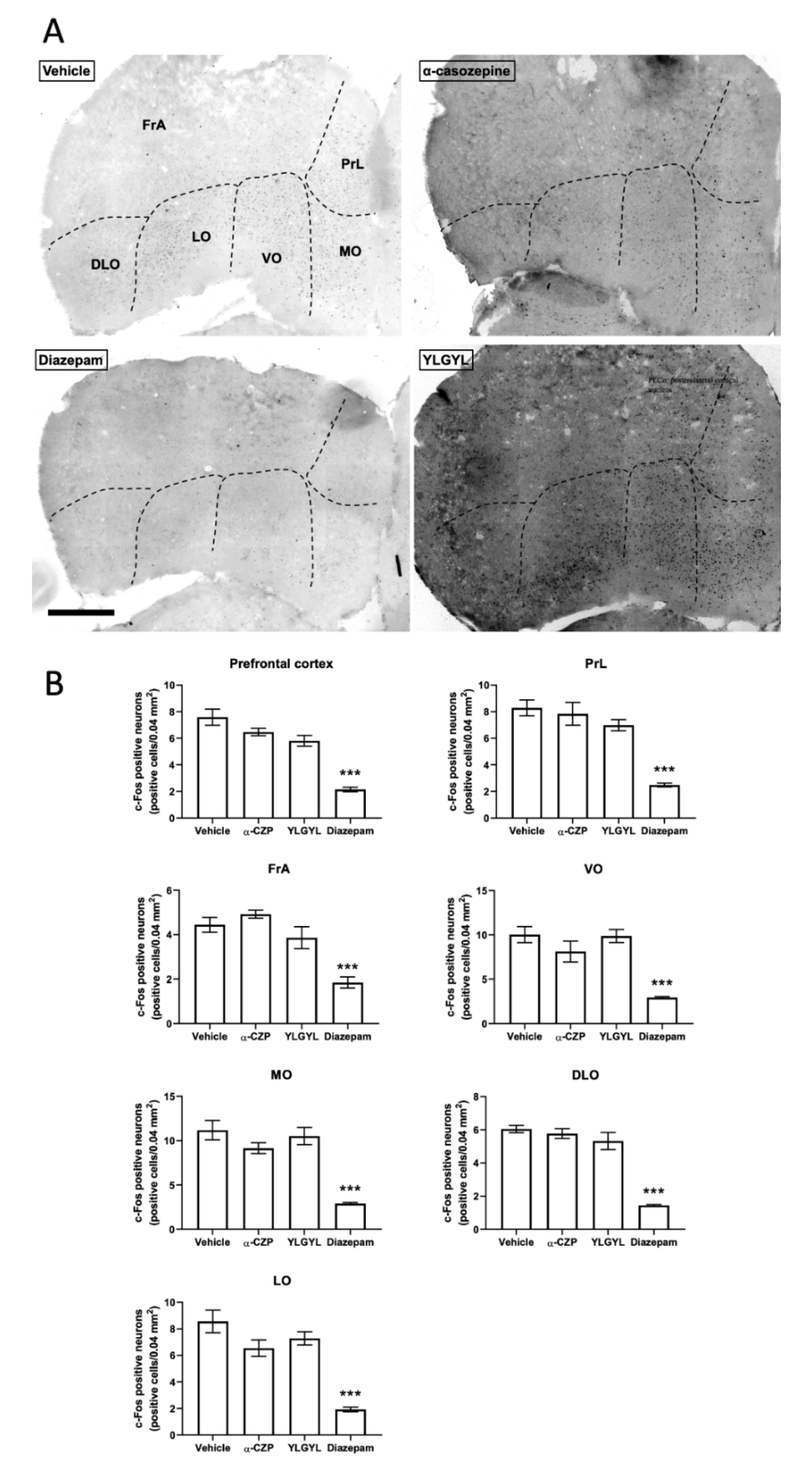
3.2. Modulation of Neuronal Activity induced by Intraperitoneal Injection of either YLGYL, α-CZP, or Diazepam in an Anxiety-Inducing Situation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miclo, L.; Perrin, E.; Driou, A.; Papadopoulos, V.; Boujrad, N.; Vanderesse, R.; Boudier, J.F.; Desor, D.; Linden, G.; Gaillard, J.-L. Characterization of alpha-casozepine, a tryptic peptide from bovine alpha(s1)-casein with benzodiazepine-like activity. FASEB J. 2001, 15, 1780–1782. [Google Scholar] [CrossRef]
- Messaoudi, M.; Lalonde, R.; Schroeder, H.; Desor, D. Anxiolytic-like effects and safety profile of a tryptic hydrolysate from bovine alpha s1-casein in rats. Fundam. Clin. Pharmacol. 2009, 23, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Beata, C.; Beaumont-Graff, E.; Coll, V.; Cordel, J.; Marion, M.; Massal, N.; Marlois, N.; Tauzin, J. Effect of alpha-casozepine (Zylkene) on anxiety in cats. J. Vet. Behav. Clin. Appl. Res. 2007, 2, 40–46. [Google Scholar] [CrossRef]
- Beata, C.; Beaumont-Graff, E.; Diaz, C.; Marion, M.; Massal, N.; Marlois, N.; Muller, G.; Lefranc-Millot, C. Effects of alpha-casozepine (Zylkene) versus selegiline hydrochloride (Selgian, Anipryl) on anxiety disorders in dogs. J. Vet. Behav. Clin. Appl. Res. 2007, 2, 175–183. [Google Scholar] [CrossRef]
- McDonnell, S.M.; Miller, J.; Vaala, W. Calming Benefit of Short-Term Alpha-Casozepine Supplementation During Acclimation to Domestic Environment and Basic Ground Training of Adult Semi-Feral Ponies. J. Equine Vet. Sci. 2013, 33, 101–106. [Google Scholar] [CrossRef]
- McDonnell, S.M.; Miller, J.; Vaala, W. Modestly improved compliance and apparent comfort of horses with aversions to mildly-aversive routine healthcare procedures following short-term alpha-casozepine supplementation. J. Equine Vet. Sci. 2014, 34, 1016–1020. [Google Scholar] [CrossRef]
- Palestrini, C.; Minero, M.; Cannas, S.; Berteselli, G.; Scaglia, E.; Barbieri, S.; Cavallone, E.; Puricelli, M.; Servida, F.; Dall’Ara, P. Efficacy of a diet containing caseinate hydrolysate on signs of stress in dogs. J. Vet. Behav. Clin. Appl. Res. 2010, 5, 309–317. [Google Scholar] [CrossRef]
- Violle, N.; Messaoudi, M.; Lefranc-Millot, C.; Desor, D.; Nejdi, A.; Demagny, B.; Schroeder, H. Ethological comparison of the effects of a bovine alpha s1-casein tryptic hydrolysate and diazepam on the behaviour of rats in two models of anxiety. Pharmacol. Biochem. Behav. 2006, 84, 517–523. [Google Scholar] [CrossRef]
- Guesdon, B.; Messaoudi, M.; Lefranc-Millot, C.; Fromentin, G.; Tomé, D.; Even, P.C. A tryptic hydrolysate from bovine milk alphaS1-casein improves sleep in rats subjected to chronic mild stress. Peptides 2006, 27, 1476–1482. [Google Scholar] [CrossRef]
- Dela Peña, I.J.I.; Kim, H.J.; de la Peña, J.B.; Kim, M.; Botanas, C.J.; You, K.Y.; Woo, T.; Lee, Y.S.; Jung, J.-C.; Kim, K.-M.; et al. A tryptic hydrolysate from bovine milk αs1-casein enhances pentobarbital-induced sleep in mice via the GABAA receptor. Behav. Brain Res. 2016, 313, 184–190. [Google Scholar] [CrossRef]
- Lanoir, D.; Canini, F.; Messaoudi, M.; Lefranc-Millot, C.; Demagny, B.; Martin, S.; Bourdon, L. Long term effects of a bovine milk alpha-s1 casein hydrolysate on healthy low and high stress responders. Stress 2002, 5, 124. [Google Scholar]
- Messaoudi, M.; Lefranc-Millot, C.; Desor, D.; Demagny, B.; Bourdon, L. Effects of a tryptic hydrolysate from bovine milk alphaS1-casein on hemodynamic responses in healthy human volunteers facing successive mental and physical stress situations. Eur. J. Nutr. 2005, 44, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Desor, D.; Kim, Y.T.; Yoon, W.J.; Kim, K.S.; Jun, J.S.; Pyun, K.H.; Shim, I. Efficacy of alphas1-casein hydrolysate on stress-related symptoms in women. Eur. J. Clin. Nutr. 2007, 61, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Cakir-Kiefer, C.; Le Roux, Y.; Balandras, F.; Trabalon, M.; Dary, A.; Laurent, F.; Gaillard, J.-L.; Miclo, L. In vitro digestibility of α-casozepine, a benzodiazepine-like peptide from bovine casein, and biological activity of its main proteolytic fragment. J. Agric. Food Chem. 2011, 59, 4464–4472. [Google Scholar] [CrossRef] [PubMed]
- Mizushige, T.; Sawashi, Y.; Yamada, A.; Kanamoto, R.; Ohinata, K. Characterization of Tyr-Leu-Gly, a novel anxiolytic-like peptide released from bovine αS-casein. FASEB J. 2013, 27, 2911–2917. [Google Scholar] [CrossRef] [PubMed]
- Benoit, S.; Chaumontet, C.; Schwarz, J.; Cakir-Kiefer, C.; Tomé, D.; Miclo, L. Mapping in mice the brain regions involved in the anxiolytic-like properties of α-casozepine, a tryptic peptide derived from bovine α s1 -casein. J. Funct. Foods 2017, 38, 464–473. [Google Scholar] [CrossRef]
- Cakir-Kiefer, C.; Miclo, L.; Balandras, F.; Dary, A.; Soligot, C.; Le Roux, Y. Transport across Caco-2 cell monolayer and sensitivity to hydrolysis of two anxiolytic peptides from αs1-casein, α-casozepine, and αs1-casein-f91-97: Effect of bile salts. J. Agric. Food Chem. 2011, 59, 11956–11965. [Google Scholar] [CrossRef]
- Kovács, K.J. c-Fos as a transcription factor: A stressful (re)view from a functional map. Neurochem. Int. 1998, 33, 287–297. [Google Scholar] [CrossRef]
- Chung, L. A Brief Introduction to the Transduction of Neural Activity into Fos Signal. Dev. Reprod. 2015, 19, 61–67. [Google Scholar] [CrossRef]
- Calhoon, G.G.; Tye, K.M. Resolving the neural circuits of anxiety. Nat. Neurosci. 2015, 18, 1394–1404. [Google Scholar] [CrossRef]
- Tovote, P.; Fadok, J.P.; Lüthi, A. Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci. 2015, 16, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Noori, H.R.; Spanagel, R.; Hansson, A.C. Neurocircuitry for modeling drug effects. Addict. Biol. 2012, 17, 827–864. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ; Bethesda: Rockville, MD, USA, 1997. [Google Scholar]
- Paxinos, G.; Franklin, K.B.J. The Mouse Brain In Stereotaxic Coordinates, 2nd ed.; Academic Press: Amsterdam, The Netherlands, 2001; ISBN 978-0125476362. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2013. [Google Scholar]
- Barbé, F.; Le Feunteun, S.; Rémond, D.; Ménard, O.; Jardin, J.; Henry, G.; Laroche, B.; Dupont, D. Tracking the in vivo release of bioactive peptides in the gut during digestion: Mass spectrometry peptidomic characterization of effluents collected in the gut of dairy matrix fed mini-pigs. Food Res. Int. 2014, 63, 147–156. [Google Scholar] [CrossRef]
- Egger, L.; Ménard, O.; Baumann, C.; Duerr, D.; Schlegel, P.; Stoll, P.; Vergères, G.; Dupont, D.; Portmann, R. Digestion of milk proteins: Comparing static and dynamic in vitro digestion systems with in vivo data. Food Res. Int. 2019, 118, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Boutrou, R.; Gaudichon, C.; Dupont, D.; Jardin, J.; Airinei, G.; Marsset-Baglieri, A.; Benamouzig, R.; Tomé, D.; Leonil, J. Sequential release of milk protein-derived bioactive peptides in the jejunum in healthy humans. Am. J. Clin. Nutr. 2013, 97, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Sanchón, J.; Fernández-Tomé, S.; Miralles, B.; Hernández-Ledesma, B.; Tomé, D.; Gaudichon, C.; Recio, I. Protein degradation and peptide release from milk proteins in human jejunum. Comparison with in vitro gastrointestinal simulation. Food Chem. 2018, 239, 486–494. [Google Scholar]
- Walther, B.; Lett, A.M.; Bordoni, A.; Tomás-Cobos, L.; Nieto, J.A.; Dupont, D.; Danesi, F.; Shahar, D.R.; Echaniz, A.; Re, R.; et al. GutSelf: Interindividual Variability in the Processing of Dietary Compounds by the Human Gastrointestinal Tract. Mol. Nutr. Food Res. 2019, 63, 1900677. [Google Scholar] [CrossRef]
- Lecouvey, M.; Frochot, C.; Miclo, L.; Orlewski, P.; Driou, A.; Linden, G.; Gaillard, J.L.; Marraud, M.; Cung, M.T.; Vanderesse, R. Two-dimensional 1H-NMR and CD structural analysis in a micellar medium of a bovine alphaS1-casein fragment having benzodiazepine-like properties. Eur. J. Biochem. 1997, 248, 872–878. [Google Scholar] [CrossRef]
- Kanegawa, N.; Suzuki, C.; Ohinata, K. Dipeptide Tyr-Leu (YL) exhibits anxiolytic-like activity after oral administration via activating serotonin 5-HT1A, dopamine D1 and GABAA receptors in mice. FEBS Lett. 2010, 584, 599–604. [Google Scholar] [CrossRef]
- Mizushige, T.; Kanegawa, N.; Yamada, A.; Ota, A.; Kanamoto, R.; Ohinata, K. Aromatic amino acid-leucine dipeptides exhibit anxiolytic-like activity in young mice. Neurosci. Lett. 2013, 543, 126–129. [Google Scholar] [CrossRef]
- Bourin, M.; Hascoët, M. The mouse light/dark box test. Eur. J. Pharmacol. 2003, 463, 55–65. [Google Scholar] [CrossRef]
- Yayeh, T.; Leem, Y.-H.; Kim, K.-M.; Jung, J.-C.; Schwarz, J.; Oh, K.-W.; Oh, S. Administration of Alphas1-Casein Hydrolysate Increases Sleep and Modulates GABAA Receptor Subunit Expression. Biomol. Ther. 2018, 26, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.J.; Lillian, A.; Hazum, E.; Cuatrecasas, P.; Chang, J.K. Morphiceptin (NH4-tyr-pro-phe-pro-COHN2): A potent and specific agonist for morphine (mu) receptors. Science 1981, 212, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Loukas, S.; Varoucha, D.; Zioudrou, C.; Streaty, R.A.; Klee, W. a Opioid activities and structures of alpha-casein-derived exorphins. Biochemistry 1983, 22, 4567–4573. [Google Scholar] [CrossRef] [PubMed]
- Barron, K.W.; Pavelka, S.M.; Garrett, K.M. Diazepam-sensitive GABA(A) receptors in the NTS participate in cardiovascular control. Brain Res. 1997, 773, 53–60. [Google Scholar] [CrossRef]
- Suzuki, M.; Nishina, M.; Nakamura, S.; Maruyama, K. Benzodiazepine-sensitive GABA(A) receptors in the commissural subnucleus of the NTS are involved in the carotid chemoreceptor reflex in rats. Auton. Neurosci. 2004, 110, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Carrive, P. The periaqueductal gray and defensive behavior: Functional representation and neuronal organization. Behav. Brain Res. 1993, 58, 27–47. [Google Scholar] [CrossRef]
- Millan, M.J. The neurobiology and control of anxious states. Prog. Neurobiol. 2003, 70, 83–244. [Google Scholar] [CrossRef]
- Saiyudthong, S.; Pongmayteegul, S.; Marsden, C.A.; Phansuwan-Pujito, P. Anxiety-like behaviour and c-fos expression in rats that inhaled vetiver essential oil. Nat. Prod. Res. 2015, 29, 2141–2144. [Google Scholar] [CrossRef]
- Shaw, D.; Norwood, K.; Leslie, J.C. Chlordiazepoxide and lavender oil alter unconditioned anxiety-induced c-fos expression in the rat brain. Behav. Brain Res. 2011, 224, 1–7. [Google Scholar] [CrossRef]
- Shoji, H.; Mizoguchi, K. Brain region-specific reduction in c-Fos expression associated with an anxiolytic effect of yokukansan in rats. J. Ethnopharmacol. 2013, 149, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Tye, K.M.; Prakash, R.; Kim, S.; Fenno, L.E.; Grosenick, L.; Zarabi, H.; Thompson, K.R.; Gradinaru, V.; Ramakrishnan, C.; Deisseroth, K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 2011, 471, 358–362. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benoit, S.; Chaumontet, C.; Schwarz, J.; Cakir-Kiefer, C.; Boulier, A.; Tomé, D.; Miclo, L. Anxiolytic Activity and Brain Modulation Pattern of the α-Casozepine-Derived Pentapeptide YLGYL in Mice. Nutrients 2020, 12, 1497. https://doi.org/10.3390/nu12051497
Benoit S, Chaumontet C, Schwarz J, Cakir-Kiefer C, Boulier A, Tomé D, Miclo L. Anxiolytic Activity and Brain Modulation Pattern of the α-Casozepine-Derived Pentapeptide YLGYL in Mice. Nutrients. 2020; 12(5):1497. https://doi.org/10.3390/nu12051497
Chicago/Turabian StyleBenoit, Simon, Catherine Chaumontet, Jessica Schwarz, Céline Cakir-Kiefer, Audrey Boulier, Daniel Tomé, and Laurent Miclo. 2020. "Anxiolytic Activity and Brain Modulation Pattern of the α-Casozepine-Derived Pentapeptide YLGYL in Mice" Nutrients 12, no. 5: 1497. https://doi.org/10.3390/nu12051497
APA StyleBenoit, S., Chaumontet, C., Schwarz, J., Cakir-Kiefer, C., Boulier, A., Tomé, D., & Miclo, L. (2020). Anxiolytic Activity and Brain Modulation Pattern of the α-Casozepine-Derived Pentapeptide YLGYL in Mice. Nutrients, 12(5), 1497. https://doi.org/10.3390/nu12051497




