Early Effect of Supplemented Infant Formulae on Intestinal Biomarkers and Microbiota: A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Objectives
2.2. Study Design
2.3. Study Population
2.4. Study Formulas, Randomisation, and Visits
2.5. Outcome Measures
2.5.1. Stool Parameters
2.5.2. Anthropometry and Body Composition
2.5.3. Iron Status
2.5.4. Digestive Tolerance
2.5.5. Adverse Events
2.6. Statistical Analysis
2.7. Ethics
3. Results
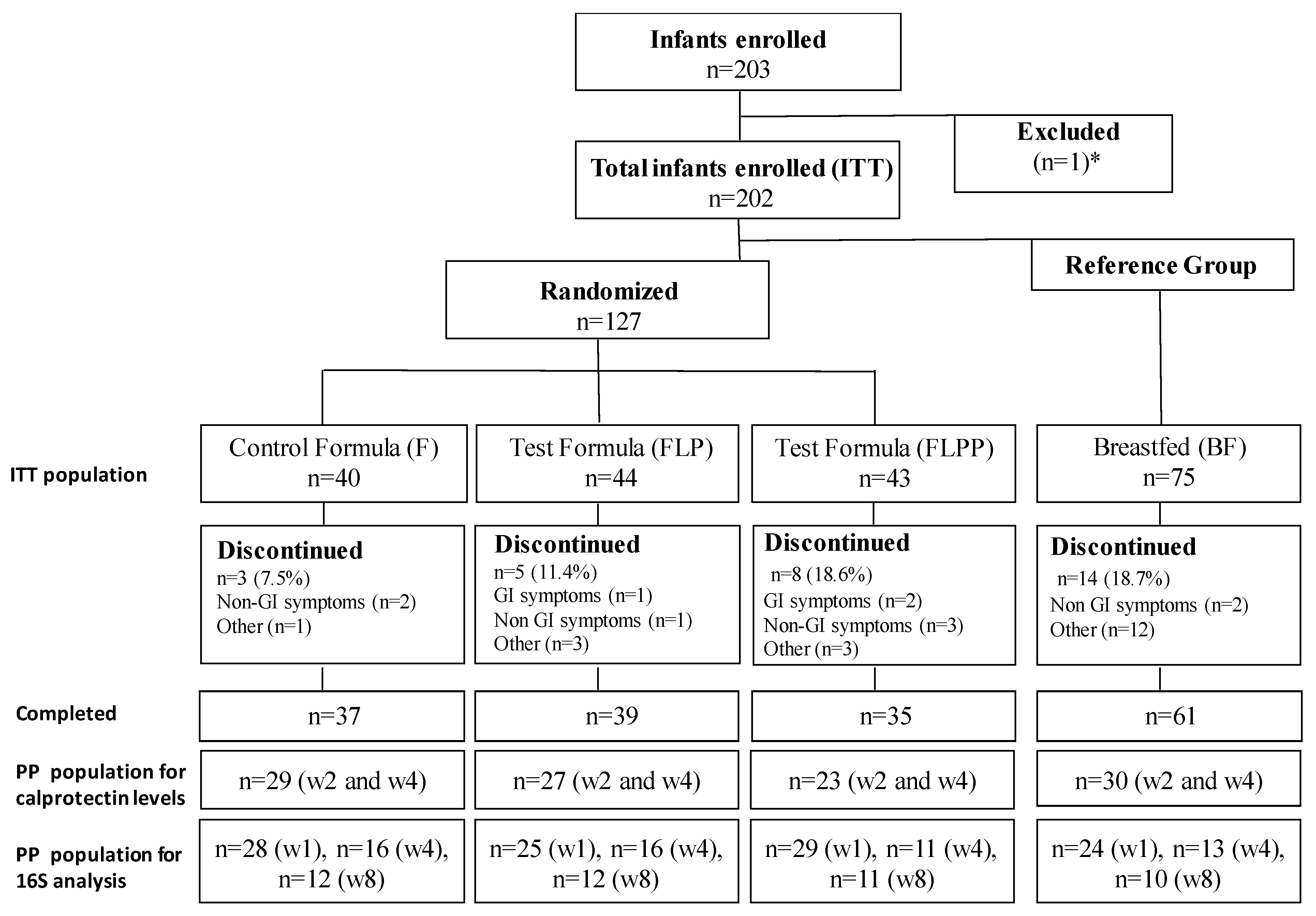
3.1. Study Population
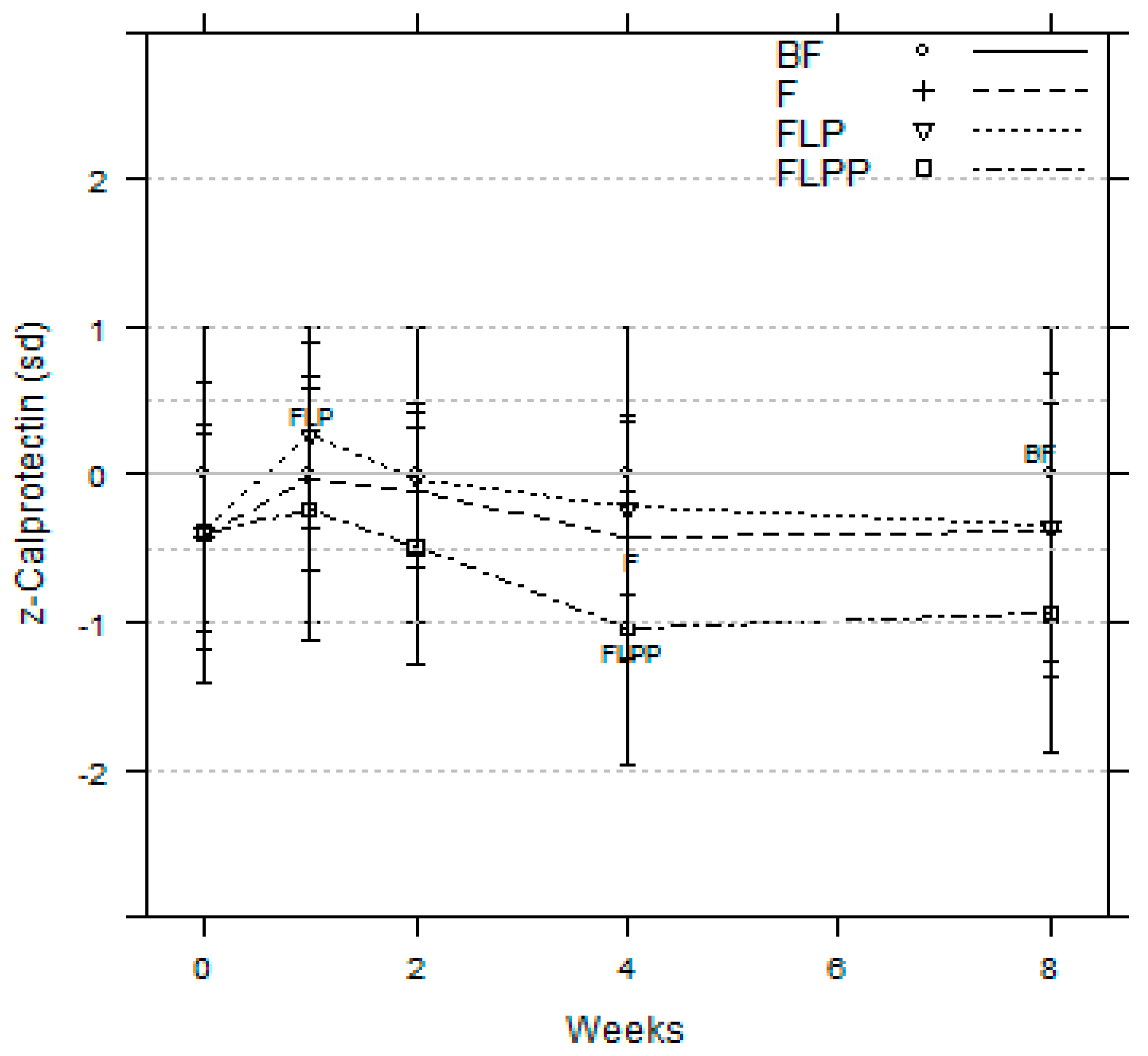
3.2. Gut Barrier Function Maturation
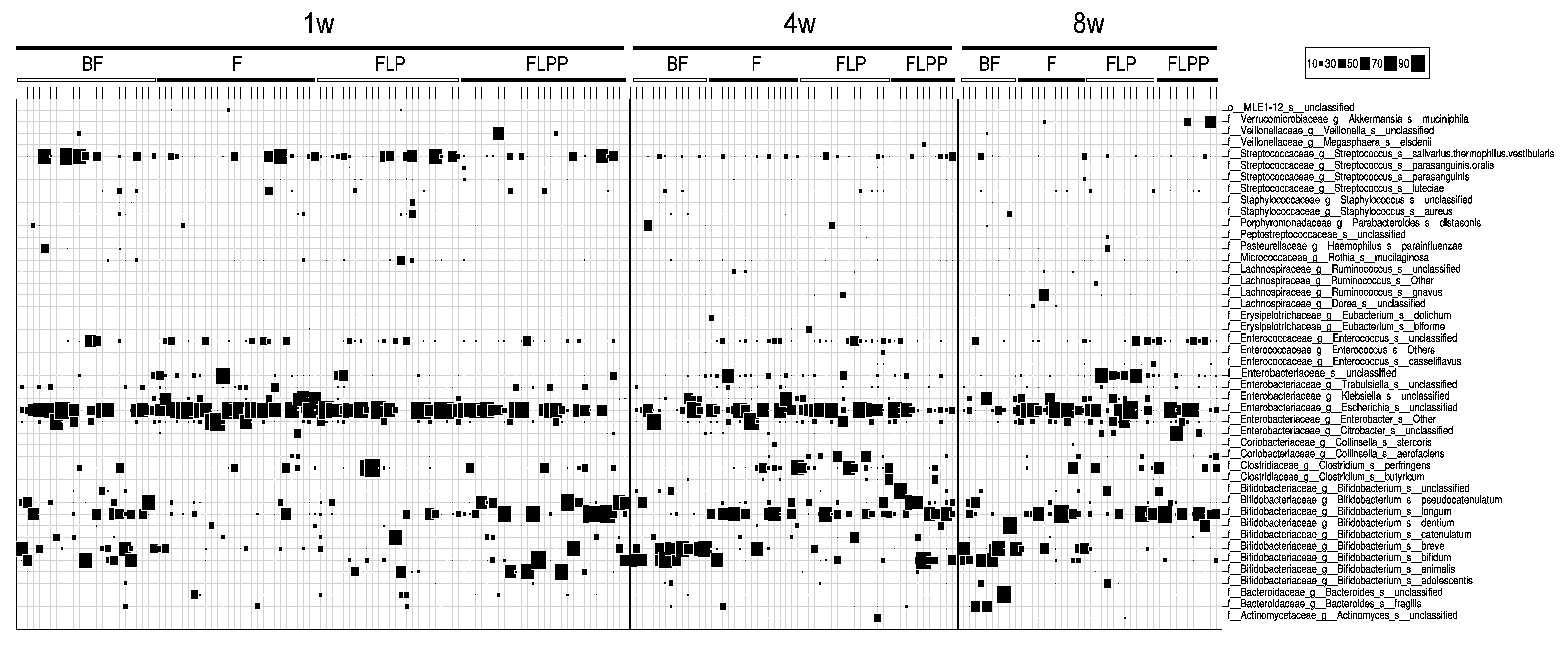
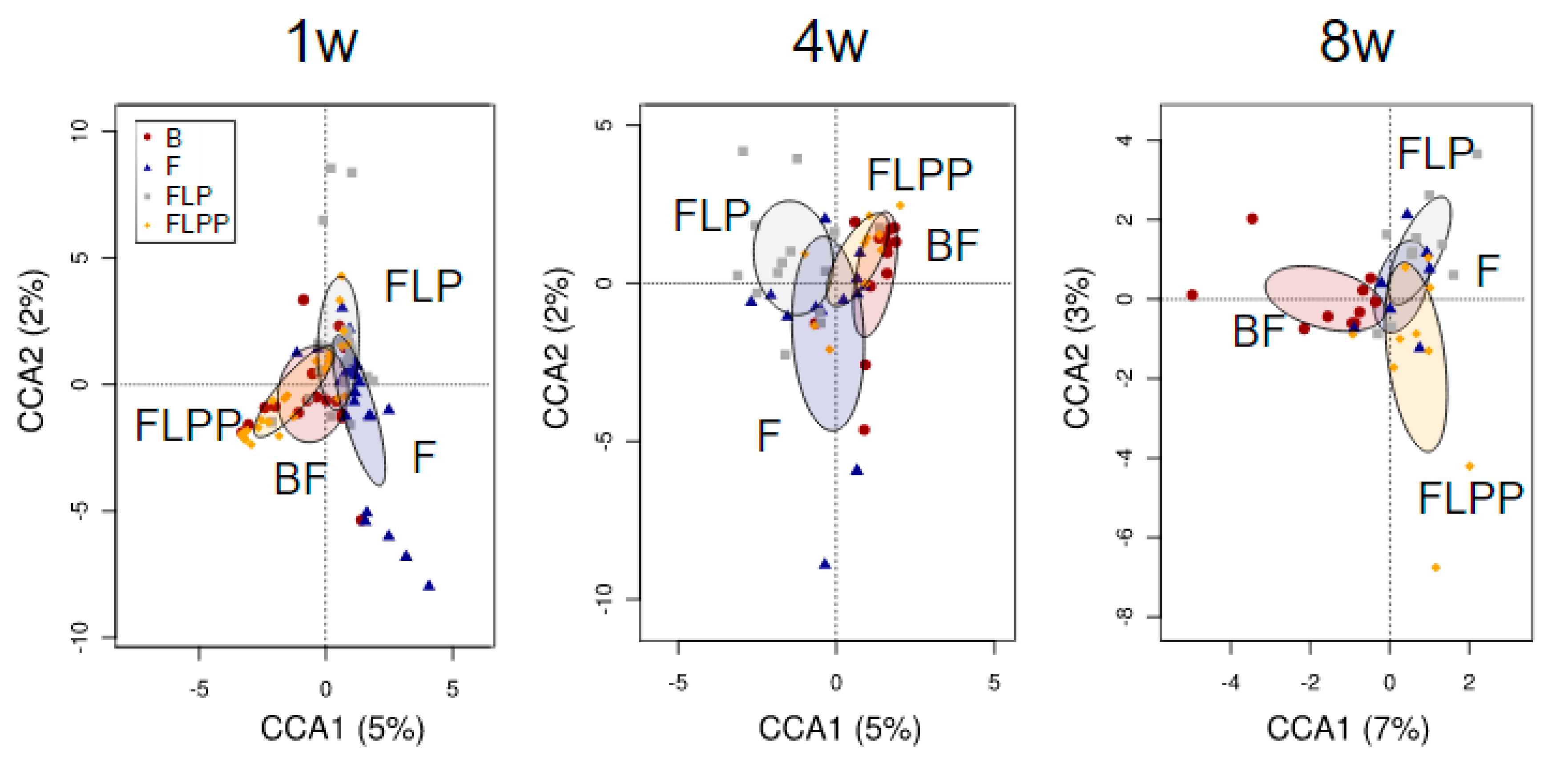
3.3. Stool Microbiota
3.4. Association between Microbiota Profiles and Calprotectin Levels
3.5. Fecal pH
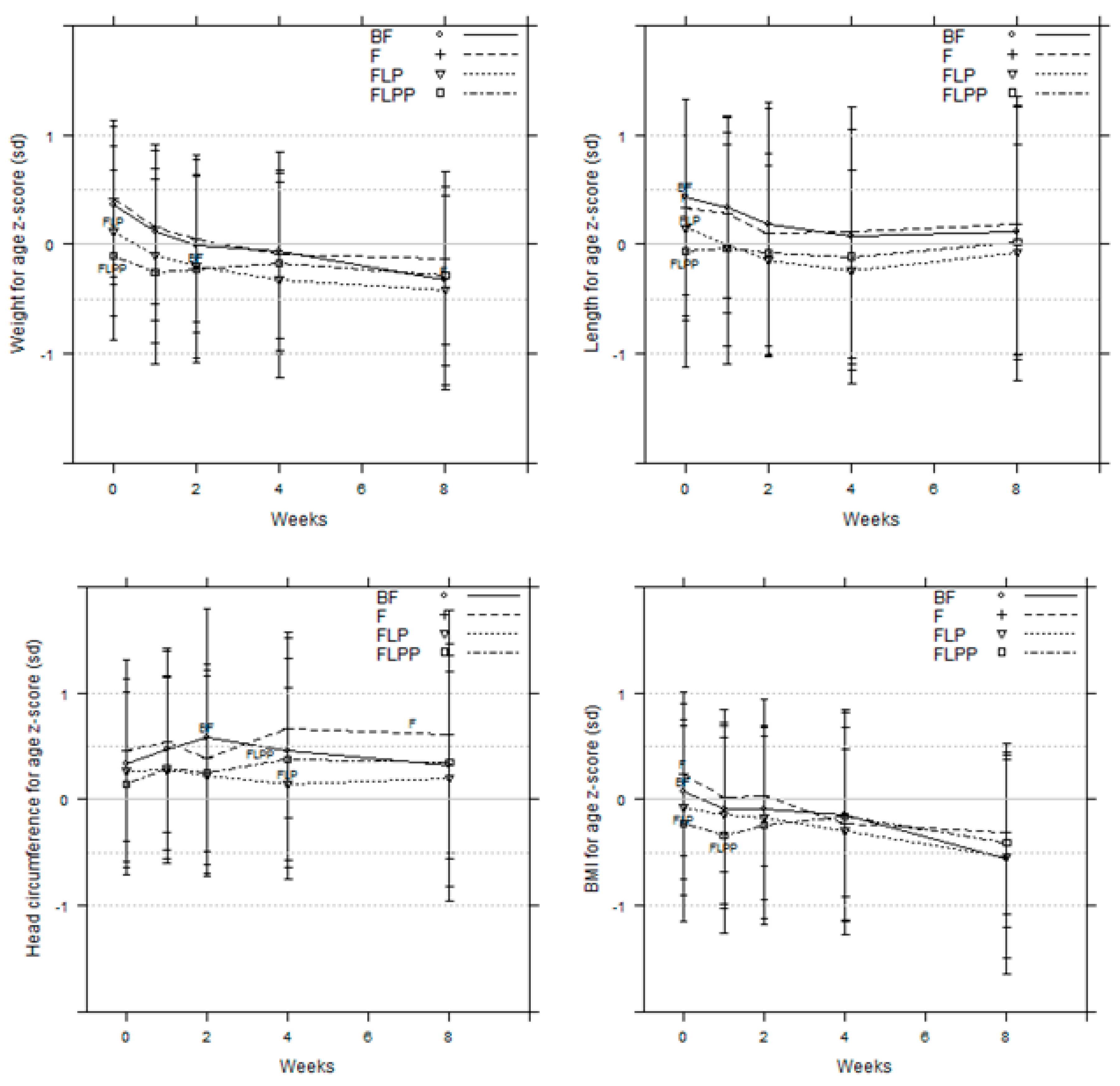
3.6. Growth (Anthropometry and Body Composition)
3.7. Iron Status
3.8. Digestive Tolerance
3.9. Adverse Events/Morbidity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tamburini, S.; Shen, N.; Wu, H.C.; Clemente, J.C. The microbiome in early life: Implications for health outcomes. Nat. Med. 2016, 22, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.M.; Hill, D.R.; Aurora, M.; Spence, J.R. Morphogenesis and maturation of the embryonic and postnatal intestine. Semin. Cell Dev. Boil. 2017, 66, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Fiebiger, U.; Bereswill, S.; Heimesaat, M.M. Dissecting the interplay between intestinal microbiota and host immunity in health and disease: Lessons learned from germfree and gnotobiotic animal models. Eur. J. Microbiol. Immunol. 2016, 6, 253–271. [Google Scholar] [CrossRef]
- Parker, A.; Lawson, M.A.; Vaux, L.; Pin, C. Host-microbe interaction in the gastrointestinal tract. Environ. Microbiol. 2017, 20, 2337–2353. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Round, J.L.; Kasper, D.L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 2008, 453, 620–625. [Google Scholar] [CrossRef]
- Chung, H.; Pamp, S.J.; Hill, J.A.; Surana, N.K.; Edelman, S.M.; Troy, E.B.; Reading, N.C.; Villablanca, E.J.; Wang, S.; Mora, J.R.; et al. Gut Immune Maturation Depends on Colonization with a Host-Specific Microbiota. Cell 2012, 149, 1578–1593. [Google Scholar] [CrossRef] [PubMed]
- Cilieborg, M.S.; Boye, M.; Sangild, P. Bacterial colonization and gut development in preterm neonates. Early Hum. Dev. 2012, 88, S41–S49. [Google Scholar] [CrossRef]
- Azad, M.B.; Konya, T.; Maughan, H.; Guttman, D.S.; Field, C.J.; Chari, R.S.; Sears, M.R.; Becker, A.B.; Scott, J.A.; Kozyrskyj, A.L. Gut microbiota of healthy Canadian infants: Profiles by mode of delivery and infant diet at 4 months. Can. Med. Assoc. J. 2013, 185, 385–394. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; Lieber, A.D.; Wu, F.; Pérez-Pérez, G.; Chen, Y.; et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 2016, 8, 343ra82. [Google Scholar] [CrossRef]
- Fallani, M.; Young, D.; A Scott, J.; Norin, E.; Amarri, S.; Adam, R.; Aguilera, M.; Khanna, S.; Gil, A.; Edwards, C.A.; et al. Intestinal Microbiota of 6-week-old Infants Across Europe: Geographic Influence Beyond Delivery Mode, Breast-feeding, and Antibiotics. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Oozeer, N.B.; Fairgrieve, R.; Clement, W. Conservative management of laryngeal dog bite. Scott. Med. J. 2013, 58, e22–e27. [Google Scholar] [CrossRef] [PubMed]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; Brandt, P.A.V.D.; Stobberingh, E.E. Factors Influencing the Composition of the Intestinal Microbiota in Early Infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Simeoni, U.; Berger, B.; Junick, J.; Blaut, M.; Pecquet, S.; Rezzonico, E.; Grathwohl, D.; Sprenger, N.; Brüssow, H.; Team, S. Gut microbiota analysis reveals a marked shift to bifidobacteria by a starter infant formula containing a synbiotic of bovine milk-derived oligosaccharides and B ifidobacterium animalis subsp. lactis CNCM I-3446. Environ. Microbiol. 2016, 18, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Yassour, M.; Vatanen, T.; Siljander, H.; Hämäläinen, A.-M.; Härkönen, T.; Ryhänen, S.; Franzosa, E.A.; Vlamakis, H.; Huttenhower, C.; Gevers, D.; et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci. Transl. Med. 2016, 8, 343ra81. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Bonucci, A.; Coppa, G.V.; Carlucci, A.; Giorgi, P.L. Intestinal permeability changes during the first month: Effect of natural versus artificial feeding. J. Pediatr. Gastroenterol. Nutr. 1995, 21, 383–386. [Google Scholar] [CrossRef]
- Colomé, G.; Sierra, C.; Blasco, J.; García, M.V.; Valverde, E.; Sánchez, E.; Blasco-Alonso, J. Intestinal permeability in different feedings in infancy. Acta Paediatr. 2007, 96, 69–72. [Google Scholar] [CrossRef]
- Weaver, L.T.; Ewing, G.; Taylor, L.C. The Bowel Habit of Milk-Fed Infants. J. Pediatr. Gastroenterol. Nutr. 1988, 7, 568–571. [Google Scholar] [CrossRef]
- Xie, L.; Innis, S.M. Genetic Variants of the FADS1 FADS2 Gene Cluster Are Associated with Altered (n-6) and (n-3) Essential Fatty Acids in Plasma and Erythrocyte Phospholipids in Women during Pregnancy and in Breast Milk during Lactation. J. Nutr. 2008, 138, 2222–2228. [Google Scholar] [CrossRef]
- Harsløf, L.B.; Larsen, L.H.; Ritz, C.; I Hellgren, L.; Michaelsen, K.F.; Vogel, U.; Lauritzen, L. FADS genotype and diet are important determinants of DHA status: A cross-sectional study in Danish infants. Am. J. Clin. Nutr. 2013, 97, 1403–1410. [Google Scholar] [CrossRef]
- Muc, M.; Kreiner, E.; Larsen, J.M.; Birch, S.; Brix, S.; Bisgaard, H.; Lauritzen, L.; Brix, S. Maternal fatty acid desaturase genotype correlates with infant immune responses at 6 months. Br. J. Nutr. 2015, 114, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P.; Decembrino, L.; Stolfi, I.; Pugni, L.; Rinaldi, M.; Cattani, S.; Romeo, M.; Messner, H.; Laforgia, N.; Vagnarelli, F.; et al. Lactoferrin and prevention of late-onset sepsis in the pre-term neonates. Early Hum. Dev. 2010, 86, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P.; Stolfi, I.; Messner, H.; Cattani, S.; Laforgia, N.; Romeo, M.G.; Bollani, L.; Rinaldi, M.; Gallo, E.; Quercia, M.; et al. Bovine Lactoferrin Prevents Invasive Fungal Infections in Very Low Birth Weight Infants: A Randomized Controlled Trial. Pediatrics 2011, 129, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, T.J.; Chea-Woo, E.; Baiocchi, N.; Pecho, I.; Campos-Sánchez, M.; Prada, A.; Valdiviezo, G.; Lluque, A.; Lai, D.; Cleary, T.G. Randomized double-blind controlled trial of bovine lactoferrin for prevention of diarrhea in children. J. Pediatr. 2012, 162, 349–356. [Google Scholar] [CrossRef]
- Pammi, M.; Abrams, S.A. Oral Lactoferrin for the Prevention of Sepsis and Necrotizing Enterocolitis in Preterm Infants; Wiley: Hoboken, NJ, USA, 2015; p. CD007137. [Google Scholar]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef]
- Watson, H.; Mitra, S.; Croden, F.C.; Taylor, M.; Wood, H.M.; Perry, S.L.; A Spencer, J.; Quirke, P.; Toogood, G.J.; Lawton, C.L.; et al. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut 2017, 67, 1974–1983. [Google Scholar] [CrossRef]
- Chichlowski, M.; German, J.B.; Lebrilla, C.B.; Mills, D.A. The Influence of Milk Oligosaccharides on Microbiota of Infants: Opportunities for Formulas. Annu. Rev. Food Sci. Technol. 2011, 2, 331–351. [Google Scholar] [CrossRef]
- Berger, B.; Porta, N.; Foata, F.; Grathwohl, D.; Delley, M.; Moine, D.; Charpagne, A.; Siegwald, L.; Descombes, P.; Alliet, P.; et al. Linking Human Milk Oligosaccharides, Infant Fecal Community Types, and Later Risk to Require Antibiotics. mBio 2020, 11. [Google Scholar] [CrossRef]
- Campeotto, F.; Butel, M.J.; Kalach, N.; Derrieux, S.; Aubert-Jacquin, C.; Barbot, L.; Francoual, C.; Dupont, C.; Kapel, N. High faecal calprotectin concentrations in newborn infants. Arch. Dis. Child. - Fetal Neonatal Ed. 2004, 89, F353–F355. [Google Scholar] [CrossRef] [PubMed]
- Summerton, C.B.; Longlands, M.G.; Wiener, K.; Shreeve, D.R. Faecal calprotectin: A marker of inflammation throughout the intestinal tract. Eur. J. Gastroenterol. Hepatol. 2002, 14, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Kapel, N.; Campeotto, F.; Kalach, N.; Baldassare, M.; Butel, M.-J.; Dupont, C. Faecal Calprotectin in Term and Preterm Neonates. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 542–547. [Google Scholar] [CrossRef] [PubMed]
- I Campbell, D.; McPhail, G.; Lunn, P.G.; Elia, M.; Jeffries, D.J. Intestinal Inflammation Measured by Fecal Neopterin in Gambian Children with Enteropathy: Association With Growth Failure, Giardia lamblia, and Intestinal Permeability. J. Pediatr. Gastroenterol. Nutr. 2004, 39, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.-M.; Knobel, R.; Ewe, K. Fecal α1-Antitrypsin in Newborn Infants. J. Pediatr. Gastroenterol. Nutr. 1997, 24, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Nissler, K.; Von Katte, I.; Huebner, A.; Henker, J. Pancreatic Elastase 1 in Feces of Preterm and Term Infants. J. Pediatr. Gastroenterol. Nutr. 2001, 33, 28–31. [Google Scholar] [CrossRef]
- David-Henriau, L.; Bui, S.; Molinari, I.; Montaudon, D.; Lamireau, T. Fecal elastase-1: A useful test in pediatric practice. Arch. Pediatr. Organe Off. Soc. Fr. Pediatr. 2005, 12, 1221–1225. [Google Scholar] [CrossRef]
- Shulman, R.J.; Buffone, G.; Wise, L. Enteric protein loss in necrotizing enterocolitis as measured by fecal α1-antitrypsin excretion. J. Pediatr. 1985, 107, 287–289. [Google Scholar] [CrossRef]
- Kapel, N.; Meillet, D.; Favennec, L.; Magne, D.; Raichvarg, D.; Gobert, J.-G. Evaluation of Intestinal Clearance and Faecal Excretion of α1-Antiproteinase and Immunoglobulins During Crohn’s Disease and Ulcerative Colitis. Clin. Chem. Lab. Med. 1992, 30, 197–202. [Google Scholar] [CrossRef]
- Majamaa, H.; Aittoniemi, J.; Miettinen, A. Increased concentration of fecal α1-antitrypsin is associated with cow’s milk allergy in infants with atopic eczema. Clin. Exp. Allergy 2001, 31, 590–592. [Google Scholar] [CrossRef]
- Rollins, N.C.; Filteau, S.M.; Coutsoudis, A.; Tomkins, A.M. Feeding mode, intestinal permeability, and neopterin excretion: A longitudinal study in infants of HIV-infected South African women. J. Acquir. Immune Defic. Syndr. (1999) 2001, 28, 132–139. [Google Scholar] [CrossRef]
- Amarri, S.; Benatti, F.; Callegari, M.; Shahkhalili, Y.; Chauffard, F.; Rochat, F.; Acheson, K.; Hager, C.; Benyacoub, J.; Galli, E.; et al. Changes of Gut Microbiota and Immune Markers During the Complementary Feeding Period in Healthy Breast-fed Infants. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Borulf, S.; Lindberg, T.; Månsson, M. Immunoreactive Anionic Trypsin and Anionic Elastase in Human Milk. Acta Paediatr. 1987, 76, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Campeotto, F.; Kapel, N.; Kalach, N.; Razafimahefa, H.; Castela, F.; Barbot, L.; Soulaines, P.; Dehan, M.; Gobert, J.G.; Dupont, C. Low levels of pancreatic elastase 1 in stools of preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2002, 86, F198–F199. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.A.; Lönnerdal, B. Fecal Alpha1-Antitrypsin in Breast-Fed Infants Is Derived from Human Milk and Is not Indicative of Enteric Protein Loss. Acta Paediatr. 1990, 79, 137–141. [Google Scholar] [CrossRef]
- Lisowska-Myjak, B.; Pachecka, J. Alpha-1-Antitrypsin and IgA in Serial Meconium and Faeces of Healthy Breast-Fed Newborns. Fetal Diagn. Ther. 2006, 22, 116–120. [Google Scholar] [CrossRef]
- Matsubara, Y.; E Gaull, G. Biopterin and neopterin in various milks and infant formulas. Am. J. Clin. Nutr. 1985, 41, 110–112. [Google Scholar] [CrossRef]
- Mohan, R.; Koebnick, C.; Schildt, J.; Mueller, M.; Radke, M.; Blaut, M. Effects of Bifidobacterium lactis Bb12 Supplementation on Body Weight, Fecal pH, Acetate, Lactate, Calprotectin, and IgA in Preterm Infants. Pediatr. Res. 2008, 64, 418–422. [Google Scholar] [CrossRef]
- Lönnerdal, B.; Erdmann, P.; Thakkar, S.K.; Sauser, J.; Destaillats, F. Longitudinal evolution of true protein, amino acids and bioactive proteins in breast milk: A developmental perspective. J. Nutr. Biochem. 2017, 41, 1–11. [Google Scholar] [CrossRef]
- Van Elburg, R.M.; Fetter, W.P.F.; Bunkers, C.M.; Heymans, H. Intestinal permeability in relation to birth weight and gestational and postnatal age. Arch. Dis. Child. Fetal Neonatal Ed. 2003, 88, F52–F55. [Google Scholar] [CrossRef]
- Available online: http://cgenome.net/calypso (accessed on 19 May 2020).
- Meli, F.; Puccio, G.; Cajozzo, C.; Ricottone, G.L.; Pecquet, S.; Sprenger, N.; Steenhout, P. Growth and safety evaluation of infant formulae containing oligosaccharides derived from bovine milk: A randomized, double-blind, noninferiority trial. BMC Pediatr. 2014, 14, 306. [Google Scholar] [CrossRef]
- Asgarshirazi, M.; Shariat, M.; Nayeri, F.; Dalili, H.; Abdollahi, A. Comparison of Fecal Calprotectin in Exclusively Breastfed and Formula or Mixed Fed Infants in the First Six Months of Life. Acta Med. Iran. 2017, 55, 53–58. [Google Scholar] [PubMed]
- Groer, M.; Ashmeade, T.; Louis-Jacques, A.; Beckstead, J.; Ji, M. Relationships of Feeding and Mother’s Own Milk with Fecal Calprotectin Levels in Preterm Infants. Breastfeed. Med. 2016, 11, 207–212. [Google Scholar] [CrossRef]
- Savino, F.; Castagno, E.; Calabrese, R.; Viola, S.; Oggero, R.; Miniero, R. High Faecal Calprotectin Levels in Healthy, Exclusively Breast-Fed Infants. Neonatology 2010, 97, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ma, J.; Geng, S.; Wang, J.; Ren, F.; Sheng, X. Comparison of the different kinds of feeding on the level of fecal calprotectin. Early Hum. Dev. 2014, 90, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Austin, S.; De Castro, C.A.; Bénet, T.; Hou, Y.; Sun, H.; Thakkar, S.K.; Vinyes-Pares, G.; Zhang, Y.; Wang, P. Temporal Change of the Content of 10 Oligosaccharides in the Milk of Chinese Urban Mothers. Nutrients 2016, 8, 346. [Google Scholar] [CrossRef]
- Keikha, M.; Bahreynian, M.; Saleki, M.; Kelishadi, R. Macro- and Micronutrients of Human Milk Composition: Are They Related to Maternal Diet? A Comprehensive Systematic Review. Breastfeed. Med. 2017, 12, 517–527. [Google Scholar] [CrossRef]
- Sprenger, N.; Lee, L.Y.; De Castro, C.A.; Steenhout, P.; Thakkar, S.K. Longitudinal change of selected human milk oligosaccharides and association to infants’ growth, an observatory, single center, longitudinal cohort study. PLoS ONE 2017, 12, e0171814. [Google Scholar] [CrossRef]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.-J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef]
- Löser, C.; Mollgaard, A.; Fölsch, U.R. Faecal elastase 1: A novel, highly sensitive, and specific tubeless pancreatic function test. Gut 1996, 39, 580–586. [Google Scholar] [CrossRef]
- Stein, J.; Jung, M.; Sziegoleit, A.; Zeuzem, S.; Caspary, W.F.; Lembcke, B. Immunoreactive elastase I: Clinical evaluation of a new noninvasive test of pancreatic function. Clin. Chem. 1996, 42, 222–226. [Google Scholar] [CrossRef]
- Pokusaeva, K.; Fitzgerald, G.F.; Van Sinderen, D. Carbohydrate metabolism in Bifidobacteria. Genes Nutr. 2011, 6, 285–306. [Google Scholar] [CrossRef] [PubMed]
- Brüssow, H.; Parkinson, S.J. You are what you eat. Nat. Biotechnol. 2014, 32, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.; Bolton, K.D.; Velaphi, S.; De Groot, N.; Emady-Azar, S.; Pecquet, S.; Steenhout, P. Early Benefits of a Starter Formula Enriched in Prebiotics and Probiotics on the Gut Microbiota of Healthy Infants Born to HIV+ Mothers: A Randomized Double-Blind Controlled Trial. Clin. Med. Insights Pediatr. 2016, 10, 119–130. [Google Scholar] [CrossRef]
| BF (n = 75) | F (n = 40) | FLP (n = 44) | FLPP (n = 43) | |
|---|---|---|---|---|
| Mothers | ||||
| Weight, kg (range) | 58.0 (52; 65) | 61.0 (54.2; 69.2) | 64 (55.5; 69.5) | 61.0 (55.0; 71.2) |
| Height, cm (range) | 166 (160; 169) | 165 (162; 170) | 165 (162; 170) | 164 (160; 170) |
| Age, years (range) | 30.0 (27.0; 33.0) | 30.0 (25.8; 33.2) | 28.5 (24.2; 30.0) | 28.5 (24.2; 33.8) |
| Former smoker, % | 11 | 20 | 27 | 14 |
| Infants | ||||
| Male gender, % | 52 | 57.5 | 38.6 | 48.8 |
| Age at enrolment, days | 1.45 ± 0.76 | 1.45 ± 0.71 | 1.52 ± 0.63 | 1.4 ± 073 |
| Weight, kg | 3.45 ± 0.37 | 3.49 ± 0.36 | 3.33 ± 0.43 | 3.24 ± 0.38 |
| Crown-heel length, cm | 50.6 ± 1.7 | 50.5 ± 1.8 | 50.0 ± 1.6 | 49.6 ± 2.0 |
| Head circumference, cm | 34.7 ± 1.2 | 34.9 ± 1.1 | 34.6 ± 1.0 | 34.5 ± 1.1 |
| Body mass index, kg/m2 | 13.5 ± 1.1 | 13.7 ± 1.0 | 13.3 ± 1.0 | 13.1 ± 1.2 |
| BF (n = 30) | F (n = 29) | FLP (n = 27) | FLPP (n = 24) | |
|---|---|---|---|---|
| Mean (range) faecal calprotectin concentrations (µg/g of faeces) | 386 (143, 1044) | 321 (174, 594) | 346 (201, 596) | 205 (90, 470) |
| p-value (versus breastfed infants) | 0.338 | 0.686 | 0.014 |
| BF | F | FLP | FLPP | |
|---|---|---|---|---|
| Calprotectin µg/g (range) | 444 (158;1251) | 289 (123; 678) | 352 (193; 641) | 152 (59; 396) † p = 0.003 ∆ p = 0.031 □ p = 0.002 |
| Elastase µg/g (range) | 725 (485; 1085) | 1084 (532; 2208) † p = 0.010 | 1129 (754; 1689) † p < 0.001 | 631 (301; 1324) ∆ p = 0.002 □ p = 0.010 |
| Alpha 1-AT mg/g (range) | 0.175 (0.084; 0.363) | 0.273 (0.169; 0.442) † p = 0.011 | 0.239 (0.165; 0.345) | 0.133 (0.062; 0.288) ∆ p = 0.002 □ p = 0.010 |
| Neopterin pmol/g (range) | 597 (367; 971) | 849 (514; 1403) † p = 0.048 | 973 (557; 1699) † p = 0.008 | 948 (683; 1317) † p = 0.002 |
| IgA µg/g (range) | 619 (283; 1354) | 78 (20; 309) † p < 0.001 | 132 (75; 234) † p < 0.001 | 117( 40; 340) † p < 0.001 |
| Permeability Index (range) | 0.774 (0.455; 1.316) | 0.635 (0.318; 1.267) | 0.647 (0.292; 1.435) | 0.783 (0.459; 1.334) |
| Time | Genus/Species | B | F | FLP | FLPP | Significance of Differences | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | Mean | Median | Mean | Median | Mean | Median | Mean | ALL | B vs. F | B vs. FLP | B vs. FLPP | F vs. FLPP | FLP vs. FLPP | ||
| 1w | Bifidobacterium | 28.3 | 36.7 | 3.0 | 7.2 | 5.3 | 14.1 | 54.2 | 46.7 | *** | *** | * | NS | *** | ** |
| Staphylococcus | 0.1 | 0.5 | 0.0 | 0.2 | 0.0 | 1.8 | 0.0 | 0.0 | *** | * | NS | *** | NS | * | |
| Klebsiella | 0.0 | 0.3 | 0.1 | 9.3 | 0.0 | 0.7 | 0.0 | 0.1 | *** | ** | NS | NS | ** | NS | |
| unclassified Enterobacteriaceae | 0.0 | 0.8 | 0.2 | 5.1 | 0.0 | 3.1 | 0.0 | 0.9 | * | ** | NS | NS | ** | NS | |
| unclassified Klebsiella | 0.0 | 0.3 | 0.1 | 9.3 | 0.0 | 0.7 | 0.0 | 0.1 | *** | ** | NS | NS | ** | NS | |
| Staphylococcus aureus | 0.0 | 0.3 | 0.0 | 0.2 | 0.0 | 1.2 | 0.0 | 0.0 | *** | NS | NS | *** | NS | * | |
| Bifidobacterium animalis | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 3.3 | 0.0 | 7.4 | ** | NS | * | * | ** | NS | |
| Streptococcus parasanguinis | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.4 | 0.0 | 0.3 | ** | NS | ** | * | NS | NS | |
| 4w | Enterococcus | 0.0 | 0.1 | 1.6 | 3.7 | 3.4 | 6.3 | 0.5 | 1.7 | *** | *** | *** | ** | NS | NS |
| Bifidobacterium | 81.0 | 61.7 | 34.5 | 32.5 | 16.0 | 23.8 | 77.1 | 61.5 | ** | * | ** | NS | ** | ** | |
| Staphylococcus | 0.2 | 0.6 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | ** | NS | ** | NS | NS | NS | |
| Clostridium | 0.0 | 0.4 | 1.0 | 9.8 | 2.5 | 14.4 | 0.0 | 4.0 | * | NS | ** | NS | NS | * | |
| unclassified Enterococcus | 0.0 | 0.0 | 1.6 | 3.7 | 2.4 | 5.6 | 0.5 | 1.7 | *** | *** | *** | ** | NS | NS | |
| Bifidobacterium breve | 35.3 | 33.7 | 0.0 | 5.0 | 0.0 | 1.1 | 0.0 | 0.2 | ** | * | ** | * | NS | NS | |
| Clostridium perfringens | 0.0 | 0.1 | 0.6 | 9.3 | 2.0 | 12.0 | 0.0 | 1.3 | ** | * | ** | NS | NS | * | |
| Staphylococcus aureus | 0.0 | 0.5 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | * | NS | * | NS | NS | NS | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castanet, M.; Costalos, C.; Haiden, N.; Hascoet, J.-M.; Berger, B.; Sprenger, N.; Grathwohl, D.; Brüssow, H.; De Groot, N.; Steenhout, P.; et al. Early Effect of Supplemented Infant Formulae on Intestinal Biomarkers and Microbiota: A Randomized Clinical Trial. Nutrients 2020, 12, 1481. https://doi.org/10.3390/nu12051481
Castanet M, Costalos C, Haiden N, Hascoet J-M, Berger B, Sprenger N, Grathwohl D, Brüssow H, De Groot N, Steenhout P, et al. Early Effect of Supplemented Infant Formulae on Intestinal Biomarkers and Microbiota: A Randomized Clinical Trial. Nutrients. 2020; 12(5):1481. https://doi.org/10.3390/nu12051481
Chicago/Turabian StyleCastanet, Mireille, Christos Costalos, Nadja Haiden, Jean-Michel Hascoet, Bernard Berger, Norbert Sprenger, Dominik Grathwohl, Harald Brüssow, Nanda De Groot, Philippe Steenhout, and et al. 2020. "Early Effect of Supplemented Infant Formulae on Intestinal Biomarkers and Microbiota: A Randomized Clinical Trial" Nutrients 12, no. 5: 1481. https://doi.org/10.3390/nu12051481
APA StyleCastanet, M., Costalos, C., Haiden, N., Hascoet, J.-M., Berger, B., Sprenger, N., Grathwohl, D., Brüssow, H., De Groot, N., Steenhout, P., Pecquet, S., Benyacoub, J., & Picaud, J.-C. (2020). Early Effect of Supplemented Infant Formulae on Intestinal Biomarkers and Microbiota: A Randomized Clinical Trial. Nutrients, 12(5), 1481. https://doi.org/10.3390/nu12051481







