Calcium Bioavailability of Opuntia ficus-indica Cladodes in an Ovariectomized Rat Model of Postmenopausal Bone Loss
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vegetal Material
2.2. Preparation of O. ficus-indica Powder
2.3. Extraction of Soluble and Insoluble Fibers from O. ficus-indica Cladodes
2.4. Determination of Calcium and Phosphorus Content in O. ficus-indica Cladodes
2.5. Experimental Design
2.6. Chemical Composition of Experimental Diets
2.7. Evaluation of Serum Estrogen Levels
2.8. Calcium Bioavailability
2.9. Assessment of Femoral Dimensions and Weight
2.10. Analyses of the Biomechanical Properties of Femurs
2.11. Determination of Calcium and Phosphorus Content in the Femurs
2.12. Analyses of Bone Mineral Density
2.13. Evaluation of the Microstructural Properties of Rat Femoral Bone
2.14. Measurement of Bone Remodeling Biochemical Biomarkers
2.15. Statistical Analyses
3. Results
3.1. Analyses of Ca and P Content in O. ficus-indica Cladodes, and the Soluble and Insoluble Fiber Extracted from O. ficus-indica
3.2. Chemical Composition and Mineral Content in Experimental Diets
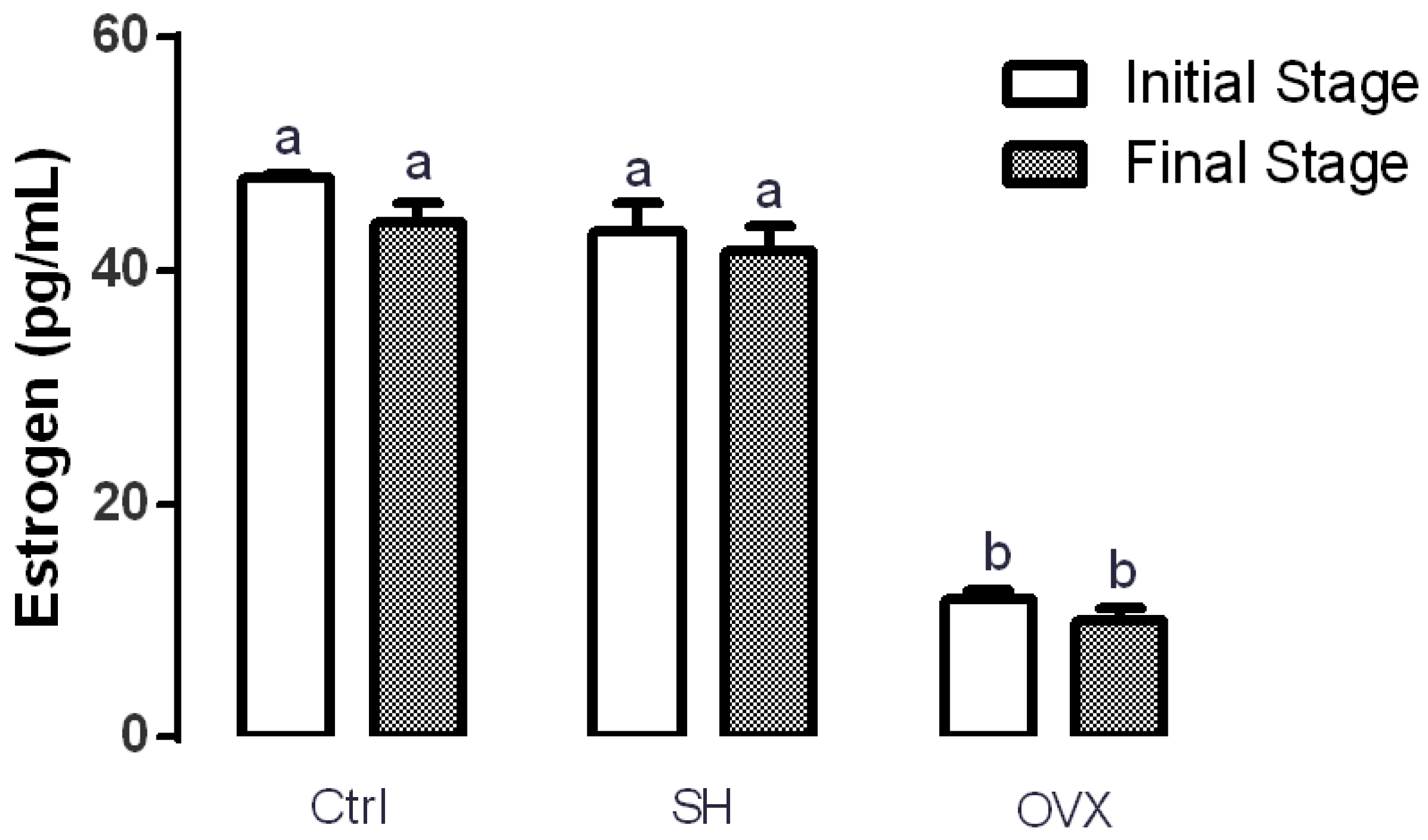
3.3. Serum Estrogen Levels in Ovariectomized Rats
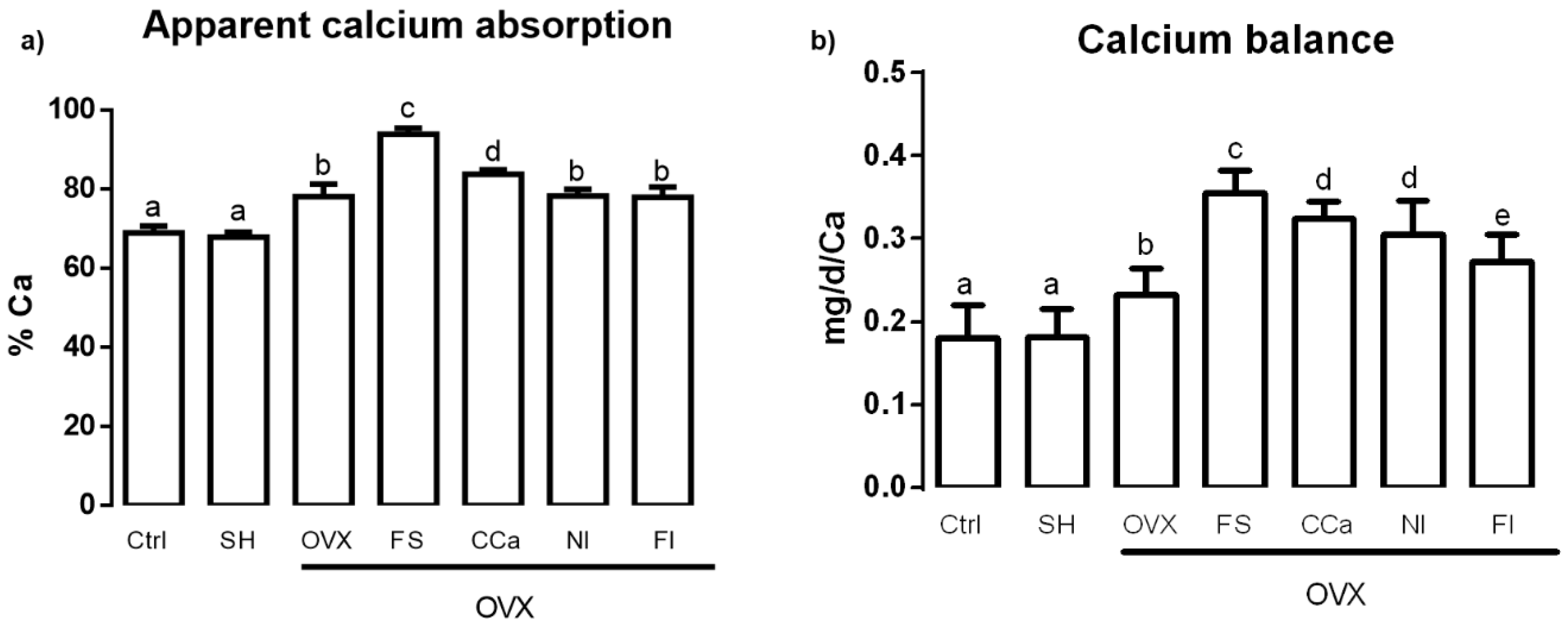
3.4. Calcium Bioavailability
3.5. Physical and Biomechanical Properties of the Femur
3.6. Mineral Content in the Femur
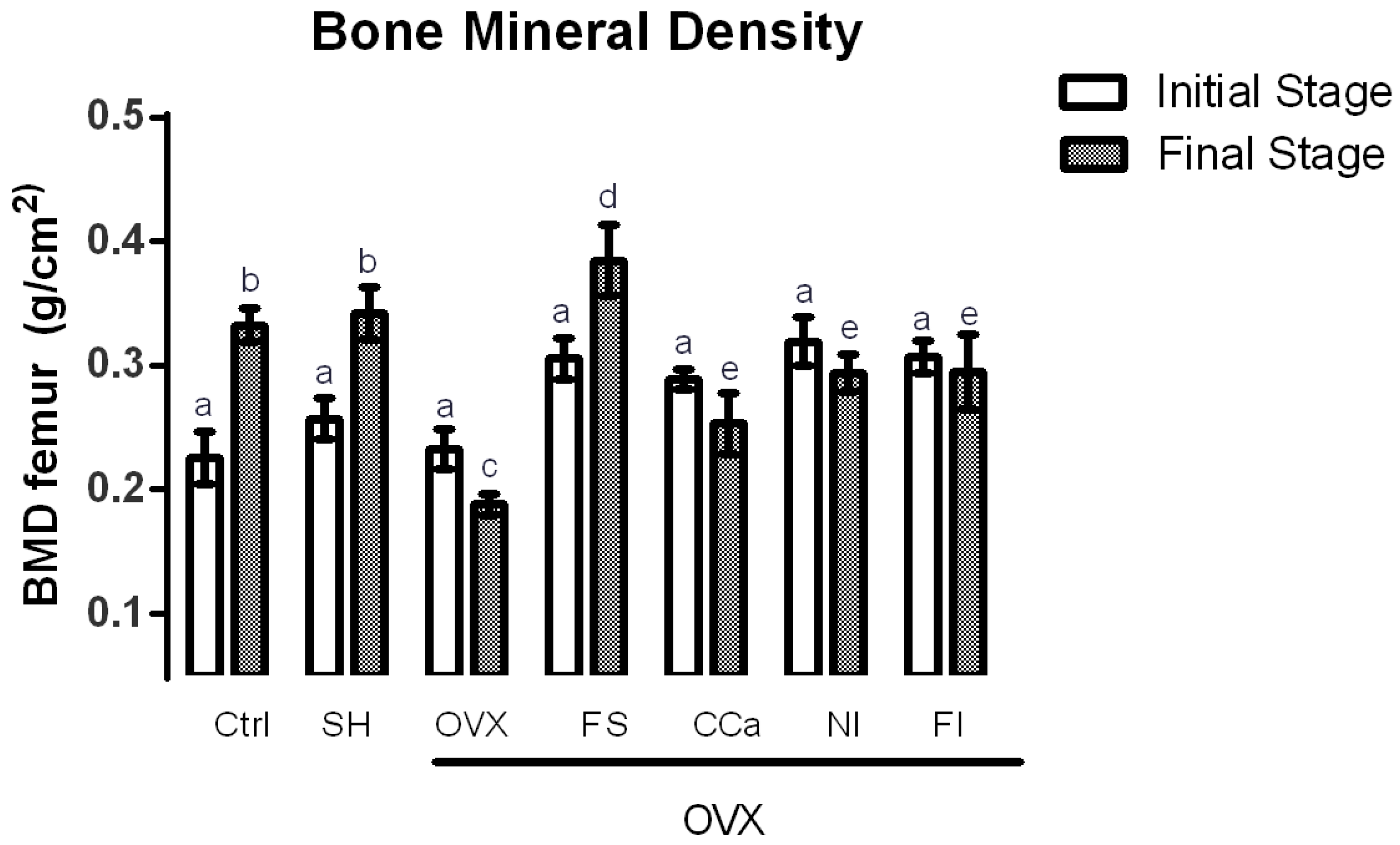
3.7. Bone Mineral Density (BMD) Measurements
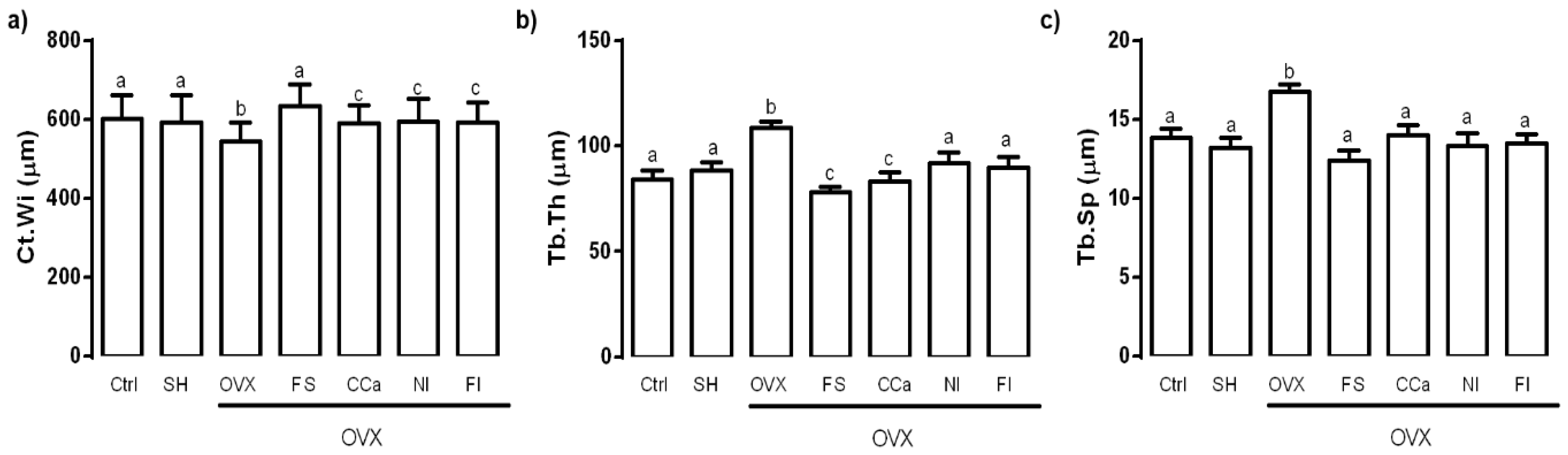
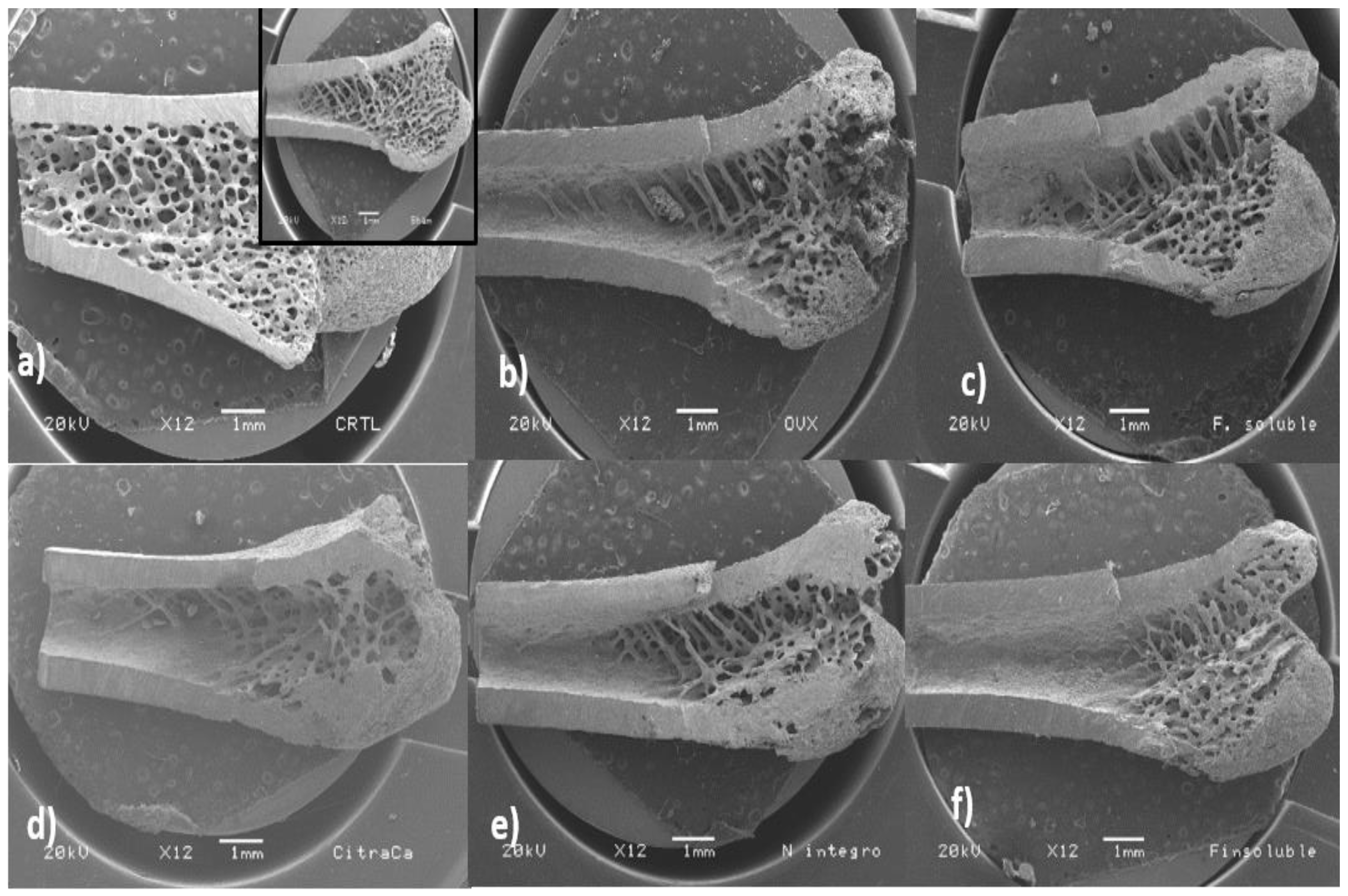
3.8. Bone Microstructure Evaluation
3.9. Determination of Biomarkers Related to Bone Remodeling
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hodges, J.K.; Cao, S.; Cladis, D.P.; Weaver, C.M. Lactose intolerance and Bone Health: The Challenge of Ensuring Adequate Calcium Intake. Nutrients 2019, 11, 718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trajanoska, K.; Rivadeneira, F. The genetic architecture of osteoporosis and fracture risk. Bone 2019, 126, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Nieto, L.; Zamora, E.; Reséndiz, A.; Camacho, S.; Espinosa, J.; Torres, R.; Aguilar, G.; Loreto, U.; Roque, I.; González, R. Consideraciones epidemiológicas de las fracturas del fémur proximal. Mediagraphic 2012, 8, 135–139. [Google Scholar]
- Khosla, S.; Melton, J.L.; Riggs, L.B. The unitary model for estrogen deficiency and the pathogenesis osteoporosis: Is a revision needed? J. Bone Miner. Res. 2011, 26, 441–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watts, N.; Bilezikian, J.P.; Camacho, P.M.; Greenspan, S.L.; Harris, S.T.; Hodgson, S.F.; Kleerekoper, M.; Luckey, M.M.; McClung, M.R.; Pollack, R.P.; et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the Diagnosis and Treatment of Postmenopausal Osteoporosis. Endocr. Pract. 2010, 16 (Suppl. 3), 4–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Setsuo Maeda, S.; Lazaretti-Castro, M. An overview on the treatment of postmenopausal osteoporosis. J. Braz. Soc. Endocr. Metabolism. 2014, 58, 162–171. [Google Scholar]
- Drake, M.T.; Clarke, B.L.; Lewiecki, E.M. The pathophysiology and treatment of Osteoporosis. Clin. Ther. 2015, 37, 1837–1850. [Google Scholar] [CrossRef]
- Hernández-Becerra, E.; Mendoza-Ávila, M.; Jiménez-Mendoza, D.; Gutiérrez-Cortez, E.; Rodríguez-García, M.E.; Rojas-Molina, J.I. Effect of Nopal (Opuntia ficus indica) consumption at different maturity stages as an only calcium source on bone mineral metabolism in growing rats. Biol. Trace Elem. Res. 2019. [Google Scholar] [CrossRef]
- Hernández-Becerra, E.; Gutiérrez-Cortez, E.; Del Real, A.; Rojas-Molina, A.; Rodríguez-García, M.; Rubio, E.; Quintero-García, M.; Rojas-Molina, I. Bone mineral density, mechanical, microstructural properties and mineral content of the femur in growing rats fed with Opuntia ficus indica as calcium source in diet. Nutrients 2017, 9, 108. [Google Scholar] [CrossRef] [Green Version]
- Rojas-Molina, I.; Gutiérrez-Cortez, E.; Bah, M.; Rojas-Molina, A.; Ibarra-Alvarado, C.; Rivera-Muñoz, E.; Del Real, A.; Aguilera-Barreiro, M.A. Characterization of Calcium Compounds in Opuntia ficus indica as a Source of Calcium for Human Diet. J. Chem. 2015. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Padilla, M.; Pérez-Torrero, E.; Hernández-Urbiola, M.I.; Hernández-Quevedo, G.; Del Real, A.; Rivera-Muñoz, E.; Rodríguez-García, M. Evaluation of oxalates and calcium in nopal pads (Opuntia ficus-indica var. redonda) at different maturity stages. J. Food Compos. Anal. 2011, 24, 38–43. [Google Scholar] [CrossRef]
- Official Methods of Analysis of the Association of Official Analytical Chemists, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000.
- Reeves, P.G.; Nielsen, F.H.; Fahey, J.G.C. AIN-93 Purified diets for laboratory rodents, final report of the American Institute of Nutrition ad hoc writing committee on reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Pomeranz, Y.; Meloan, C. Carbohydrates. In Food Analysis. Theory and Practice, 3rd ed.; Chapman & Hall Inc.: New York, NY, USA, 1994; pp. 625–669. [Google Scholar]
- Choi, K.O.; Lee, I.; Paik, S.Y.; Kim, D.E.; Lim, J.D.; Kang, W.S.; Ko, S. Ultrafine Angelica gigas powder normalizes ovarian hormone levels and has antiosteoporosis properties in ovariectomized rats: Particle size effect. J. Med. Food. 2012, 15, 863–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Lai, W.P.; Leung, P.C.; Wu, C.F.; Yao, X.S.; Wong, M.S. Effects of Fructus Ligustri Lucidi extract on bone turnover and calcium balance in ovariectomized rats. Biol. Pharm. Bull. 2006, 29, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Zafar, T.A.; Weaver, C.M.; Zhao, Y.; Martin, B.R.; Wastney, M.E. Nondigestible oligosaccharides increase calcium absorption and supress bone resorption in ovariectomized rats. Nutr. J. 2003, 17, 339–402. [Google Scholar]
- Jimenez-Mendoza, D.; Espinosa-Arbelaez, D.G.; Giraldo-Betancur, A.L.; Hernandez-Urbiola, M.I.; Vargas-Vazquez, D.; Rodriguez-Garcia, M.E. Single x-ray transmission system for bone mineral density determination. Rev. Sci. Instrum. 2011, 82, 125105. [Google Scholar] [CrossRef]
- García-Vieyra, M.I.; Del Real, A.; López, M.G. Agave fructans, their effect on mineral absorption and bone mineral content. J. Med. Food. 2014, 17, 1247–1255. [Google Scholar] [CrossRef]
- Lee, Y.S.; Choi, C.W.; Kim, J.J.; Ganapathi, A.; Udayakumar, R.; Kim, S. Determination of mineral content in methanolic safflower (Carthamus tinctorius L.) seed extract and its effect on osteoblast markers. Int. J. Mol. Sci. 2009, 10, 292–305. [Google Scholar] [CrossRef] [Green Version]
- National Institutes of Health. Calcium: Dietary Supplement Fact Sheet. Office of Dietary Supplements. Available online: https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/ (accessed on 24 September 2018).
- Dike, N.K.; Chung-Ching, L.; Hardin, R.R.; Bruce, W.H. The aged rat model of ovarian hormone deficiency bone loss. Endocrinology 1988, 124, 7–15. [Google Scholar]
- Liu, B.; Wu, P.; Bringhurts, R.F.; Pein-Wen, W.; Jeng-Tzung, W. Estrogen inhibition of PTH-stimulated osteoclast formation and attachment in vitro: Involvement of both PKA and PKC. Endocrinology 2002, 2, 627–635. [Google Scholar] [CrossRef]
- Carrillo-López, N.; Román-García, P.; Rodríguez-Rebollar, A.; Fernández-Martín, J.L.; Naves-Díaz, M.; Cannata-Andía, J.B. Indirect regulation of PTH by estrogens may require FGF23. J. Am. Soc. Nephrol. 2009, 20, 2009–2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.W.; Liang, B.; Shi, X.L.; Wang, H. Opg/Rankl mRNA Dynamic expression in the bone tissue of ovariectomized rats with osteoporosis. Genet. Mol. Res. 2015, 14, 9215–9224. [Google Scholar] [CrossRef] [PubMed]
- Whisner, C.M.; Castillo, L.F. Prebiotics, bone and mineral metabolism. Calcif. Tissue Int. 2018, 102, 443–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavanda, I.; Isay-Saad, S.M.; Colli, C. Prebiotics and their effect on calcium bioavailability. Nutr. J. 2011, 24, 333–344. [Google Scholar]
- Escudero, E.; González-Sánchez, P. La fibra dietética. Nutr. Hosp. 2006, 21, 61–72. [Google Scholar]
- Cashman, K. Prebiotics and calcium bioavailability. Curr. Issues Intest. Microbiol. 2003, 4, 21–32. [Google Scholar]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Pacifici, R. Bone remodeling and the microbiome. Cold Spring Harb. Perspect. Med. 2018. [Google Scholar] [CrossRef]
- Goss, S.L.; Lemons, K.A.; Kerstetter, J.E.; Bogner, R.H. Determination of calcium salt solubility with changes in pH and P(CO2) simulating varying gastrointestinal environments. J. Pharm. Pharmacol. 2007, 59, 1485–1492. [Google Scholar] [CrossRef]
- Wang, H.; Bua, P.; Capodice, J. A comparative study of calcium absorption following a single serving administration of calcium carbonate powder versus calcium citrate tablets in healthy premenopausal women. Food. Nutr. Res. 2014. [Google Scholar] [CrossRef] [Green Version]
- González, M.J.; Olmos-Martínez, J.M. Fisiopatología de la osteoporosis y mecanismo de acción de la PTH. Rev. Osteoporos. Metab. Miner. 2010, 2, 85–117. [Google Scholar]
- Gibson, G.R.; Probert, H.M.; Loo, J.V.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piri, F.; Khosravi, A.; Moayeri, A.; Moradipour, A.; Derakhshan, S. The effects of dietary supplements of calcium, vitamin D and estrogen hormone on serum levels of OPG and RANKL cytokines and their relationship with increased bone density in rats. J. Clin. Diagn. Res. 2016, 10, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Quarles, L.D. How fibroblast growth factor 23 works. J. Am. Soc. Nephrol. 2007, 18, 1637–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wetmore, J.B. The link between estrogen and fibroblast growth factor 23. Am. J. Kidney Dis. 2011. [Google Scholar] [CrossRef]
- McConn, M.M.; Nakata, P.A. Oxalate reduces calcium availability in the pads of the Prickly Pear cactus through formation of calcium oxalate crystals. J. Agric. Food Chem. 2004, 52, 1371–1374. [Google Scholar] [CrossRef]
- Kressel, G.; Maike, W.; Hahn, A. Bioavailability and solubility of different calcium-salts as a basis for calcium enrichment of beverages. J. Food Nutr. Res. 2010, 1, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Guede, D.; González, P.; Caeiro, J.R. Biomeccánica y hueso (I): Conceptos básicos y ensayos mecánicos clásicos. Rev. Osteoporos. Metab. Miner. 2013, 5, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Clarke, B. Normal bone anatomy and phisiology. Clin. J. Am. Soc. Nephrol. 2008, 3, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Martin, B.R.; Mohammad, S.; Legette, L. Diet calcium level but not calcium supplement particle size affects bone density and mechanical properties in ovariectomized rats. Nutr. J. 2009. [Google Scholar] [CrossRef] [Green Version]
- Reddy, P.N.; Lakshmana, M. Prevention of bone loss in calcium deficient ovariectomized rats by OST-6, a herbal preparation. J. Ethnopharmacol. 2003, 84, 259–264. [Google Scholar] [CrossRef]
- Azuma, Y.; Oue, Y.; Kanatani, H.; Ohta, T.; Kiyoki, M.; Kmoriya, K. Effects of continuous alendronate treatment on bone mass and mechanical properties in ovariectomized rats: Comparison with pamidronate and etidronate in growing rats. J. Pharmacol. Exp. Ther. 1998, 286, 128–135. [Google Scholar] [PubMed]
- Koshihara, M.; Masuyama, R.; Uehara, M.; Suzuki, K. Reduction in dietary calcium/phosphorus ratio reduces bone mass and strength in ovariectomized rats enchancing bone turnover. Biosci. Biotechnol. Biochem. 2005, 69, 1970–1977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koshihara, M.; Masuyama, R.; Uehara, M.; Suzuki, K. Effect of dietary calcium: Phosphorus ratio on bone mineralization and intestinal calcium absorption in ovariectomized rats. Biofactors 2008, 22, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Wu, J.; Wang, S.; Wei, Y.; Chen, F.; Chen, J.; Shi, J.; Xia, J. Pycnogenol treatment inhibits bone mineral density los and trabecular deterioration in ovariectomized rats. Int. J. Clin. Exp. Med. 2015, 8, 10893–10901. [Google Scholar] [PubMed]
- Zhu, L.; Zhao, X.; Lu, X. Ovariectomy-associated changes in bone mineral density and bone marrow haematopoiesis in rats. Int. J. Exp. Pathol. 2009, 90, 512–519. [Google Scholar]
- Rodríguez-González, S.; Martínez-Flores, H.E.; Chávez-Moreno, C.K.; Macías-Rodríguez, L.I.; Zavala-Mendoza, E.; Garnica-Romo, M.G.; Chacón-García, L. Extraction and characterization of mucilage from wild species of Opuntia. J. Food Process Eng. 2014, 37, 285–292. [Google Scholar] [CrossRef]
- McGarvie, D.; Parolis, H. Methylation analysis of the mucilage of Opuntia ficus-indica. Carbohydr. Res. 1981, 88, 305–314. [Google Scholar] [CrossRef]
- Ariefdjohan, M.W.; Brown-Esters, O.N.; Savaiano, D.A. Intestinal microflora and diet in health. In Nutrition in the Prevention and Treatment of Disease, 3rd ed.; Coulston, A.M., Boushey, C.J., Ferruzzi, M.G., Eds.; Elsevier, Inc.: San Diego, CA, USA, 2013; pp. 719–738. [Google Scholar]
- Weaver, C.M. Diet, gut microbiome, and bone health. Curr. Osteoporos. Rep. 2015, 2, 125–130. [Google Scholar] [CrossRef]
- Chonan, O.; Watanuki, M. The effect of 6′-galactooligosaccharides on bone mineralization of rats adapted to different levels of dietary calcium. Int. J. Vitam. Nutr. Res. 1996, 66, 244–249. [Google Scholar]
- Rashka, L.; Daniel, H. Diet composition and age determine the effects of inulin-type fructans on intestinal calcium absorption in rat. Eur. J. Nutr. 2005, 44, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Conesa, D.; López, G.; Abellán, P.; Ros, G. Bioavailability of calcium, magnesium and phosphorus in rats fed probiotic, prebiotic and symbiotic powder follow-up infant formulas and their effect on physiological and nutritional parameters. J. Sci. Food Agric. 2006, 86, 2327–2336. [Google Scholar] [CrossRef]
- Pérez-Conesa, D.; López, G.; Ros, G. Effects of probiotic, prebiotic and symbiotic follow-up infant formulas on large intestine morphology and bone mineralization in rats. J. Sci. Food Agric. 2007, 87, 1059–1068. [Google Scholar] [CrossRef]
- Weaver, C.M.; Martin, B.R.; Nakatsu, C.H.; Armstrong, A.P.; Clavijo, A.; McCabe, L.D.; McCabe, G.P.; Duignan, S.; Schoterman, M.H.; van den Heuvel, E.G. Galactooligosaccharides improve mineral absorption and bone properties in growing rats through gut fermentation. J. Agric. Food Chem. 2011, 59, 6501–6510. [Google Scholar] [CrossRef] [PubMed]
- Scholz-Ahrens, K.; Schaafsma, G.; van den Heuvel, E.; Schrezenmeir, J. Effects of prebiotics on mineral metabolism. Am. J. Clin. Nutr. 2001, 73, 459–464. [Google Scholar] [CrossRef] [Green Version]
- Scholz-Ahrens, K.E.; Acil, Y.; Schrezenmeir, J. Effect of oligofructose or dietary calcium on repeated calcium and phosphorus balances, bone mineralization and trabecular structure in ovariectomized rats. Br. J. Nutr. 2002, 88, 365–377. [Google Scholar] [CrossRef]
- Bala, Y.; Bui, Q.M.; Wang, X.F.; Luliano, S.; Wang, Q.; Ghasem-Zadeh, A.; Rozental, T.D.; Bouxsein, M.L.; Zebaze, R.M.; Seeman, E. Trabecular and cortical microstructure and fragility of the distal radius women. J. Bone. Miner. Res. 2015, 30, 21–629. [Google Scholar] [CrossRef]
- National Osteoporosis Foundation. Balancing the Benefits and Risks of Osteoporosis Treatment. Available online: https://www.nof.org/preventing-fractures/nutrition-for-bone-health/webinar-series/ (accessed on 12 November 2019).
- Beto, J.A. The role of calcium in human aging. Clin. Nutr. Res. 2015, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Orimo, H. The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J. Nippon. Med. Sch. 2010, 77, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Grigoryan, A.V.; Dimitrova, A.A.; Kostov, K.G.; Russeva, A.L.; Atanasova, M.A.; Blagev, A.B.; Betova, T.M.; Trifonov, R.G. Changes of serum concentrations of alkaline phosphatase and metalloproteinase-9 in ovariectomized wistar rat model of osteoporosis. J. Biomed. Clin. Res. 2017, 10, 32–36. [Google Scholar] [CrossRef] [Green Version]
- Wheater, G.; Elshahaly, M.; Tuck, S.P.; Datta, H.K.; Laar, J.M. The clinical utility of bone marker measurements in osteoporosis. J. Transl. Med. 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patti, A.; Gennari, L.; Merlotti, D.; Dotta, F. Endocrine actions of osteocalcin. Int. J. Endocrinol. 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ce, C.; Zhou, L.; Yu, D.; Zhao, Y.; Yang, N. Serum osteocalcin levels and bone mineral density in ovariectomized rats. Int. J. Innov. Sci. Eng. Technol. 2014, 5, 1–8. [Google Scholar]
- Usha, K.; Nandeesh, B.N. Physiology of Bone Formation Remodeling, and Metabolism; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Sartori, M.; Giavaresi, G.; Parrilli, A.; Ferrari, A.; Aldini, N.N.; Morra, M.; Cassinelli, C.; Bollati, D.; Fini, M. Collagen type I coating stimulates bone regeneration and osteointegration of titanium implants in the osteopenic rat. Int. Orthop. 2015, 39, 2041–2052. [Google Scholar] [CrossRef] [PubMed]

| Groups | Control (Ctrl) | Calcium Citrate (CCa) | O. ficus-indica Powder (NI) | Soluble Dietary Fiber (FS) | Insoluble Dietary Fiber (FI) | |
|---|---|---|---|---|---|---|
| Ingredients | ||||||
| Corn starch | 621 | 621 | 621 | 621 | 621 | |
| Sucrose | 140 | 140 | 140 | 140 | 140 | |
| Casein a | 100 | 100 | 100 | 100 | 100 | |
| Soybean oil | 40 | 40 | 40 | 40 | 40 | |
| Fiber b | 50 | 50 | 33 | 43 | 35 | |
| MixMin c | 10 | 10 | 10 | 10 | 10 | |
| MixVit d | 35 | 35 | 35 | 35 | 35 | |
| L-Cystine | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | |
| Choline bitartrate | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | |
| CaCO3 e | 12.5 | - | - | - | - | |
| Calcium citrate | - | 0.207 | - | - | - | |
| O. ficus-indica powder | - | - | 48 | - | - | |
| Soluble fiber extracted from O. ficus-indica | - | - | - | 86 | - | |
| Insoluble fiber extracted from O. ficus-indica | - | - | - | - | 105 | |
| Content | Ctrl | CCa | NI | FS | FI |
|---|---|---|---|---|---|
| Moisture | 4.50 ± 0.02 a | 4.38 ± 0.02 a | 4.49 ± 0.02 a | 4.45 ± 0.03 a | 5.00 ± 0.06 a |
| Ashes | 3.24 ± 0.01 a | 3.28 ± 0.02 a | 3.60 ± 0.08 a | 3.19 ± 0.03 a | 3.37 ± 0.01 a |
| Carbohydrates ** | 76.90 ± 7.69 a | 73.98 ± 7.39 a | 80.65 ± 8.0 a | 74.92 ± 7.49 a | 77.70 ± 7.77 a |
| Protein ** | 11.94 ± 0.10 a | 13.30 ± 0.91 a | 11.58 ± 0.30 a | 11.68 ± 1.03 a | 11.54 ± 0.10 a |
| Lipids ** | 3.43 ± 0.37 a | 3.06 ± 0.10 a | 3.68 ± 0.11 a | 3.76 ± 0.16 a | 3.39 ± 0.14 a |
| cal/kg diet * | 386.21 ± 0.16 a | 394.67 ± 0.17 a | 376.02 ± 0.15 a | 371.21 ± 0.15 a | 378.42 ± 0.18 a |
| Magnesium | 0.06 ± 0.01 a | 0.05 ± 0.01 a | 0.11 ± 0.02 b | 0.08 ± 0.01 c | 0.09 ± 0.01 c |
| Potassium | 0.29 ± 0.01 a | 0.20 ± 0.01 a | 0.46 ± 0.02 b | 0.33 ± 0.01 c | 0.34 ± 0.02 c |
| Calcium | 0.59 ± 0.03 a | 0.54 ± 0.02 a | 0.55 ± 0.03 a | 0.55 ± 0.06 a | 0.54 ± 0.03 a |
| Phosphorus | 0.33 ± 0.02 a | 0.28 ± 0.01 b | 0.29 ± 0.01 b | 0.29 ± 0.02 b | 0.29 ± 0.01 b |
| Ca/P ratio | 1.79 ± 0.12 a | 1.89 ± 0.07 a | 1.86 ± 0.10 a | 1.87 ± 0.20 a | 1.83 ± 0.11 a |
| Parameters | Ctrl | SH | OVX | FS | CCa | NI | FI |
|---|---|---|---|---|---|---|---|
| Length (cm) | 3.6 ± 0.16 a | 3.5 ± 0.15 a | 3.3 ± 0.25 b | 3.6 ± 0.12 a | 3.6 ± 0.08 a | 3.6 ± 0.13 a | 3.6 ± 0.13 a |
| Weight (g) | 0.92 ± 0.16 a | 0.94 ± 0.03 a | 0.97 ± 0.09 a | 0.95 ± 0.12 a | 0.96 ± 0.07 a | 0.97 ± 0.05 a | 0.94 ± 0.08 a |
| Width (mm) | 4.0 ± 0.10 a | 4.0 ± 0.09 a | 4.0 ± 0.10 a | 4.0 ± 0.11 a | 4.0 ± 0.15 a | 4.0 ± 0.10 a | 4.0 ± 0.10 a |
| Thickness (mm) | 5.1 ± 0.05 a | 5.1 ± 0.03 a | 5.0 ± 0.05 a | 5.1 ± 0.04 a | 5.2 ± 0.06 a | 5.1 ± 0.05 a | 5.1 ± 0.05 a |
| Compression test Fmax (N) | 610.3 ± 46.6 a | 634 ± 45.8 a | 496.1 ± 24.9 b | 606.5 ± 15.8 a | 555.9 ± 14.8 c | 530.9 ± 37.7 c | 533.4 ± 26.5 c |
| Three-point bending test Pmax (N) | 86.3 ± 5.5 a | 90.6 ± 1.1 a | 65.7 ± 3.5 b | 89.9 ± 4.3 a | 85.4 ± 4.4 a | 74.87 ± 3.6 c | 73.0 ± 5.9 c |
| E (N/mm2) | 545.3 ± 82.5 a | 556.1 ± 23.7 a | 439.3 ± 15.4 b | 596.1 ± 78.4 c | 556.6 ± 19.6 a | 539.3 ± 15.9 a | 476.2 ± 19.7 b |
| Group | Ca (%) | P (%) | K (%) | Mg (%) | Ca/P Ratio |
|---|---|---|---|---|---|
| Ctrl | 22.64 ± 0.20 a | 17.66 ± 0.13 a | 0.21 ± 0.04 a | 0.74 ± 0.04 a | 1.28 ± 0.06 a |
| SH | 23.87 ± 0.19 a | 18.46 ± 0.14 a | 0.21 ± 0.06 a | 0.73 ± 0.02 a | 1.29 ± 0.01 a |
| OVX | 16.98 ± 0.98 b | 5.18 ± 0.05 b | 0.14 ± 0.10 b | 0.51 ± 0.02 b | 3.27 ± 0.16 b |
| FS | 22.91 ± 0.24 a | 18.60 ± 0.12 a | 0.19 ± 0.04 a | 0.43 ± 0.02 b | 1.23 ± 0.06 a |
| CCa | 21.13 ± 0.18 a | 17.99 ± 0.14 a | 0.18 ± 0.09 a | 0.44 ± 0.02 b | 1.17 ± 0.05 c |
| NI | 20.41 ± 0.22 c | 18.32 ± 0.09 a | 0.20 ± 0.04 a | 0.47 ± 0.02 b | 1.11 ± 0.08 c |
| FI | 20.11 ± 0.22 c | 18.77 ± 0.15 a | 0.19 ± 0.04 a | 0.45 ± 0.02 b | 1.07 ± 0.05 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintero-García, M.; Gutiérrez-Cortez, E.; Rojas-Molina, A.; Mendoza-Ávila, M.; Del Real, A.; Rubio, E.; Jiménez-Mendoza, D.; Rojas-Molina, I. Calcium Bioavailability of Opuntia ficus-indica Cladodes in an Ovariectomized Rat Model of Postmenopausal Bone Loss. Nutrients 2020, 12, 1431. https://doi.org/10.3390/nu12051431
Quintero-García M, Gutiérrez-Cortez E, Rojas-Molina A, Mendoza-Ávila M, Del Real A, Rubio E, Jiménez-Mendoza D, Rojas-Molina I. Calcium Bioavailability of Opuntia ficus-indica Cladodes in an Ovariectomized Rat Model of Postmenopausal Bone Loss. Nutrients. 2020; 12(5):1431. https://doi.org/10.3390/nu12051431
Chicago/Turabian StyleQuintero-García, Michelle, Elsa Gutiérrez-Cortez, Alejandra Rojas-Molina, Monsserrat Mendoza-Ávila, Alicia Del Real, Efraín Rubio, Daniel Jiménez-Mendoza, and Isela Rojas-Molina. 2020. "Calcium Bioavailability of Opuntia ficus-indica Cladodes in an Ovariectomized Rat Model of Postmenopausal Bone Loss" Nutrients 12, no. 5: 1431. https://doi.org/10.3390/nu12051431







