Discordant Dose-Dependent Metabolic Effects of Eicosapentanoic Acid in Diet-Induced Obese Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Diets and Study Design
2.2. Triglyceride Assay
2.3. Red Blood Cells Fatty Acid Composition
2.4. Glucose and Insulin Tolerance Tests
2.5. Body Composition
2.6. Metabolic Cages and Energetics
2.7. Plasma Insulin and Adipokine Measurements
2.8. Statistical Analysis
3. Results
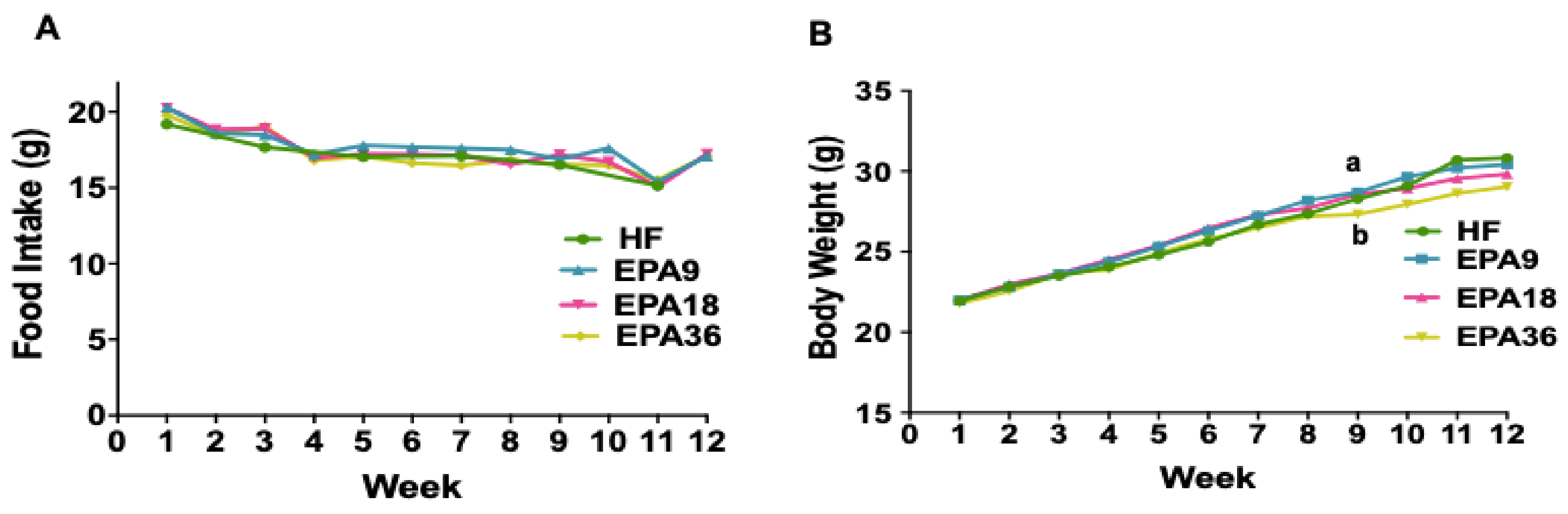
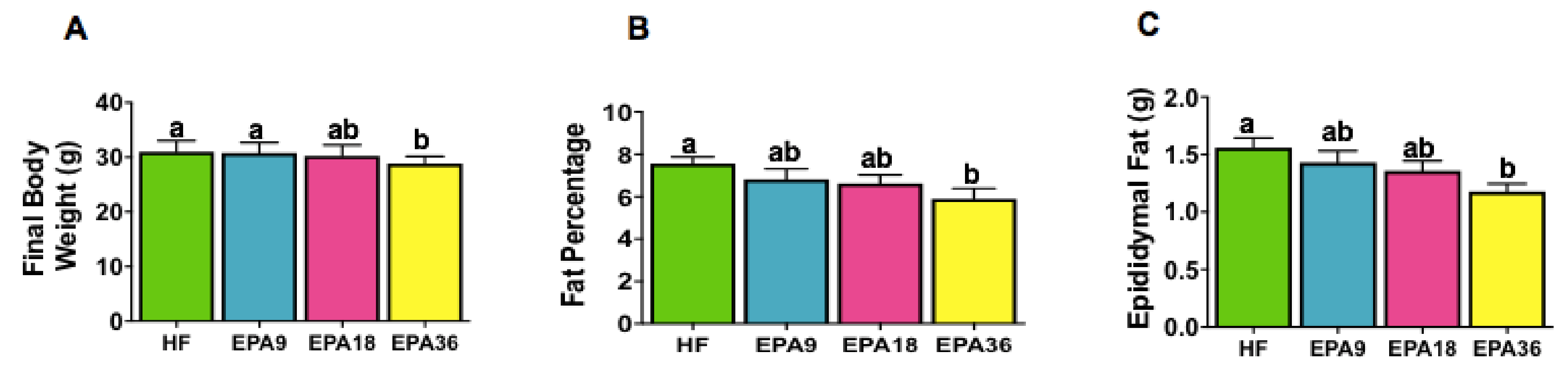
3.1. Effects of EPA on Food Intake, Body Weight, Fat Pad Weight, and Body Fat Percentage
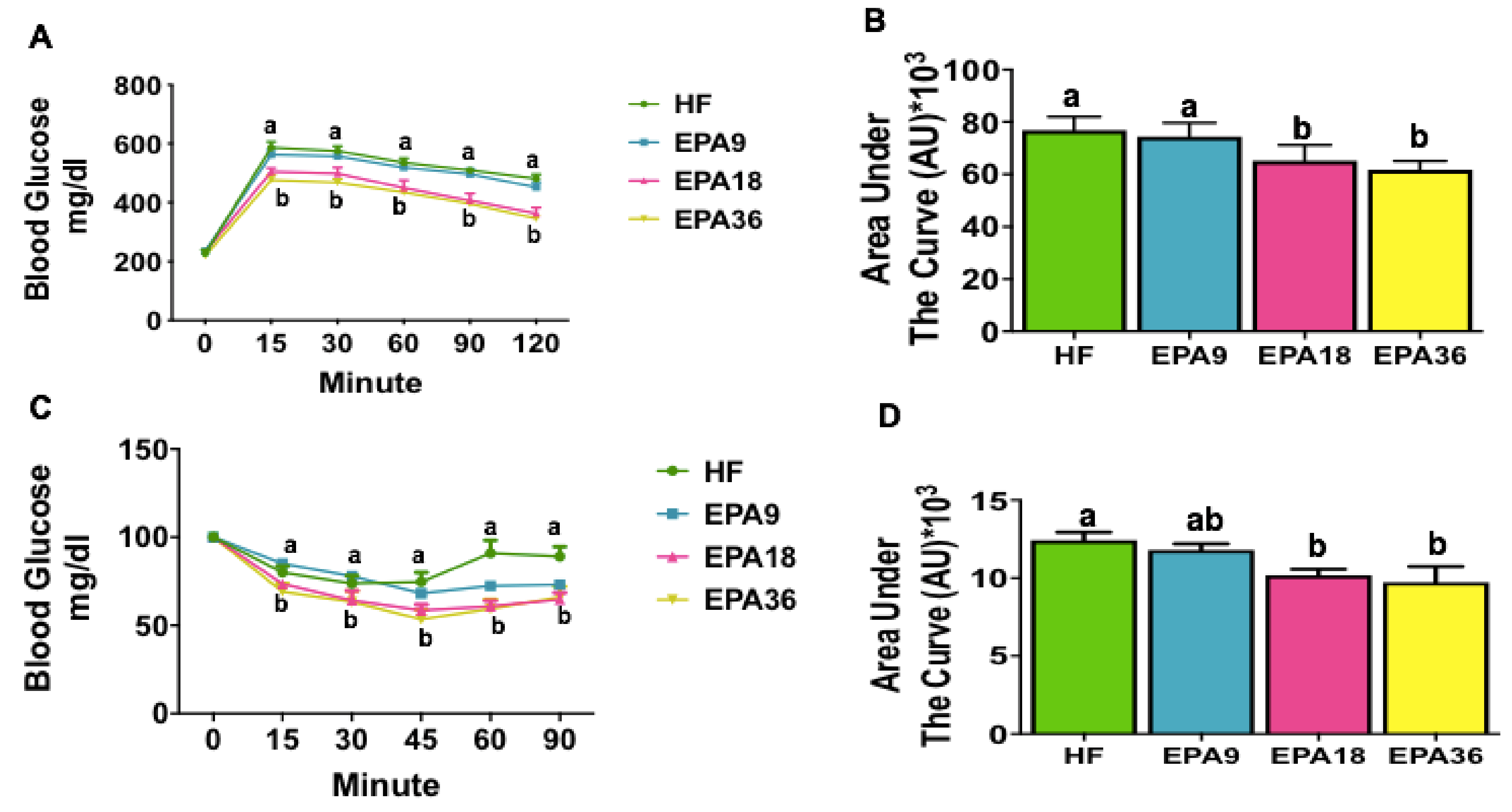
3.2. Effects of EPA on Glucose Homeostasis
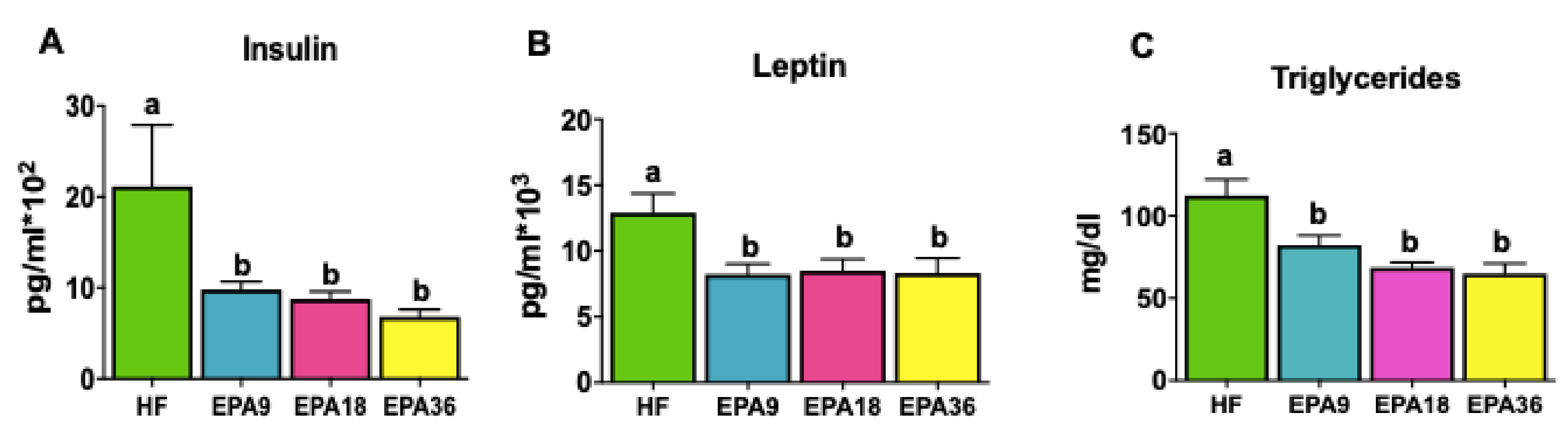
3.3. Effects of EPA on Obesity-Associated Metabolic Hormones and Triglycerides
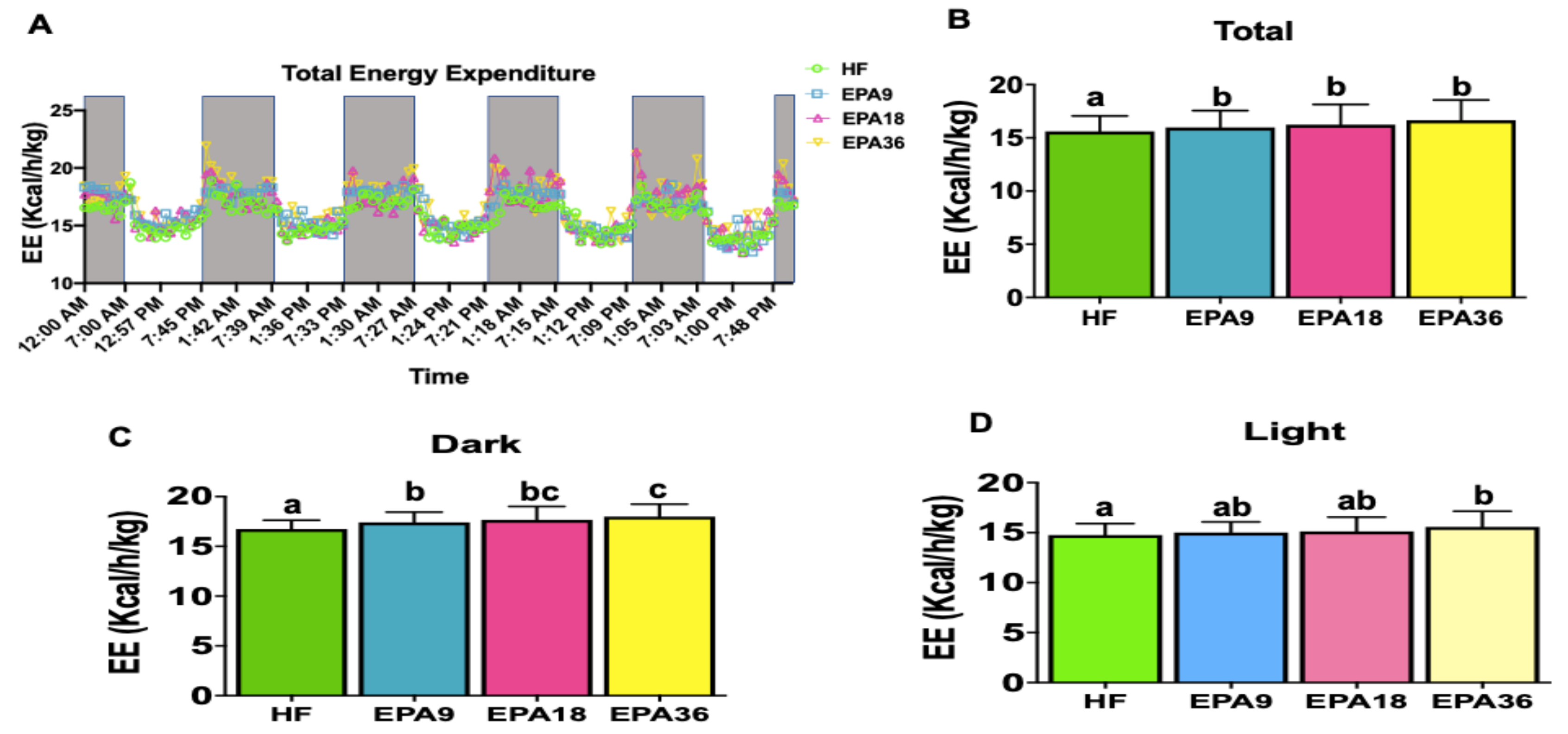
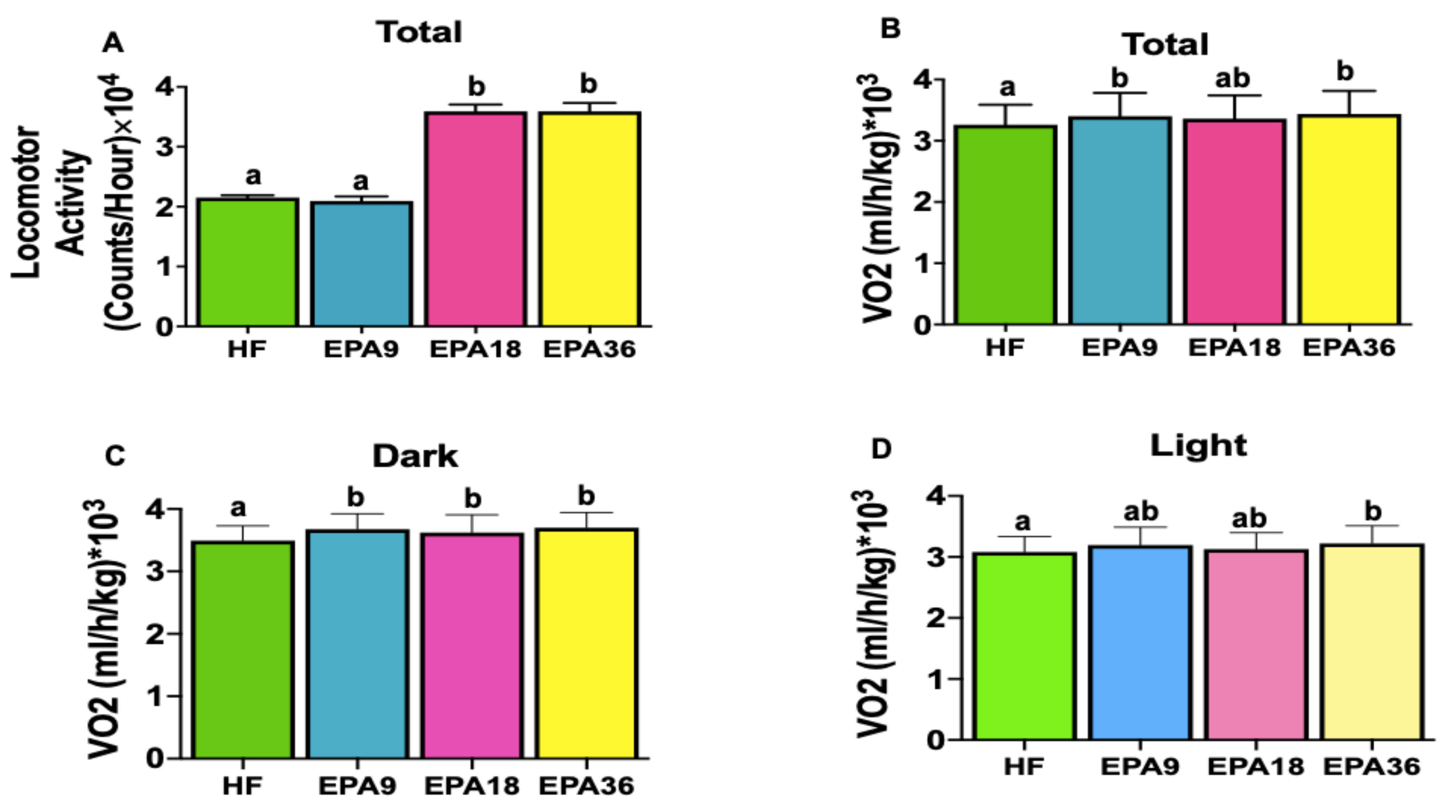
3.4. Effect of EPA on Energy Homeostasis
3.5. Fatty Acid Analysis in Mice Serum with Different Diets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Polyzos, S.A.; Mantzoros, C.S. Obesity: seize the day, fight the fat. Metab. Clin. Exp. 2019, 92, 1–5. [Google Scholar] [CrossRef]
- Lean, M.E.J.; Astrup, A.; Roberts, S.B. Making progress on the global crisis of obesity and weight management. BMJ 2018, 361, k2538. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: the linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Unamuno, X.; Gomez-Ambrosi, J.; Rodriguez, A.; Becerril, S.; Fruhbeck, G.; Catalan, V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur. J. Clin. Investig. 2018, 48, e12997. [Google Scholar] [CrossRef] [PubMed]
- Reilly, S.M.; Saltiel, A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017, 13, 633. [Google Scholar] [CrossRef]
- Kenney, E.L.; Wintner, S.; Lee, R.M.; Austin, S.B. Obesity Prevention Interventions in US Public Schools: Are Schools Using Programs That Promote Weight Stigma? Prev. Chronic Dis. 2017, 14, E142. [Google Scholar] [CrossRef] [PubMed]
- Jayarathne, S.; Koboziev, I.; Park, O.-H.; Oldewage-Theron, W.; Shen, C.-L.; Moustaid-Moussa, N. Anti-Inflammatory and Anti-Obesity Properties of Food Bioactive Components: Effects on Adipose Tissue. Prev. Nutr. Food Sci. 2017, 22, 251–262. [Google Scholar] [CrossRef] [PubMed]
- De Mello, A.H.; Uberti, M.F.; de Farias, B.X.; de Souza, N.A.R.; Rezin, G.T. n-3 PUFA and obesity: From peripheral tissues to the central nervous system. Br. J. Nutr. 2018, 119, 1312–1323. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.D.; Howe, P.R. Long-chain omega-3 polyunsaturated fatty acids may be beneficial for reducing obesity-a review. Nutrients 2010, 2, 1212–1230. [Google Scholar] [CrossRef] [PubMed]
- Golub, N.; Geba, D.; Mousa, S.A.; Williams, G.; Block, R.C. Greasing the wheels of managing overweight and obesity with omega-3 fatty acids. Med. Hypotheses 2011, 77, 1114–1120. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot Essent Fat. Acids 2006, 75, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.F.; Sinclair, A.J.; Kaur, G.; Li, D. Differential effects of EPA, DPA and DHA on cardio-metabolic risk factors in high-fat diet fed mice. Prostaglandins Leukot Essent Fat. Acids 2018, 136, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance - A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.E.; Kim, J.S.; Das, A. Emerging class of omega-3 fatty acid endocannabinoids & their derivatives. Prostaglandins Other Lipid Mediat. 2019, 143, 106337. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef]
- Burdge, G. Alpha-linolenic acid metabolism in men and women: nutritional and biological implications. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 137–144. [Google Scholar] [CrossRef]
- Flachs, P.; Rossmeisl, M.; Bryhn, M.; Kopecky, J. Cellular and molecular effects of n−3 polyunsaturated fatty acids on adipose tissue biology and metabolism. Clin. Sci. 2009, 116, 1–16. [Google Scholar] [CrossRef]
- González-Périz, A.; Horrillo, R.; Ferré, N.; Gronert, K.; Dong, B.; Morán-Salvador, E.; Titos, E.; Martínez-Clemente, M.; López-Parra, M.; Arroyo, V.; et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by ω-3 fatty acids: a role for resolvins and protectins. FASEB J. 2009, 23, 1946–1957. [Google Scholar] [CrossRef]
- Carracedo, M.; Artiach, G.; Arnardottir, H.; Back, M. The resolution of inflammation through omega-3 fatty acids in atherosclerosis, intimal hyperplasia, and vascular calcification. Semin. Immunopathol. 2019, 41, 757–766. [Google Scholar] [CrossRef]
- Allam-Ndoul, B.; Guénard, F.; Barbier, O.; Vohl, M.-C. Effect of n-3 fatty acids on the expression of inflammatory genes in THP-1 macrophages. Lipids Health Dis. 2016, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Kalupahana, N.S.; Claycombe, K.; Newman, S.J.; Stewart, T.; Siriwardhana, N.; Matthan, N.; Lichtenstein, A.H.; Moustaid-Moussa, N. Eicosapentaenoic Acid Prevents and Reverses Insulin Resistance in High-Fat Diet-Induced Obese Mice via Modulation of Adipose Tissue Inflammation. J. Nutr. 2010, 140, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Todorcevic, M.; Hodson, L. The Effect of Marine Derived n-3 Fatty Acids on Adipose Tissue Metabolism and Function. J. Clin. Med. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Umemoto, T.; Kakei, M.; Momomura, S.-i.; Kawakami, M.; Ishikawa, S.-e.; Hara, K. Eicosapentaenoic acid shows anti-inflammatory effect via GPR120 in 3T3-L1 adipocytes and attenuates adipose tissue inflammation in diet-induced obese mice. Nutr. Metab. 2017, 14, 33. [Google Scholar] [CrossRef]
- Pahlavani, M.; Razafimanjato, F.; Ramalingam, L.; Kalupahana, N.S.; Moussa, H.; Scoggin, S.; Moustaid-Moussa, N. Eicosapentaenoic acid regulates brown adipose tissue metabolism in high-fat-fed mice and in clonal brown adipocytes. J. Nutr. Biochem. 2017, 39, 101–109. [Google Scholar] [CrossRef]
- LeMieux, M.J.; Kalupahana, N.S.; Scoggin, S.; Moustaid-Moussa, N. Eicosapentaenoic Acid Reduces Adipocyte Hypertrophy and Inflammation in Diet-Induced Obese Mice in an Adiposity-Independent Manner. J. Nutr. 2014, 145, 411–417. [Google Scholar] [CrossRef]
- Khan, R.S.; Chokshi, A.; Drosatos, K.; Jiang, H.; Yu, S.; Harris, C.R.; Schulze, P.C.; Homma, S.; Blaner, W.S.; Shulman, G.I.; et al. Fish oil selectively improves heart function in a mouse model of lipid-induced cardiomyopathy. J. Cardiovasc. Pharm. 2013, 61, 345–354. [Google Scholar] [CrossRef]
- Pinel, A.; Pitois, E.; Rigaudiere, J.-P.; Jouve, C.; De Saint-Vincent, S.; Laillet, B.; Montaurier, C.; Huertas, A.; Morio, B.; Capel, F. EPA prevents fat mass expansion and metabolic disturbances in mice fed with a Western diet. J. Lipid Res. 2016, 57, 1382–1397. [Google Scholar] [CrossRef]
- Kunz, H.E.; Dasari, S.; Lanza, I.R. EPA and DHA elicit distinct transcriptional responses to high-fat feeding in skeletal muscle and liver. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E460–E472. [Google Scholar] [CrossRef]
- Du, S.; Jin, J.; Fang, W.; Su, Q. Does Fish Oil Have an Anti-Obesity Effect in Overweight/Obese Adults? A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2015, 10, e0142652. [Google Scholar] [CrossRef]
- Miller, P.E.; Van Elswyk, M.; Alexander, D.D. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. Am. J. Hypertens. 2014, 27, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Skulas-Ray Ann, C.; Wilson Peter, W.F.; Harris William, S.; Brinton Eliot, A.; Kris-Etherton Penny, M.; Richter Chesney, K.; Jacobson Terry, A.; Engler Mary, B.; Miller, M.; Robinson Jennifer, G.; et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory From the American Heart Association. Circulation 2019, 140, e673–e691. [Google Scholar] [CrossRef] [PubMed]
- O’Fallon, J.V.; Busboom, J.R.; Nelson, M.L.; Gaskins, C.T. A direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils, and feedstuffs. J. Anim. Sci. 2007, 85, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Wijayatunga, N.N.; Sams, V.G.; Dawson, J.A.; Mancini, M.L.; Mancini, G.J.; Moustaid-Moussa, N. Roux-en-Y gastric bypass surgery alters serum metabolites and fatty acids in patients with morbid obesity. Diabetes Metab. Res. Rev. 2018, 34, e3045. [Google Scholar] [CrossRef]
- Ayala, J.E.; Samuel, V.T.; Morton, G.J.; Obici, S.; Croniger, C.M.; Shulman, G.I.; Wasserman, D.H.; McGuinness, O.P. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis. Model. Mech. 2010, 3, 525–534. [Google Scholar] [CrossRef]
- Qaid, M.M.; Abdelrahman, M.M. Role of insulin and other related hormones in energy metabolism—A review. Cogent Food Agric. 2016, 2, 1267691. [Google Scholar] [CrossRef]
- Azab, N.; Abdel-Aziz, T.; Ahmed, A.; El-deen, I.M. Correlation of serum resistin level with insulin resistance and severity of retinopathy in type 2 diabetes mellitus. J. Saudi Chem. Soc. 2016, 20, 272–277. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Khaza’ai, H.; Abed, Y.; Rahmat, A.; Ismail, P.; Ranneh, Y. Role of fish oil in human health and possible mechanism to reduce the inflammation. Inflammopharmacology 2015, 23, 79–89. [Google Scholar] [CrossRef]
- Soni, N.; Ross, A.B.; Scheers, N.; Nookaew, I.; Gabrielsson, B.G.; Sandberg, A.-S. The Omega-3 Fatty Acids EPA and DHA, as a Part of a Murine High-Fat Diet, Reduced Lipid Accumulation in Brown and White Adipose Tissues. Int. J. Mol. Sci. 2019, 20, 5895. [Google Scholar] [CrossRef]
- Drouin, G.; Rioux, V.; Legrand, P. The n-3 docosapentaenoic acid (DPA): A new player in the n-3 long chain polyunsaturated fatty acid family. Biochimie 2019, 159, 36–48. [Google Scholar] [CrossRef]
- Tian, Y.; Katsuki, A.; Romanazzi, D.; Miller, M.R.; Adams, S.L.; Miyashita, K.; Hosokawa, M. Docosapentaenoic Acid (22:5n-3) Downregulates mRNA Expression of Pro-inflammatory Factors in LPS-activated Murine Macrophage Like RAW264.7 Cells. J. Oleo Sci. 2017, 66, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Dai, Z.; Cao, Y.; Shen, Q.; Zhang, Y. Docosapentaenoic acid (DPA, 22:5n-3) ameliorates inflammation in an ulcerative colitis model. Food Funct. 2019, 10, 4199–4209. [Google Scholar] [CrossRef] [PubMed]
- Albracht-Schulte, K.; Gonzalez, S.; Jackson, A.; Wilson, S.; Ramalingam, L.; Kalupahana, N.S.; Moustaid-Moussa, N. Eicosapentaenoic Acid Improves Hepatic Metabolism and Reduces Inflammation Independent of Obesity in High-Fat-Fed Mice and in HepG2 Cells. Nutrients 2019, 11, 599. [Google Scholar] [CrossRef] [PubMed]
- Rossmeisl, M.; Medrikova, D.; van Schothorst, E.M.; Pavlisova, J.; Kuda, O.; Hensler, M.; Bardova, K.; Flachs, P.; Stankova, B.; Vecka, M.; et al. Omega-3 phospholipids from fish suppress hepatic steatosis by integrated inhibition of biosynthetic pathways in dietary obese mice. Biochim. Biophys. Acta 2014, 1841, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Philp, L.K.; Heilbronn, L.K.; Janovska, A.; Wittert, G.A. Dietary enrichment with fish oil prevents high fat-induced metabolic dysfunction in skeletal muscle in mice. PLoS ONE 2015, 10, e0117494. [Google Scholar] [CrossRef]
- Dai, Y.; Zhou, N.; Yang, F.; Zhou, S.; Sha, L.; Wang, J.; Li, X. Effects of postnatal overfeeding and fish oil diet on energy expenditure in rats. Pediatric Res. 2017, 83, 156–163. [Google Scholar] [CrossRef]
- Cussons, A.J.; Watts, G.F.; Mori, T.A.; Stuckey, B.G. Omega-3 fatty acid supplementation decreases liver fat content in polycystic ovary syndrome: a randomized controlled trial employing proton magnetic resonance spectroscopy. J. Clin. Endocrinol. Metab. 2009, 94, 3842–3848. [Google Scholar] [CrossRef]
- Wong, A.T.; Chan, D.C.; Barrett, P.H.; Adams, L.A.; Watts, G.F. Supplementation with n3 fatty acid ethyl esters increases large and small artery elasticity in obese adults on a weight loss diet. J. Nutr. 2013, 143, 437–441. [Google Scholar] [CrossRef]
- Mohammadi, E.; Rafraf, M.; Farzadi, L.; Asghari-Jafarabadi, M.; Sabour, S. Effects of omega-3 fatty acids supplementation on serum adiponectin levels and some metabolic risk factors in women with polycystic ovary syndrome. Asia Pac. J. Clin. Nutr. 2012, 21, 511–518. [Google Scholar]
- Rafraf, M.; Mohammadi, E.; Asghari-Jafarabadi, M.; Farzadi, L. Omega-3 fatty acids improve glucose metabolism without effects on obesity values and serum visfatin levels in women with polycystic ovary syndrome. J. Am. Coll. Nutr. 2012, 31, 361–368. [Google Scholar] [CrossRef]
- Islam, R.; Trépanier, M.-O.; Milenkovic, M.; Horsfall, W.; Salahpour, A.; Bazinet, R.P.; Ramsey, A.J. Vulnerability to omega-3 deprivation in a mouse model of NMDA receptor hypofunction. npj Schizophrenia 2017, 3, 12. [Google Scholar] [CrossRef]
- Crochemore, I.C.; Souza, A.F.; de Souza, A.C.; Rosado, E.L. omega-3 polyunsaturated fatty acid supplementation does not influence body composition, insulin resistance, and lipemia in women with type 2 diabetes and obesity. Nutr. Clin. Pr. 2012, 27, 553–560. [Google Scholar] [CrossRef] [PubMed]
- DeFina, L.F.; Marcoux, L.G.; Devers, S.M.; Cleaver, J.P.; Willis, B.L. Effects of omega-3 supplementation in combination with diet and exercise on weight loss and body composition. Am. J. Clin. Nutr. 2011, 93, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Jannas-Vela, S.; Roke, K.; Boville, S.; Mutch, D.M.; Spriet, L.L. Lack of effects of fish oil supplementation for 12 weeks on resting metabolic rate and substrate oxidation in healthy young men: A randomized controlled trial. PLoS ONE 2017, 12, e0172576. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Abdelmalek, M.F.; Suzuki, A.; Cummings, O.W.; Chojkier, M. No Significant Effects of Ethyl-Eicosapentanoic Acid on Histologic Features of Nonalcoholic Steatohepatitis in a Phase 2 Trial. Gastroenterology 2014, 147, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.B.S. Direct and indirect effects of leptin on adipocyte metabolism. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2014, 1842, 414–423. [Google Scholar] [CrossRef]
- Morrison, C. Interaction Between Exercise and Leptin in the Treatment of Obesity. Diabetes 2008, 57, 534. [Google Scholar] [CrossRef][Green Version]
- Magkos, F. Metabolically healthy obesity: what’s in a name? Am. J. Clin. Nutr. 2019, 110, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Bortolin, R.C.; Vargas, A.R.; Gasparotto, J.; Chaves, P.R.; Schnorr, C.E.; Martinello, K.B.; Silveira, A.K.; Rabelo, T.K.; Gelain, D.P.; Moreira, J.C.F. A new animal diet based on human Western diet is a robust diet-induced obesity model: comparison to high-fat and cafeteria diets in term of metabolic and gut microbiota disruption. Int. J. Obes. 2018, 42, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Drescher, H.K.; Weiskirchen, R.; Fülöp, A.; Hopf, C.; de San Román, E.G.; Huesgen, P.F.; de Bruin, A.; Bongiovanni, L.; Christ, A.; Tolba, R.; et al. The Influence of Different Fat Sources on Steatohepatitis and Fibrosis Development in the Western Diet Mouse Model of Non-alcoholic Steatohepatitis (NASH). Front. Physiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Medicine, I.o. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005; p. 1358. [Google Scholar] [CrossRef]
- Nair, A.; Morsy, M.A.; Jacob, S. Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug Dev. Res. 2018. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Dou, D.; Ran, X.; Kang, T. Neuroprotective effect of arctigenin against neuroinflammation and oxidative stress induced by rotenone. RSC Adv. 2018, 8, 2280–2292. [Google Scholar] [CrossRef]
- Banfi, S.; Gusarova, V.; Gromada, J.; Cohen, J.C.; Hobbs, H.H. Increased thermogenesis by a noncanonical pathway in ANGPTL3/8-deficient mice. Proc. Natl. Acad. Sci. USA 2018, 115, E1249–E1258. [Google Scholar] [CrossRef]
- Fernandez-Verdejo, R.; Ravussin, E.; Speakman, J.R.; Galgani, J.E. Progress and challenges in analyzing rodent energy expenditure. Nat. Methods 2019, 16, 797–799. [Google Scholar] [CrossRef] [PubMed]
- Virtue, S.; Even, P.; Vidal-Puig, A. Below thermoneutrality, changes in activity do not drive changes in total daily energy expenditure between groups of mice. Cell Metab. 2012, 16, 665–671. [Google Scholar] [CrossRef]
- Orringer, C.E. Icosapent ethyl: Where will it fit into guideline-based medical therapy for high risk atherosclerotic cardiovascular disease? Trends Cardiovasc. Med. 2019. [Google Scholar] [CrossRef]
- Michos, E.D.; McEvoy, J.W.; Blumenthal, R.S. Lipid Management for the Prevention of Atherosclerotic Cardiovascular Disease. N. Engl. J. Med. 2019, 381, 1557–1567. [Google Scholar] [CrossRef]
| Procedure | Start of Diet (Mice Age 5–6 weeks) | GTT | Body Composition | ITT | Metabolic Cage | Euthanasia |
|---|---|---|---|---|---|---|
| Time (weeks) | 0 | 10 | 11 | 12 | 13 | 14 |
| HF | EPA9 | EPA18 | EPA36 | p-Value | |
|---|---|---|---|---|---|
| Saturated Fatty Acids | 40.94 ± 3.7 | 46.22 ± 4.1 | 46.76 ± 4.2 | 50.17 ± 4.6 | NS |
| C15:0 pentadecanoic acid | 0.22 ± 0.22 | 0.53 ± 0.27 | 0.70 ± 0.05 | 0.48 ± 0.24 | NS |
| C16:0 palmitic acid | 25.52 ± 0.77 a | 28.76 ± 0.37 b | 29.84 ± 0.61 b | 32.29 ± 0.64bc | <0.01 |
| C18:0 octadecanoic acid | 15.20 ± 0.16 a | 16.93 ± 0.18 ab | 16.22 ± 0.05 ab | 17.40 ± 0.27 b | <0.02 |
| Monosaturated Fatty Acids | 26.36 ± 2.3 | 19.12 ± 2 | 19.61 ± 2 | 17.72 ± 2.3 | NS |
| C17:1 Heptadecenoic acid | 0.160 ± 0.27 | 0.35 ± 0.70 | 0.45 ± 0.19 | 0.00 | NS |
| C18:1, trans Elaidic acid | 12.29 ± 1.56 a | 5.16 ± 0.91b | 5.60 ± 1.45 b | 2.71 ± 0.43 b | <0.01 |
| C18:1, cis oleic acid | 13.91 ± 1.72 | 13.61 ± 0.19 | 13.56 ± 0.35 | 15.01 ± 0.27 | NS |
| PUFA | 25.51 ± 1.35 | 32.4 ± 0.52 | 30.36 ± 0.82 | 28.9 ± 0.92 | NS |
| ω6 Fatty Acids | 22.07 ± 1.9 | 22.56 ± 1.4 | 18.04 ± 1.2 | 14.19 ± 1 | NS |
| C18:2, trans linolelaidic acid | 0.80 ± 0.40 | 1.14 ± 0.01 | 1.20 ± 0.02 | 1.11 ± 0.02 | NS |
| C18:2, cis linoleic acid | 10.32 ± 0.81 ab | 10.76 ± 0.16 a | 9.51 ± 0.11 ab | 8.83 ± 0.11 b | <0.04 |
| C20:3 eicosatrienoic acid | 0.00 a | 0.59 ± 0.02 b | 0.16 ± 0.16 a | 0.00a | <0.003 |
| C20:4 arachidonic acid | 10.95 ± 5.16 | 10.07 ± 0.11 | 7.17 ± 0.19 | 4.25 ± 0.09 | NS |
| ω3 Fatty Acids | 3.44 ± 0.8 | 9.84 ± 0.1 | 12.32 ± 0.5 | 14.71 ± 1.4 | NS |
| C20:5 eicosapentaenoic acid (EPA) | 0.00 a | 4.66 ± 0.09 b | 7.31 ± 0.37 c | 10.58 ± 0.16 d | <0.0001 |
| C22:6 docosahexaenoic acid (DHA) | 3.44 ± 0.17 a | 5.18 ± 0.05 b | 5.01 ± 0.10 b | 4.13 ± 0.06 c | <0.0001 |
| Body Weight | Fat Percentage | Glycemia (GTT) | Triglycerides | Fasting Insulin | Fasting Leptin | Energy Expenditure | Oxygen Consumption | Locomotor Activity | |
|---|---|---|---|---|---|---|---|---|---|
| EPA9 | NS | NS | NS | Down | Down | Down | Up | Up | NS |
| EPA18 | NS | NS | Down | Down | Down | Down | Up | NS | Up |
| EPA36 | Down | Down | Down | Down | Down | Down | Up | Up | Up |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pahlavani, M.; Ramalingam, L.; Miller, E.K.; Davis, H.; Scoggin, S.; Moustaid-Moussa, N. Discordant Dose-Dependent Metabolic Effects of Eicosapentanoic Acid in Diet-Induced Obese Mice. Nutrients 2020, 12, 1342. https://doi.org/10.3390/nu12051342
Pahlavani M, Ramalingam L, Miller EK, Davis H, Scoggin S, Moustaid-Moussa N. Discordant Dose-Dependent Metabolic Effects of Eicosapentanoic Acid in Diet-Induced Obese Mice. Nutrients. 2020; 12(5):1342. https://doi.org/10.3390/nu12051342
Chicago/Turabian StylePahlavani, Mandana, Latha Ramalingam, Emily K. Miller, Hanna Davis, Shane Scoggin, and Naima Moustaid-Moussa. 2020. "Discordant Dose-Dependent Metabolic Effects of Eicosapentanoic Acid in Diet-Induced Obese Mice" Nutrients 12, no. 5: 1342. https://doi.org/10.3390/nu12051342
APA StylePahlavani, M., Ramalingam, L., Miller, E. K., Davis, H., Scoggin, S., & Moustaid-Moussa, N. (2020). Discordant Dose-Dependent Metabolic Effects of Eicosapentanoic Acid in Diet-Induced Obese Mice. Nutrients, 12(5), 1342. https://doi.org/10.3390/nu12051342






