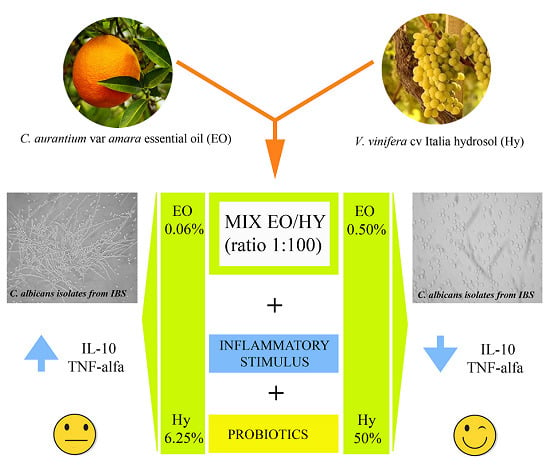
Potent In Vitro Activity of Citrus aurantium Essential Oil and Vitis vinifera Hydrolate Against Gut Yeast Isolates from Irritable Bowel Syndrome Patients—The Right Mix for Potential Therapeutic Use
Abstract
1. Introduction
2. Materials and Methods
2.1. EO and Hy Natural Substances
2.2. Fungal and Bacterial Organisms
2.3. Broth Microdilution Susceptibility Testing
2.4. Peripheral Blood Mononuclear Cell (PBMC) Collection and ELISA Testing
2.5. DPPH (2,2-Diphenyl-1-Picryl-Hydrazyl-Hydrate) Assay
2.6. GC-MS Analysis
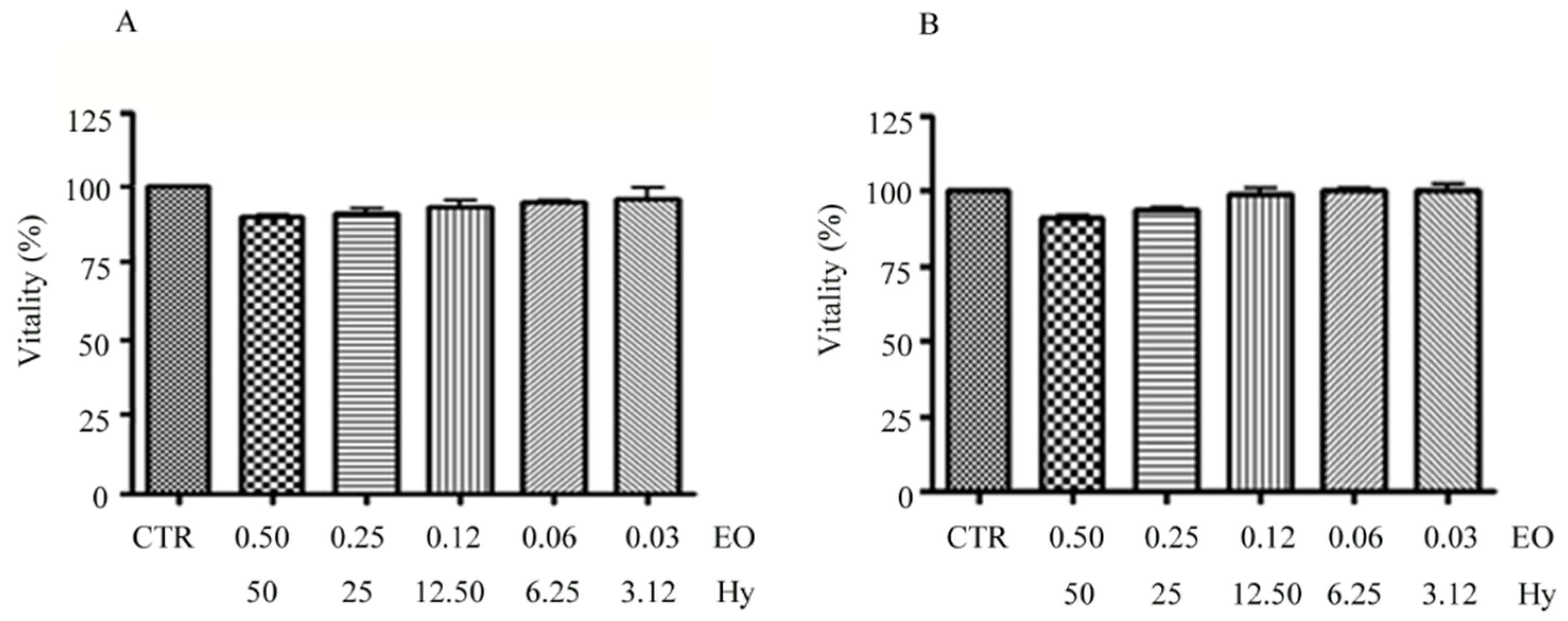
2.7. Cytotoxicity Assays
2.8. Statistical Analysis
3. Results
3.1. Antimicrobial Activities of EOs
3.2. Immunomodulatory and Antioxidant Activities of the V. vinifera cv Italia Hy
3.3. Effectiveness of the C. aurantium var. Amara EO and V. vinifera cv Italia Hy Combination
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Canavan, C.; West, J.; Card, T. The epidemiology of irritable bowel syndrome. Clin. Epidemiol. 2014, 6, 71–80. [Google Scholar] [PubMed]
- Abdul Rani, R.; Raja Ali, R.A.; Lee, Y.Y. Irritable bowel syndrome and inflammatory bowel disease overlap syndrome: Pieces of the puzzle are falling into place. Intest. Res. 2016, 14, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.D.; Spiegel, B.M.; Hays, R.D.; Melmed, G.Y.; Bolus, R.; Khanna, D.; Khanna, P.P.; Chang, L. Gastrointestinal symptom severity in irritable bowel syndrome, inflammatory bowel disease and the general population. Neurogastroenterol. Motil. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Petitpierre, M.; Gumowski, P.; Girard, J.P. Irritable bowel syndrome and hypersensitivity to food. Ann. Allergy 1985, 54, 538–540. [Google Scholar]
- Santelmann, H.; Howard, J.M. Yeast metabolic products, yeast antigens and yeasts as possible triggers for irritable bowel syndrome. Eur. J. Gastroenterol. Hepatol. 2005, 17, 21–26. [Google Scholar] [CrossRef]
- Cayzeele-Decherf, A.; Pélerin, F.; Leuillet, S.; Douillard, B.; Housez, B.; Cazaubiel, M.; Jacobson, G.K.; Jüsten, P.; Desreumaux, P. Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: An individual subject meta-analysis. World J. Gastroenterol. 2017, 23, 336–344. [Google Scholar] [CrossRef]
- Spiller, R.; Pélerin, F.; Cayzeele Decherf, A.; Maudet, C.; Housez, B.; Cazaubiel, M.; Jüsten, P. Randomized double blind placebo-controlled trial of Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: Improvement in abdominal pain and bloating in those with predominant constipation. United Eur. Gastroenterol. J. 2016, 4, 353–362. [Google Scholar] [CrossRef]
- Choi, C.H.; Jo, S.Y.; Park, H.J.; Chang, S.K.; Byeon, J.S.; Myung, S.J. A randomized, double-blind, placebo-controlled multicenter trial of Saccharomyces boulardii in irritable bowel syndrome: Effect on quality of life. J. Clin. Gastroenterol. 2011, 45, 679–683. [Google Scholar] [CrossRef]
- Cruz-Aguliar, R.M.; Wantia, N.; Clavel, T.; Vehreschild, M.J.G.T.; Buch, T.; Bajbouj, M.; Haller, D.; Busch, D.; Schmid, R.M.; Stein-Thoeringer, C.K. An open-labeled study on fecal microbiota transfer in irritable bowel syndrome patients reveals improvement in abdominal pain associated with the relative abundance of Akkermansia Muciniphila. Digestion 2018, 13, 1–12. [Google Scholar] [CrossRef]
- Sokol, H.; Leducq, V.; Aschard, H.; Pham, H.P.; Jegou, S.; Landman, C.; Cohen, D.; Liguori, G.; Bourrier, A.; Nion-Larmurier, I.; et al. Fungal microbiota dysbiosis in IBD. Gut 2017, 66, 1039–1048. [Google Scholar] [CrossRef]
- Di Vito, M.; Fracchiolla, G.; Mattarelli, P.; Modesto, M.; Tamburro, A.; Padula, F.; Agatensi, L.; Giorlandino, F.R.; Girolamo, A.; Carbonara, G.G.; et al. Probiotic and Tea Tree Oil treatments improve therapy of vaginal candidiasis: A preliminary clinical study. Med. J. Obstet. Gynecol. 2016, 4, 1090–1096. [Google Scholar]
- Bashashati, M.; Rezaei, N.; Shafieyoun, A.; McKernan, D.P.; Chang, L.; Öhman, L.; Quigley, E.M.; Schmulson, M.; Sharkey, K.A.; Simrén, M. Cytokine imbalance in irritable bowel syndrome: A systematic review and meta-analysis. Neurogastroenterol. Motil. 2014, 26, 1036–1048. [Google Scholar] [CrossRef] [PubMed]
- Bennet, S.M.; Polster, A.; Törnblom, H.; Isaksson, S.; Capronnier, S.; Tessier, A.; Le Nevé, B.; Simrén, M.; Öhman, L. Global cytokine profiles and association with clinical characteristics in patients with irritable bowel syndrome. Am. Gastroenterol. 2016, 111, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, A.; Bruninga, K.; Fields, J.; Keshavarzian, A. Irritable bowel syndrome: An update on therapeutic modalities. Expert Opin. Investig. Drugs 2001, 10, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Peckham, E.J.; Nelson, E.A.; Greenhalgh, J.; Cooper, K.; Roberts, E.R.; Agrawal, A. Homeopathy for treatment of irritable bowel syndrome. Cochrane Database Syst. Rev. 2013, 13, CD009710. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Portincasa, P.; Maes, N.; Albert, A. Efficacy of bio-optimized extracts of turmeric and essential fennel oil on the quality of life in patients with irritable bowel syndrome. Ann. Gastroenterol. 2018, 31, 685–691. [Google Scholar] [CrossRef]
- Botschuijver, S.; Roeselers, G.; Levin, E.; Jonkers, D.M.; Welting, O.; Heinsbroek, S.E.M.; de Weerd, H.H.; Boekhout, T.; Fornai, M.; Masclee, A.A.; et al. Intestinal fungal dysbiosis is associated with visceral hypersensitivity in patients with irritable bowel syndrome and rats. Gastroenterology 2017, 153, 1026–1039. [Google Scholar] [CrossRef]
- Mahboubi, M. Therapeutic Potential of Zataria multiflora Boiss in Treatment of Irritable Bowel Syndrome (IBS). J. Diet. Suppl. 2019, 16, 119–128. [Google Scholar] [CrossRef]
- Mosaffa-Jahromi, M.; Lankarani, K.B.; Pasalar, M.; Afsharypuor, S.; Tamaddon, A.M. Efficacy and safety of enteric coated capsules of anise oil to treat irritable bowel syndrome. J. Ethnopharmacol. 2016, 194, 937–946. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Scribano, M.L.; Kohn, A.; Caporaso, N.; Festi, D.; Campanale, M.C.; Di Rienzo, T.; Guarino, M.; Taddia, M.; et al. curcumin and fennel essential oil improve symptoms and quality of life in patients with Irritable Bowel Syndrome. A. J. Gastrointestin. Liver Dis. 2016, 25, 151–157. [Google Scholar] [CrossRef]
- Thompson, A.; Meah, D.; Ahmed, N.; Conniff-Jenkins, R.; Chileshe, E.; Phillips, C.O.; Claypole, T.C.; Forman, D.W.; Row, P.E. Comparison of the antibacterial activity of essential oils and extracts of medicinal and culinary herbs to investigate potential new treatments for irritable bowel syndrome. BMC Complement. Altern. Med. 2013, 28, 338. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Roy, P.K.; Miah, A.R.; Mollick, S.H.; Khan, M.R.; Mahmud, M.C.; Khatun, S. Efficacy of Peppermint oil in diarrhea predominant IBS—A double blind randomized placebo—Controlled study. Mymensingh Med. J. 2013, 22, 27–30. [Google Scholar] [PubMed]
- D’Amato, S.; Serio, A.; Chaves Lopez, C.; Paparella, A. Hydrosols: Biological activity and potential as antimicrobials for food applications. Food Control 2018, 86, 126–137. [Google Scholar] [CrossRef]
- Strati, F.; Di Paola, M.; Stefanini, I.; Albanese, D.; Rizzetto, L.; Lionetti, P.; Calabrò, A.; Jousson, O.; Donati, C.; Cavalieri, D.; et al. Age and gender affect the composition of fungal population of the human gastrointestinal tract. Front. Microbiol. 2016, 7, 1227. [Google Scholar] [CrossRef]
- Mattarelli, P.; Epifano, F.; Minardi, P.; Di Vito, M.; Modesto, M.; Barbanti, L.; Bellardi, M.G. Chemical composition and antimicrobial activity of essential oils from aerial parts of Monarda didyma and Monarda fistulosa cultivated in Italy. J. Essent. Oil Bear. Plants 2017, 20, 76–86. [Google Scholar] [CrossRef]
- Radicioni, G.; Stringaro, A.; Molinari, A.; Nocca, G.; Longhi, R.; Pirolli, D.; Scarano, E.; Iavarone, F.; Manconi, B.; Cabras, T.; et al. Characterization of the cell penetrating properties of a human salivary proline-rich peptide. Biochim. Biophys. Acta 2015, 1848, 2868–2877. [Google Scholar] [CrossRef] [PubMed]
- Agah, S.; Taleb, A.M.; Moeini, R.; Gorji, N.; Nikbakht, H. Cumin extract for symptom control in patients with irritable bowel syndrome: A case series. Middle East J. Dig. Dis. 2013, 5, 217–222. [Google Scholar]
- Committee on Herbal Medicinal Products (HMPC). Assessment Report on Cinnamomum Verum J.S. Presl, Cortex and Corticis Aetheroleum; Committee on Herbal Medicinal Products: Amsterdam, The Netherlands, 2011; EMA/HMPC/246773/2009.
- Dosoky, N.S.; Setzer, W.N. Biological activities and safety of Citrus spp. essential oils. Int. J. Mol. Sci. 2018, 19, 1966. [Google Scholar] [CrossRef]
- Di Vito, M.; Modesto, M.; Girolamo, A.; Ballardini, M.; Tamburro, A.; Meledandri, M.; Mondello, F. In vitro activity of tea tree oil vaginal suppositories against Candida spp. and probiotic vaginal microbiota. Phytother. Res. 2015, 29, 1628–1633. [Google Scholar] [CrossRef]
- Petretto, G.L.; Fancello, F.; Zara, S.; Foddai, M.; Mangia, N.P.; Sanna, M.L.; Omer, E.A.; Menghini, L.; Chessa, M.; Pintore, G. Antimicrobial activity against beneficial microorganisms and chemical composition of essential oil of Mentha suaveolens ssp. insularis grown in Sardinia. J. Food Sci. 2014, 79, M369–M377. [Google Scholar] [CrossRef]
- Shipradeep Karmakar, S.; Sahay Khare, R.; Ojha, S.; Kundu, K.; Kundu, S. Development of probiotic candidate in combination with essential oils from medicinal plant and their effect on enteric pathogens: A review. Gastroenterol. Res. Pract. 2012, 2012, 457150. [Google Scholar] [CrossRef] [PubMed]
- Sciavilla, P.; Strati, F.; Prati, G.M.; Fornari, F.; Modesto, M.; Luiselli, D.; Cavalieri, D.; De Filippo, C.; Mattarelli, P. Metagenomic characterisation of gut microbiota in IBS patients. UEG WEEK (United European Gastroenterology Week). United Eur. Gastroent. 2016, 4, 649. [Google Scholar]
- Miller, S.A.; White, J.A.; Chowdhury, R.; Gales, D.N.; Tameru, B.; Tiwari, A.K.; Samuel, T. Effects of consumption of whole grape powder on basal NF-κB signaling and inflammatory cytokine secretion in a mouse model of inflammation. J. Nutr. Intermed. Metab. 2018, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Terra, X.; Pallarés, V.; Ardèvol, A.; Bladé, C.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, J.; Arola, L.; Blay, M. Modulatory effect of grape-seed procyanidins on local and systemic inflammation in diet-induced obesity rats. J. Nutr. Biochem. 2011, 22, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Committee on Herbal Medicinal Products (HMPC). Assessment Report on Vitis vinifera L., folium; Committee on Herbal Medicinal Products: Amsterdam, The Netherlands, 2017; EMA/HMPC/464682/2016.
- Ohman, L.; Simrén, M. Pathogenesis of IBS: Role of inflammation, immunity and neuroimmune interactions. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 163–173. [Google Scholar] [CrossRef]
- Noble, S.M.; Gianetti, B.A.; Witchley, J.N. Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat. Rev. Microbiol. 2017, 15, 96–108. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Enrich-Capó, N.; Aldeguer, X.; Sabat-Mir, M.; Duncan, S.H.; Garcia-Gil, L.J.; Martinez-Medina, M. Alterations in the abundance and co-occurrence of Akkermansia muciniphila and Faecalibacterium prausnitzii in the colonic mucosa of inflammatory bowel disease subjects. Front. Cell. Infect. Microbiol. 2018, 8, 281. [Google Scholar] [CrossRef]
- Kim, Y.W.; Kim, M.J.; Chung, B.Y.; Bang du, Y.; Lim, S.K.; Choi, S.M.; Lim, D.S.; Cho, M.C.; Yoon, K.; Kim, H.S.; et al. Safety evaluation and risk assessment of d-Limonene. J. Toxicol. Environ. Health B Crit. Rev. 2013, 16, 17–38. [Google Scholar] [CrossRef]
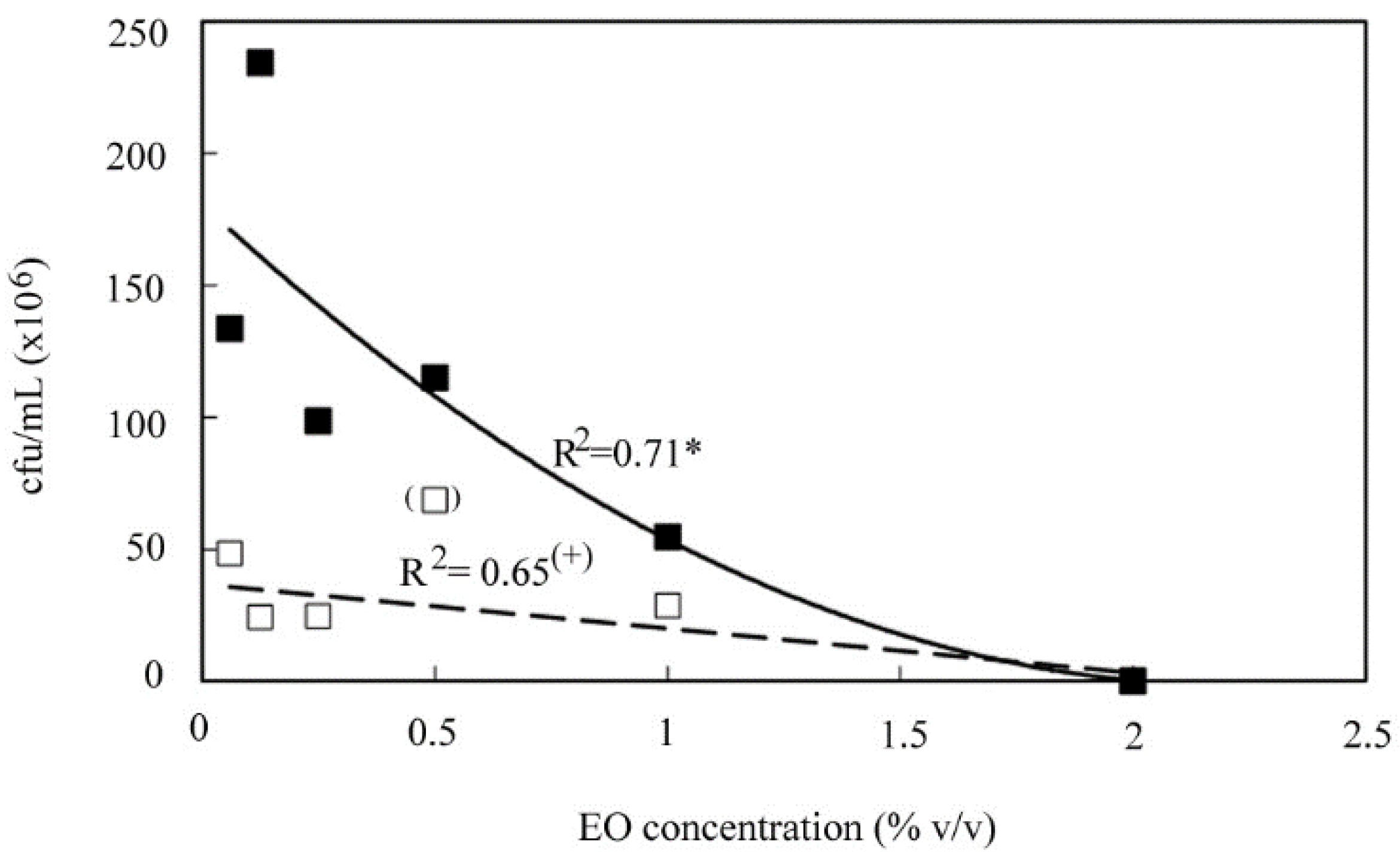

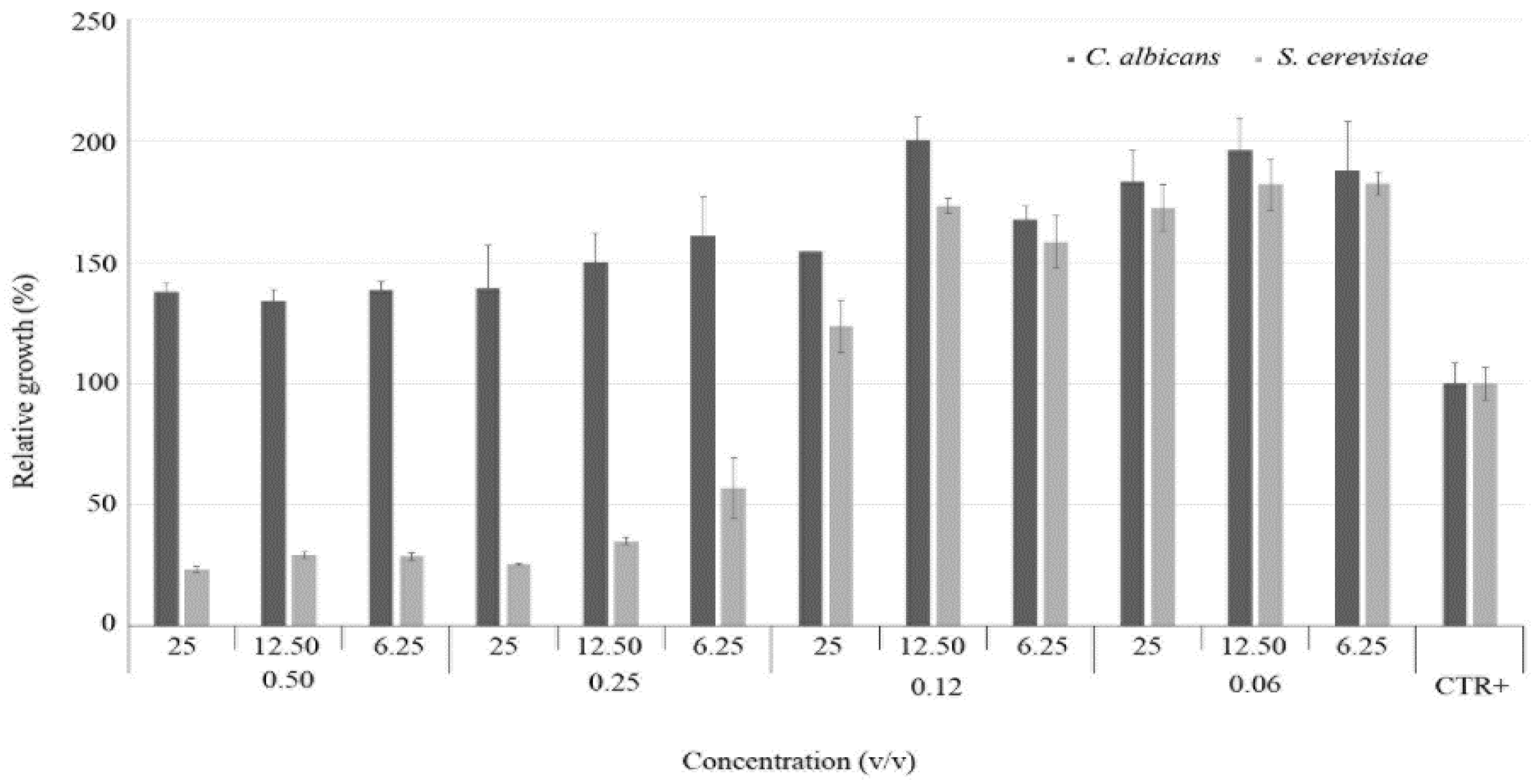
| Designation | Isolates | MIC (% v/v) | |||||
|---|---|---|---|---|---|---|---|
| CL1 | CL-f2 | CR3 | CA-a4 | CZ5 | MD6 | ||
| 3.1 | C. albicans | >0.50 | >0.50 | >0.50 | >0.50 | ≤0.06 | 0.25 |
| 3.2 | C. albicans | >0.50 | >0.50 | >0.50 | 0.50 | ≤0.06 | 0.25 |
| 3.3 | C. albicans | >0.50 | >0.50 | >0.50 | 0.50 | ≤0.06 | 0.25 |
| 3.4 | C. albicans | >0.50 | >0.50 | >0.50 | 0.50 | ≤0.06 | 0.25 |
| 3.5 | C. albicans | >0.50 | >0.50 | >0.50 | 0.50 | ≤0.06 | 0.25 |
| 3.6 | C. albicans | >0.50 | >0.50 | >0.50 | 0.06 | ≤0.06 | 0.25 |
| 3.7 | Pikia kudriavzeii | >0.50 | >0.50 | >0.50 | 0.25 | ≤0.06 | 0.25 |
| 3.8 | C. albicans | >0.50 | >0.50 | >0.50 | 0.50 | ≤0.06 | 0.25 |
| 3.9 | C. albicans | >0.50 | >0.50 | >0.50 | 0.50 | ≤0.06 | 0.25 |
| 3.11 | C. albicans | >0.50 | >0.50 | >0.50 | 0.50 | ≤0.06 | 0.25 |
| 3.12 | C. albicans | >0.50 | >0.50 | >0.50 | 0.50 | ≤0.06 | 0.25 |
| 3.14 | C. albicans | >0.50 | >0.50 | >0.50 | 0.50 | ≤0.06 | 0.25 |
| 3.15 | C. albicans | >0.50 | >0.50 | >0.50 | >0.50 | ≤0.06 | 0.25 |
| 3.16 | C. albicans | >0.50 | >0.50 | >0.50 | 0.50 | ≤0.06 | 0.25 |
| 3.17 | C. albicans | >0.50 | >0.50 | >0.50 | 0.50 | ≤0.06 | 0.25 |
| 3.18 | C. albicans | >0.50 | >0.50 | >0.50 | 0.50 | ≤0.06 | 0.25 |
| 3.19 | C. albicans | >0.50 | >0.50 | >0.50 | 0.50 | ≤0.06 | 0.50 |
| 5.2 | C. parapsilosis | >0.50 | >0.50 | >0.50 | 0.50 | ≤0.06 | 0.12 |
| 5.3 | C. zeylanoides | >0.50 | >0.50 | >0.50 | 0.50 | ≤0.06 | 0.12 |
| 5.4 | C. parapsilosis | >0.50 | >0.50 | >0.50 | >0.50 | ≤0.06 | 0.25 |
| 5.5 | C. parapsilosis | >0.50 | >0.50 | >0.50 | >0.5 | ≤0.06 | 0.25 |
| 5.6 | C. parapsilosis | >0.50 | >0.50 | >0.50 | >0.50 | ≤0.06 | 0.25 |
| 8.1 | C. albicans | >0.50 | >0.50 | >0.50 | >0.50 | ≤0.06 | 0.25 |
| 9.1 | C. albicans | >0.50 | >0.50 | >0.50 | >0.50 | ≤0.06 | 0.25 |
| 10.1 | C. albicans | >0.50 | >0.50 | >0.50 | >0.50 | ≤0.06 | 0.50 |
| 10.2 | C. albicans | >0.50 | >0.50 | >0.50 | >0.50 | ≤0.06 | 0.50 |
| 10.3 | C. albicans | >0.50 | >0.50 | >0.50 | >0.50 | ≤0.06 | 0.25 |
| 10.4 | C. albicans | >0.50 | >0.50 | >0.50 | >0.50 | ≤0.06 | 0.25 |
| 10.5 | C. albicans | >0.50 | >0.50 | >0.50 | >0.50 | ≤0.06 | 0.25 |
| 10.6 | C. albicans | >0.50 | >0.50 | >0.50 | >0.50 | ≤0.06 | 0.25 |
| 10.7 | C. albicans | >0.50 | >0.50 | >0.50 | >0.50 | ≤0.06 | 0.25 |
| 10.8 | C. albicans | >0.50 | >0.50 | >0.50 | >0.50 | ≤0.06 | 0.25 |
| 11.1 | C. albicans | >0.50 | >0.50 | >0.50 | >0.50 | ≤0.06 | 0.25 |
| 11.2 | C. albicans | >0.50 | >0.50 | >0.50 | >0.50 | ≤0.06 | 0.25 |
| 14.1 | C. albicans | >0.50 | >0.50 | >0.50 | >0.50 | ≤0.06 | 0.25 |
| 18.2 | C. glabrata | >0.50 | >0.50 | >0.50 | >0.50 | ≤0.06 | 0.50 |
| 18.5 | C. glabrata | >0.50 | >0.50 | >0.50 | >0.50 | ≤0.06 | 0.50 |
| 18.6 | C. glabrata | >0.50 | >0.50 | >0.50 | >0.50 | ≤0.06 | 0.50 |
| Strain | Probiotic Commercial Strains | MIC (% v/v) | ||
| CA-a 1 | CZ 2 | MD 3 | ||
| CBS5926 | S. boulardii | >4.00 | ≤0.06 | 0.50 |
| LA3SACCO | L. acidophilus | >4.00 | ≤0.06 | 2.00 |
| LA-14 | L. acidophilus | >4.00 | ≤0.06 | 0.50 |
| ATCC14917 | L. plantarum | >4.00 | ≤0.06 | 1.00 |
| DSMZ 20021 | L. rhamnosus | >4.00 | ≤0.06 | 0.50 |
| R0215 | L. casei | >4.00 | ≤0.06 | 1.00 |
| R0052 | L. helveticus | 0.12 | ≤0.06 | 0.12 |
| Designation | Beneficial Clinical Strains | |||
| 13.0 | Galactomyces species | 2.00 | ≤0.06 | 0.25 |
| 17.1 | Galactomyces species | >4.00 | ≤0.06 | 2.00 |
| 17.5 | Galactomyces species | 4.00 | ≤0.06 | 0.50 |
| 17.7 | Galactomyces species | >4.00 | ≤0.06 | 0.50 |
| 17.8 | Galactomyces species | >4.00 | ≤0.06 | 0,50 |
| 17.9 | Galactomyces species | >4.00 | ≤0.06 | 0.50 |
| 17.10 | Galactomyces species | 4.00 | ≤0.06 | 0.50 |
| 17.11 | Galactomyces species | 4.00 | ≤0.06 | 1.00 |
| 14.3 | S. cerevisiae | 1.00 | ≤0.06 | 0.25 |
| 14.5 | S. cerevisiae | 1.00 | ≤0.06 | 0.25 |
| 14.9 | S. cerevisiae | 1.00 | ≤0.06 | 0.50 |
| 14.11 | S. cerevisiae | 2.00 | ≤0.06 | 0.25 |
| Simple | VV-Hy1 | CA-EO2 | Mean (± St. Dev.)3 | |
|---|---|---|---|---|
| (%v/v) | (%v/v) | IL-10 | TNF-α | |
| PBMCs | - | - | 2.60 (± 0.07) | 47.98 (± 2.80) |
| 50.00 | - | 16.40 (± 0.80) | 199.50 (± 12.30) | |
| 25.00 | - | 53.80 (± 10.20) | 375.80 (± 7.20) | |
| 12.50 | - | 14.51 (± 0.60) | 1126.80 (± 14.80) | |
| 6.25 | - | 3.28 (± 0.30) | 620.60 (± 33.20) | |
| PBMCs + LPS | - | - | 518.00 (± 3.00) | 2002.90 (±14.40) |
| 50.00 | - | 5.93 (± 0.30) | 238.70 (± 7.50) | |
| 25.00 | - | 90.90 (± 3.60) | 1846.30 (± 20.30) | |
| 12.50 | - | 83.70 (± 4.20) | 2221.50 (± 2.60) | |
| 6.25 | - | 46.30 (± 0.90) | 2148.20 (± 195.6) | |
| 50.00 | 0.5 | 0.64 (± 0.14) | 0.64 (± 0.14) | |
| 25.00 | 0.25 | 119.00 (± 0.46) | 977.91 (± 16.60) | |
| 12.50 | 0.12 | 269.53 (± 4.07) | 1901.90 (± 0.00) | |
| 6.25 | 0.06 | 403.23 (± 37.65) | 2143.01 (± 25.09) | |
| Designation | Fungal Species | C. aurantium va. amara (% v/v) EO + V. vinifera Hy | |||||
|---|---|---|---|---|---|---|---|
| 0.50 + 50 | 0.25 + 25 | 0.12 + 12.5 | 0.06 + 6.25 | 0.03 + 3.17 | CTR | ||
| 0.3R 1 | C. tropicalis | Y | H | H | H | H | H++ |
| 0.4R 1 | C. tropicalis | H++ (no adhesion) | H++ (no adhesion) | H++ (no adhesion) | H++ (no adhesion) | H++ (no adhesion) | H++ |
| 3.1 | C. albicans | Y | H | H+ | H+ | H+ | + |
| 10.1 | C. albicans | Y | H | H+ | H+ | H++ | H++ |
| 3.15 | C. albicans | Y | Y | Y | Y | H+ | H++ |
| 8.5 | C. glabrata | Y | Y | Y | Y | Y | Y |
| 3.7 | C. krusei | Y | Y | Y | Y | Y | Y |
| 5.1 | C. parapsilosis | Y | Y | Y | Y | Y | Y |
| Name 1 | LRI 2 | LRIlit 3 | %CA-EO 4 | %VV-Hy 5 |
|---|---|---|---|---|
| α-pinene | 1038 | 1040 | 0.57 | 0.39 |
| β-pinene | 1134 | 1130 | 0.73 | - |
| sabinene | 1152 | 1156 | 0.36 | - |
| β-myrcene | 1171 | 1167 | 2.37 | - |
| limonene | 1232 | 1235 | 93.93 | 17.68 |
| γ-terpinene | 1270 | 1263 | 0.26 | - |
| o-cymene | 1295 | 1287 | 0.15 | - |
| cis-linaloloxide | 1433 | 1420 | - | 0.85 |
| decanal | 1520 | 1515 | 0.22 | - |
| β-linalol | 1553 | 1547 | 0.21 | 38.81 |
| linalyl acetate | 1572 | 1575 | 0.77 | - |
| α-terpineol | 1705 | 1700 | 0.22 | 7.55 |
| borneol | 1724 | 1717 | - | 0.69 |
| cis-geraniol | 1831 | 1823 | - | 25.40 |
| humulene-1,2-epoxide | 2018 | NA* | - | 2.55 |
| d-nerolidol | 2030 | 2023 | 0.21 | - |
| thymol | 2198 | 2189 | - | 1.75 |
| carvacrol | 2231 | 2225 | - | 4.33 |
| SUM | 100.00 | 100.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Vito, M.; Bellardi, M.G.; Sanguinetti, M.; Mondello, F.; Girolamo, A.; Barbanti, L.; Garzoli, S.; Sabatino, M.; Ragno, R.; Vitali, A.; et al. Potent In Vitro Activity of Citrus aurantium Essential Oil and Vitis vinifera Hydrolate Against Gut Yeast Isolates from Irritable Bowel Syndrome Patients—The Right Mix for Potential Therapeutic Use. Nutrients 2020, 12, 1329. https://doi.org/10.3390/nu12051329
Di Vito M, Bellardi MG, Sanguinetti M, Mondello F, Girolamo A, Barbanti L, Garzoli S, Sabatino M, Ragno R, Vitali A, et al. Potent In Vitro Activity of Citrus aurantium Essential Oil and Vitis vinifera Hydrolate Against Gut Yeast Isolates from Irritable Bowel Syndrome Patients—The Right Mix for Potential Therapeutic Use. Nutrients. 2020; 12(5):1329. https://doi.org/10.3390/nu12051329
Chicago/Turabian StyleDi Vito, Maura, Maria Grazia Bellardi, Maurizio Sanguinetti, Francesca Mondello, Antonietta Girolamo, Lorenzo Barbanti, Stefania Garzoli, Manuela Sabatino, Rino Ragno, Alberto Vitali, and et al. 2020. "Potent In Vitro Activity of Citrus aurantium Essential Oil and Vitis vinifera Hydrolate Against Gut Yeast Isolates from Irritable Bowel Syndrome Patients—The Right Mix for Potential Therapeutic Use" Nutrients 12, no. 5: 1329. https://doi.org/10.3390/nu12051329
APA StyleDi Vito, M., Bellardi, M. G., Sanguinetti, M., Mondello, F., Girolamo, A., Barbanti, L., Garzoli, S., Sabatino, M., Ragno, R., Vitali, A., Palucci, I., Posteraro, B., Gasbarrini, A., Prati, G. M., Aragona, G., Mattarelli, P., & Bugli, F. (2020). Potent In Vitro Activity of Citrus aurantium Essential Oil and Vitis vinifera Hydrolate Against Gut Yeast Isolates from Irritable Bowel Syndrome Patients—The Right Mix for Potential Therapeutic Use. Nutrients, 12(5), 1329. https://doi.org/10.3390/nu12051329