Efficacy of Vitamins on Cognitive Function of Non-Demented People: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Criteria for Study Inclusion/Exclusion
2.2. Search Strategy and Study Selection
2.3. Outcomes
2.4. Data Extraction and Assessment of the Methodological Quality
2.5. Statistical Analyses
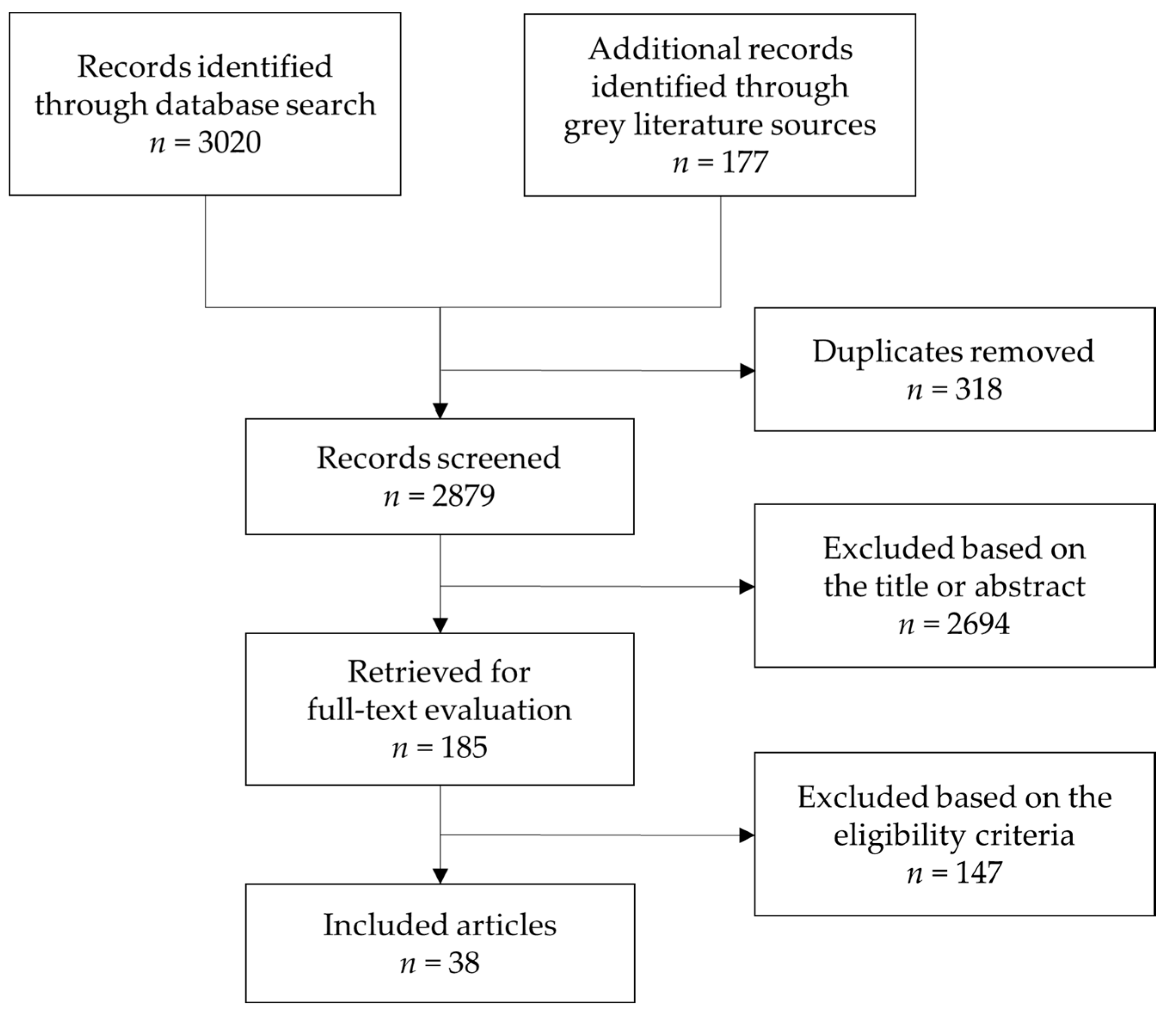
3. Results
3.1. Study Selection
3.2. Study Characteristics
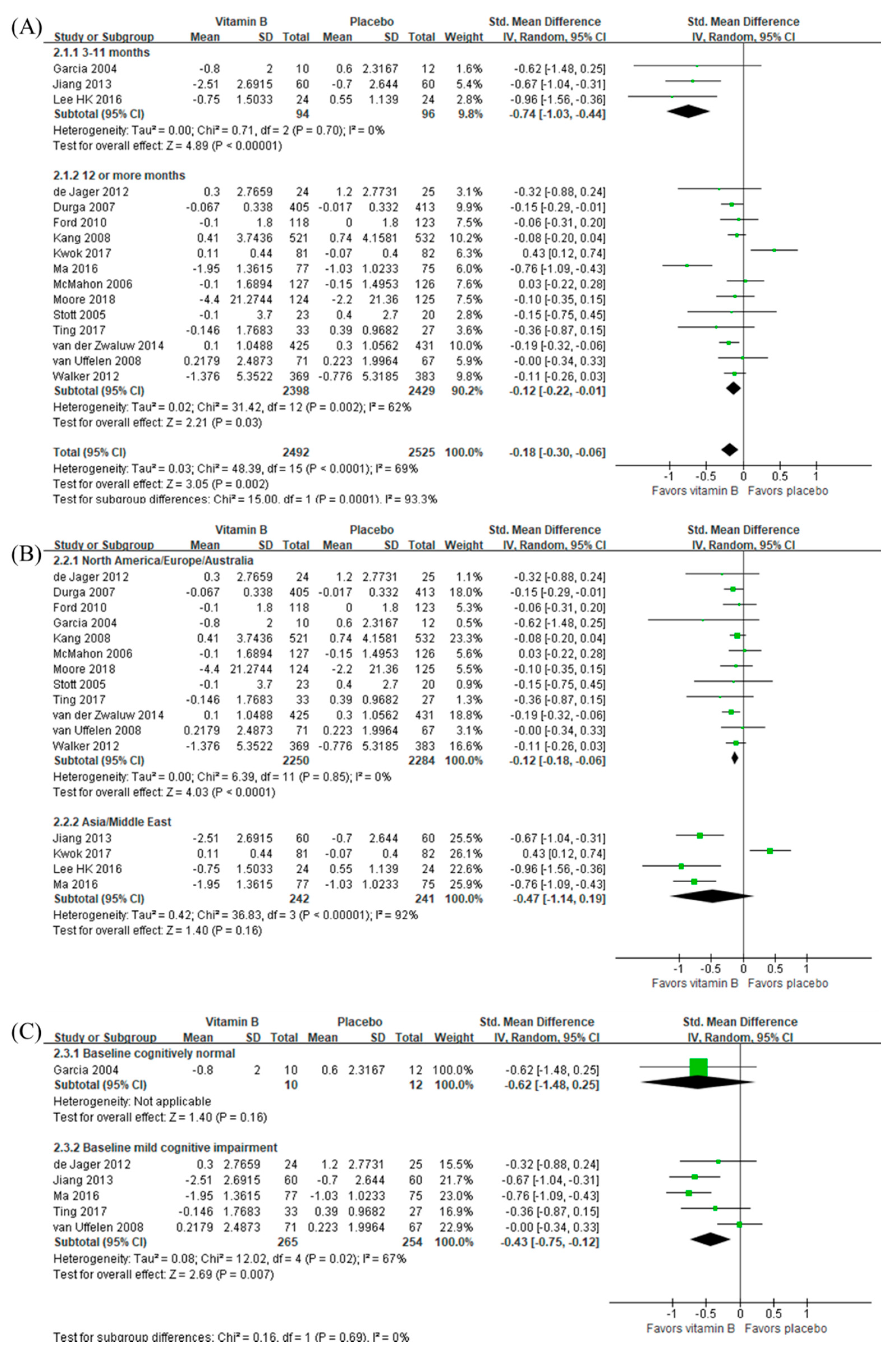
3.2.1. B Vitamins
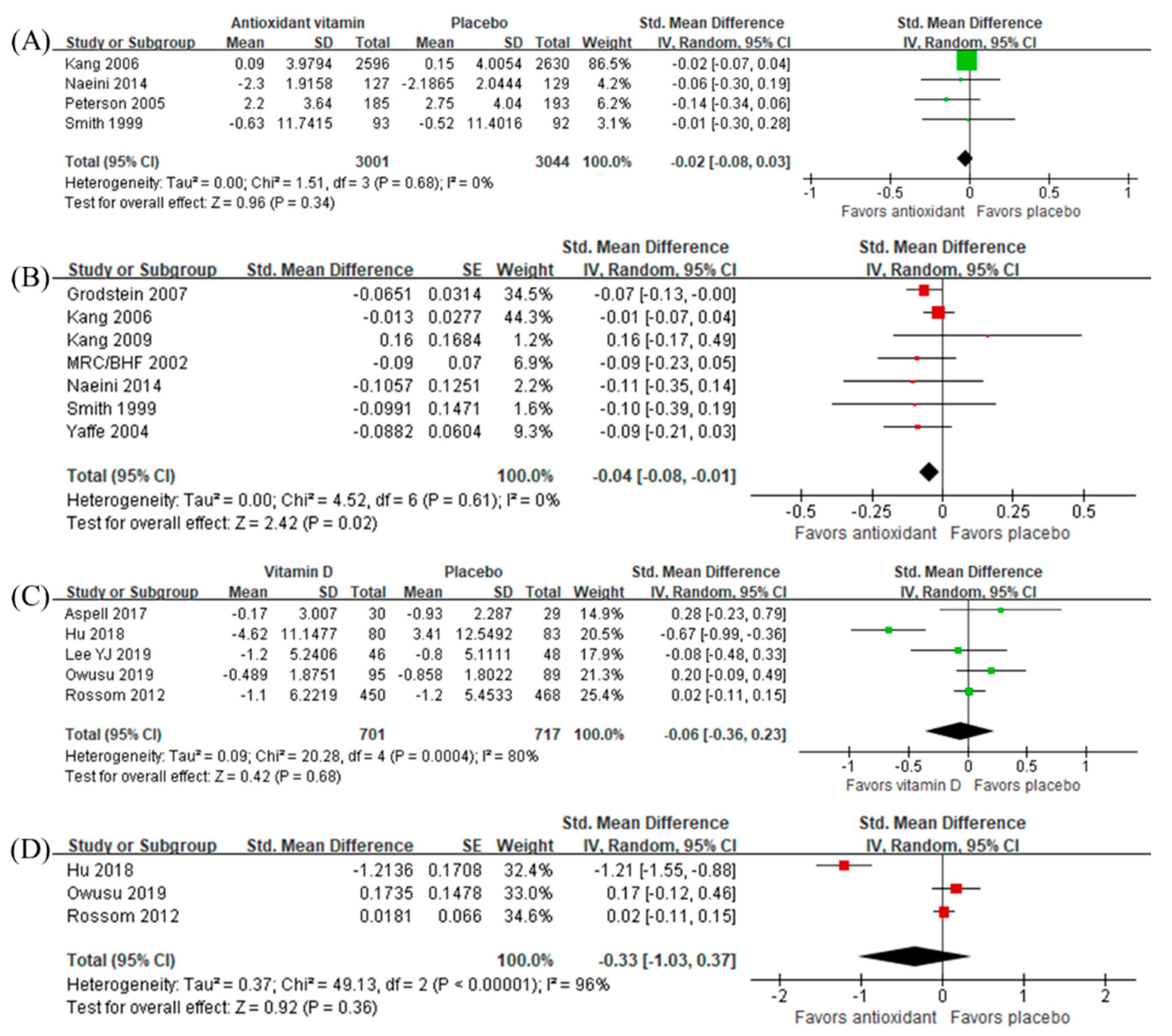
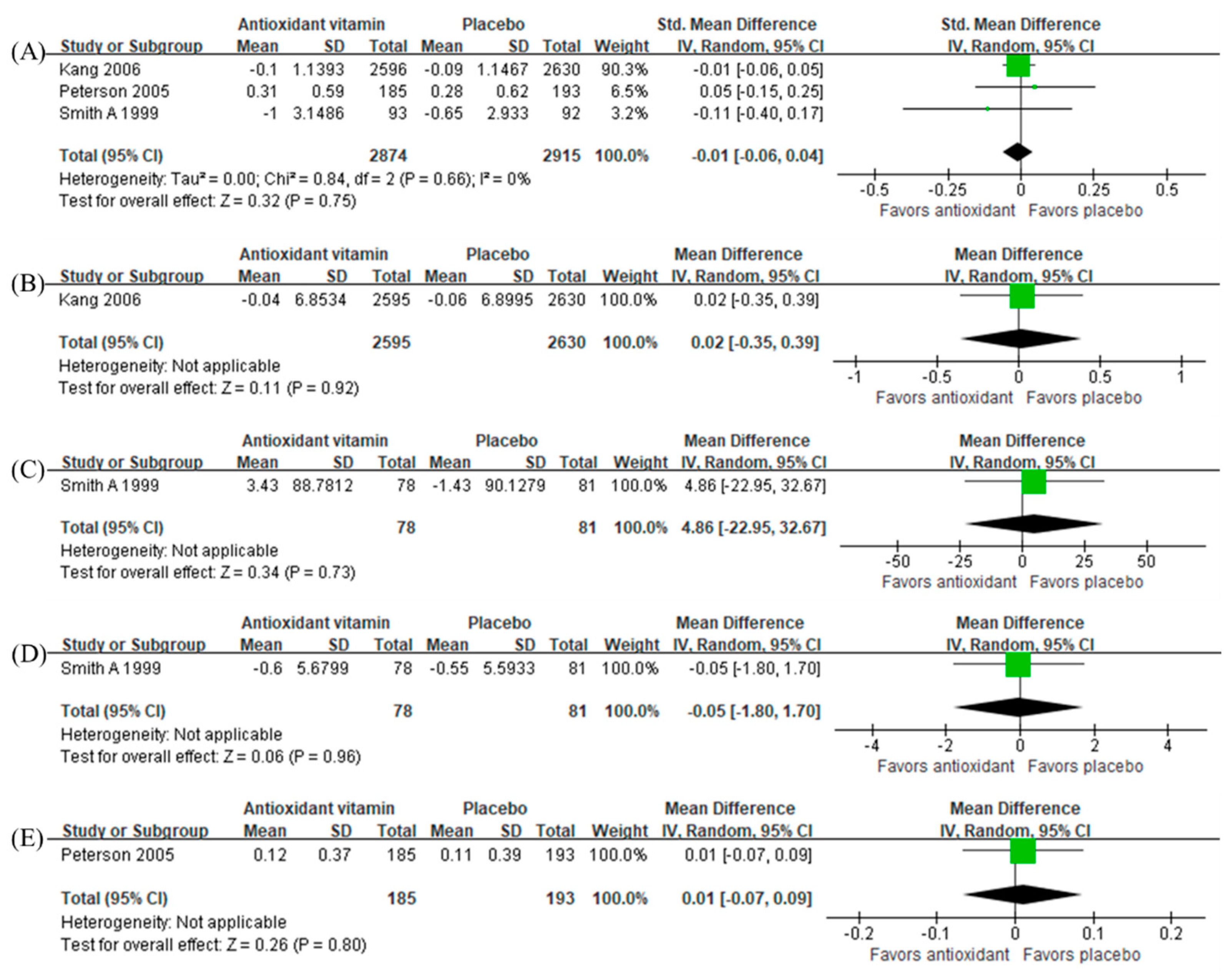
3.2.2. Antioxidant Vitamins
3.2.3. Vitamin D
3.3. Cognitive Test Outcomes
3.4. Methodological Quality
3.5. Intervention Effects of B Vitamins
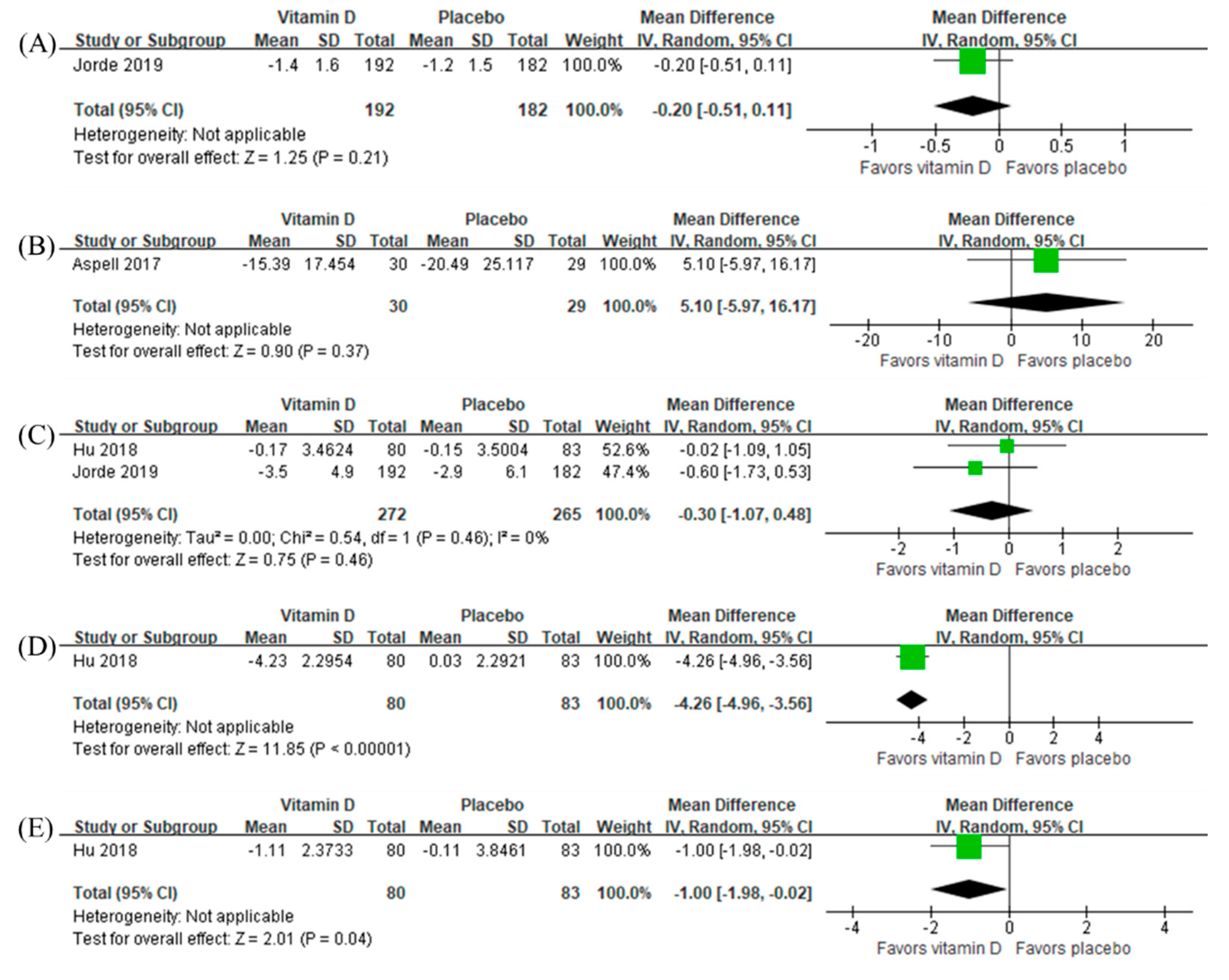
3.6. Intervention Effects of Antioxidant Vitamins and Vitamin D
3.7. Sensitivity Analyses and Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Clarke, R.; Smith, A.D.; Jobst, K.A.; Refsum, H.; Sutton, L.; Ueland, P.M. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch. Neurol. 1998, 55, 1449–1455. [Google Scholar] [CrossRef]
- Hankey, G.J.; Eikelboom, J.W. Homocysteine and vascular disease. Lancet 1999, 354, 407–413. [Google Scholar] [CrossRef]
- Refsum, P.M.U.H. Plasma homocysteine, a risk factor for vascular disease: Plasma levels in health, disease, and drug therapy. J. Lab. Clin. Med. 1989, 114, 473–501. [Google Scholar]
- Jang, S.; Han, J.W.; Shin, J.; Kim, T.H.; Kwak, K.P.; Kim, K.; Kim, B.J.; Kim, S.G.; Kim, J.L.; Kim, T.H. Normal-But-Low Serum Folate Levels and the Risks for Cognitive Impairment. Psychiatry Investig. 2019, 16, 532. [Google Scholar] [CrossRef] [PubMed]
- Root, M.; Ravine, E.; Harper, A. Flavonol intake and cognitive decline in middle-aged adults. J. Med. Food 2015, 18, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Rutjes, A.W.; Denton, D.A.; Di Nisio, M.; Chong, L.Y.; Abraham, R.P.; Al-Assaf, A.S.; Anderson, J.L.; Malik, M.A.; Vernooij, R.W.; Martínez, G. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- McCleery, J.; Abraham, R.P.; Denton, D.A.; Rutjes, A.W.; Chong, L.Y.; Al-Assaf, A.S.; Griffith, D.J.; Rafeeq, S.; Yaman, H.; Malik, M.A. Vitamin and mineral supplementation for preventing dementia or delaying cognitive decline in people with mild cognitive impairment. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef]
- Wald, D.S.; Kasturiratne, A.; Simmonds, M. Effect of folic acid, with or without other B vitamins, on cognitive decline: Meta-analysis of randomized trials. Am. J. Med. 2010, 123, 522–527.e2. [Google Scholar] [CrossRef]
- McGrattan, A.M.; McEvoy, C.T.; McGuinness, B.; McKinley, M.C.; Woodside, J.V. Effect of dietary interventions in mild cognitive impairment: A systematic review. Br. J. Nutr. 2018, 120, 1388–1405. [Google Scholar] [CrossRef]
- Doets, E.L.; van Wijngaarden, J.P.; Szczecińska, A.; Dullemeijer, C.; Souverein, O.W.; Dhonukshe-Rutten, R.A.; Cavelaars, A.E.; van’t Veer, P.; Brzozowska, A.; de Groot, L.C. Vitamin B12 intake and status and cognitive function in elderly people. Epidemiol. Rev. 2013, 35, 2–21. [Google Scholar] [CrossRef]
- Forbes, S.C.; Holroyd-Leduc, J.M.; Poulin, M.J.; Hogan, D.B. Effect of nutrients, dietary supplements and vitamins on cognition: A systematic review and meta-analysis of randomized controlled trials. Can. J. Geriatr. 2015, 18, 231. [Google Scholar] [CrossRef] [PubMed]
- D’Cunha, N.M.; Georgousopoulou, E.N.; Dadigamuwage, L.; Kellett, J.; Panagiotakos, D.B.; Thomas, J.; McKune, A.J.; Mellor, D.D.; Naumovski, N. Effect of long-term nutraceutical and dietary supplement use on cognition in the elderly: A 10-year systematic review of randomised controlled trials. Br. J. Nutr. 2018, 119, 280–298. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-M.; Yu, J.-T.; Wang, H.-F.; Jiang, T.; Wang, J.; Meng, X.-F.; Tan, C.-C.; Wang, C.; Tan, L. Efficacy of vitamins B supplementation on mild cognitive impairment and Alzheimer’s disease: A systematic review and meta-analysis. Curr. Alzheimer Res. 2014, 11, 844–852. [Google Scholar] [PubMed]
- Travica, N.; Ried, K.; Sali, A.; Scholey, A.; Hudson, I.; Pipingas, A. Vitamin C status and cognitive function: A systematic review. Nutrients 2017, 9, 960. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. Cochrane handbook for systematic reviews of interventions; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 4. [Google Scholar]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Fu, R.; Holmer, H.K. Change Score or Followup Score? An Empirical Evaluation of the Impact of Choice of Mean Difference Estimates. Research White Paper. (Prepared by the Oregon Evidence-based Practice Center under Contract No. 290-2007-10057-I.) AHRQ Publication No. 15-EHC016-EF. Rockville, MD: Agency for Healthcare Research and Quality. 2015. Available online: www.effectivehealthcare.ahrq.gov/reports/final.cfm (accessed on 17 February 2020).
- Cochrane Collaboration. Review Manager Version 5.3; The Nordic Cochrane Centre: Copenhagen, Denmark, 2014. [Google Scholar]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Lee, H.K.; Kim, S.Y.; Sok, S.R. Effects of multivitamin supplements on cognitive function, serum homocysteine level, and depression of Korean older adults with mild cognitive impairment in care facilities. J. Nurs. Scholarsh. 2016, 48, 223–231. [Google Scholar] [CrossRef]
- Cheng, D.; Kong, H.; Pang, W.; Yang, H.; Lu, H.; Huang, C.; Jiang, Y. B vitamin supplementation improves cognitive function in the middle aged and elderly with hyperhomocysteinemia. Nutr. Neurosci. 2016, 19, 461–466. [Google Scholar] [CrossRef]
- Ting, S.K.S.; Earnest, A.; Li, H.; Hameed, S.; Chang, H.M.; Chen, C.L.H.; Tan, E.-K. B vitamins and cognition in subjects with small vessel disease: A substudy of VITATOPS, a randomized, placebo-controlled trial. J. Neurol. Sci. 2017, 379, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Pulman, K.; Zanibbi, K.; Day, A.; Galarneau, L.; Freedman, M. Cobalamin Reduces Homocysteine in Older Adults on Folic Acid–Fortified Diet: A Pilot, Double-Blind, Randomized, Placebo-Controlled Trial. J. Am. Geriatr. Soc. 2004, 52, 1410–1412. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Porter, K.; Doherty, L.; Hughes, C.; Ward, M.; Hoey, L.; Strain, J.; Pentieva, K.; McNulty, H. A randomised controlled trial of B-vitamin supplementation on neuropsychiatric performance: Results from the BrainHOP trial. Proc. Nutr. Soc. 2018, 77. [Google Scholar] [CrossRef]
- Jiang, B.; Ding, C.; Yao, G.; Yao, C.; Zhang, Y.; Ge, J.; Qiu, E. Intervention effect of folic acid and vitamin B12 on vascular cognitive impairment compliacted with hyperhomocystenemia. J. Med. Biochem. 2013, 33, 169–174. [Google Scholar] [CrossRef][Green Version]
- Kang, J.H.; Cook, N.; Manson, J.; Buring, J.E.; Albert, C.M.; Grodstein, F. A trial of B vitamins and cognitive function among women at high risk of cardiovascular disease. Am. J. Clin. Nutr. 2008, 88, 1602–1610. [Google Scholar] [CrossRef]
- Stott, D.J.; MacIntosh, G.; Lowe, G.D.; Rumley, A.; McMahon, A.D.; Langhorne, P.; Tait, R.C.; O’Reilly, D.S.J.; Spilg, E.G.; MacDonald, J.B. Randomized controlled trial of homocysteine-lowering vitamin treatment in elderly patients with vascular disease. Am. J. Clin. Nutr. 2005, 82, 1320–1326. [Google Scholar] [CrossRef]
- Andreeva, V.A.; Kesse-Guyot, E.; Barberger-Gateau, P.; Fezeu, L.; Hercberg, S.; Galan, P. Cognitive function after supplementation with B vitamins and long-chain omega-3 fatty acids: Ancillary findings from the SU. FOL. OM3 randomized trial. Am. J. Clin. Nutr. 2011, 94, 278–286. [Google Scholar] [CrossRef]
- Kwok, T.; Lee, J.; Ma, R.C.; Wong, S.Y.; Kung, K.; Lam, A.; Ho, C.; Lee, V.; Harrison, J.; Lam, L. A randomized placebo controlled trial of vitamin B12 supplementation to prevent cognitive decline in older diabetic people with borderline low serum vitamin B12. Clin. Nutr. 2017, 36, 1509–1515. [Google Scholar] [CrossRef]
- de Jager, C.A.; Oulhaj, A.; Jacoby, R.; Refsum, H.; Smith, A.D. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: A randomized controlled trial. Int. J. Geriatr. Psychiatry 2012, 27, 592–600. [Google Scholar] [CrossRef]
- Ford, A.; Flicker, L.; Alfonso, H.; Thomas, J.; Clarnette, R.; Martins, R.; Almeida, O. Vitamins B12, B6, and folic acid for cognition in older men. Neurology 2010, 75, 1540–1547. [Google Scholar] [CrossRef]
- Lewerin, C.; Matousek, M.; Steen, G.; Johansson, B.; Steen, B.; Nilsson-Ehle, H. Significant correlations of plasma homocysteine and serum methylmalonic acid with movement and cognitive performance in elderly subjects but no improvement from short-term vitamin therapy: A placebo-controlled randomized study. Am. J. Clin. Nutr. 2005, 81, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- McMahon, J.A.; Green, T.J.; Skeaff, C.M.; Knight, R.G.; Mann, J.I.; Williams, S.M. A controlled trial of homocysteine lowering and cognitive performance. N. Engl. J. Med. 2006, 354, 2764–2772. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.; Rogers, G.; Weiner, D.; Livingston, K.; Selhub, J.; Jacques, P.; Rosenberg, I.; Troen, A. B-Vitamin therapy for kidney transplant recipients lowers homocysteine and improves selective cognitive outcomes in the randomized FAVORIT Ancillary Cognitive Trial. J. Prev. Alzheimer’s Dis. 2017, 4, 174. [Google Scholar]
- van Uffelen, J.G.; Chinapaw, M.J.; van Mechelen, W.; Hopman-Rock, M. Walking or vitamin B for cognition in older adults with mild cognitive impairment? A randomised controlled trial. Br. J. Sports Med. 2008, 42, 344–351. [Google Scholar] [CrossRef]
- Eussen, S.J.; de Groot, L.C.; Joosten, L.W.; Bloo, R.J.; Clarke, R.; Ueland, P.M.; Schneede, J.; Blom, H.J.; Hoefnagels, W.H.; van Staveren, W.A. Effect of oral vitamin B-12 with or without folic acid on cognitive function in older people with mild vitamin B-12 deficiency: A randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2006, 84, 361–370. [Google Scholar] [CrossRef]
- van der Zwaluw, N.L.; Dhonukshe-Rutten, R.A.; van Wijngaarden, J.P.; Brouwer-Brolsma, E.M.; van de Rest, O.; In’t Veld, P.H.; Enneman, A.W.; van Dijk, S.C.; Ham, A.C.; Swart, K.M. Results of 2-year vitamin B treatment on cognitive performance: Secondary data from an RCT. Neurology 2014, 83, 2158–2166. [Google Scholar] [CrossRef]
- Walker, J.G.; Batterham, P.J.; Mackinnon, A.J.; Jorm, A.F.; Hickie, I.; Fenech, M.; Kljakovic, M.; Crisp, D.; Christensen, H. Oral folic acid and vitamin B-12 supplementation to prevent cognitive decline in community-dwelling older adults with depressive symptoms—the Beyond Ageing Project: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 194–203. [Google Scholar] [CrossRef]
- Durga, J.; van Boxtel, M.P.; Schouten, E.G.; Kok, F.J.; Jolles, J.; Katan, M.B.; Verhoef, P. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: A randomised, double blind, controlled trial. Lancet 2007, 369, 208–216. [Google Scholar] [CrossRef]
- Ma, F.; Wu, T.; Zhao, J.; Song, A.; Liu, H.; Xu, W.; Huang, G. Folic acid supplementation improves cognitive function by reducing the levels of peripheral inflammatory cytokines in elderly Chinese subjects with MCI. Sci. Rep. 2016, 6, 37486. [Google Scholar] [CrossRef]
- Deijen, J.; Van der Beek, E.; Orlebeke, J.; Van den Berg, H. Vitamin B-6 supplementation in elderly men: Effects on mood, memory, performance and mental effort. Psychopharmacology 1992, 109, 489–496. [Google Scholar] [CrossRef]
- Dangour, A.D.; Allen, E.; Clarke, R.; Elbourne, D.; Fletcher, A.E.; Letley, L.; Richards, M.; Whyte, K.; Uauy, R.; Mills, K. Effects of vitamin B-12 supplementation on neurologic and cognitive function in older people: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Clark, R.; Nutt, D.; Haller, J.; Hayward, S.; Perry, K. Anti-oxidant vitamins and mental performance of the elderly. Hum. Psychopharm. Clin. 1999, 14, 459–471. [Google Scholar] [CrossRef]
- Smith, A.P.; Clark, R.; Nutt, D.; Haller, J.; Hayward, S.; Perry, K. Vitamin C, mood and cognitive functioning in the elderly. Nutr. Neurosci. 1999, 2, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Cook, N.; Manson, J.; Buring, J.E.; Grodstein, F. A randomized trial of vitamin E supplementation and cognitive function in women. Arch. Intern. Med. 2006, 166, 2462–2468. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Thomas, R.G.; Grundman, M.; Bennett, D.; Doody, R.; Ferris, S.; Galasko, D.; Jin, S.; Kaye, J.; Levey, A. Vitamin E and donepezil for the treatment of mild cognitive impairment. N. Engl. J. Med. 2005, 352, 2379–2388. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Cook, N.R.; Manson, J.E.; Buring, J.E.; Albert, C.M.; Grodstein, F. Vitamin E, Vitamin C, Beta Carotene, and Cognitive Function Among Women With or at Risk of Cardiovascular Disease: The Women’s Antioxidant and Cardiovascular Study. Circulation 2009. [Google Scholar] [CrossRef] [PubMed]
- Grodstein, F.; Kang, J.H.; Glynn, R.J.; Cook, N.R.; Gaziano, J.M. A randomized trial of beta carotene supplementation and cognitive function in men: The Physicians’ Health Study II. Arch. Intern. Med. 2007, 167, 2184–2190. [Google Scholar] [CrossRef]
- Naeini, A.A.; Elmadfa, I.; Djazayery, A.; Barekatain, M.; Ghazvini, M.A.; Djalali, M.; Feizi, A. The effect of antioxidant vitamins E and C on cognitive performance of the elderly with mild cognitive impairment in Isfahan, Iran: A double-blind, randomized, placebo-controlled trial. Eur. J. Nutr. 2014, 53, 1255–1262. [Google Scholar] [CrossRef]
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet 2002, 360, 23–33. [Google Scholar] [CrossRef]
- Yaffe, K.; Age-related Eye Disease Study Research Group. Impact of antioxidants, zinc, and copper on cognition in the elderly: A randomized, controlled trial. Neurology 2004, 63, 1705–1707. [Google Scholar]
- Lee, Y.-J.; Sohng, K.-Y. Effects of Vitamin D on Depression, Cognitive Function, and Physical Function in Elderly Individuals Living Alone. Int. J. Gerontol. 2019, 13, 196–201. [Google Scholar]
- Aspell, N.; Healy, M.; Mc Partlin, J.; Lawlor, B.; O’Sullivan, M. Effects of vitamin D supplementation on cognitive function in healthy, community dwelling older adults: Results from a randomised double-blind placebo-controlled pilot trial. Proc. Nutr. Soc. 2017, 76. [Google Scholar] [CrossRef]
- Owusu, J.E.; Islam, S.; Katumuluwa, S.S.; Stolberg, A.R.; Usera, G.L.; Anwarullah, A.A.; Shieh, A.; Dhaliwal, R.; Ragolia, L.; Mikhail, M.B. Cognition and Vitamin D in Older African-American Women–Physical performance and Osteoporosis prevention with vitamin D in older African Americans Trial and Dementia. J. Am. Geriatr. Soc. 2019, 67, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Rossom, R.C.; Espeland, M.A.; Manson, J.E.; Dysken, M.W.; Johnson, K.C.; Lane, D.S.; LeBlanc, E.S.; Lederle, F.A.; Masaki, K.H.; Margolis, K.L. Calcium and vitamin D supplementation and cognitive impairment in the women’s health initiative. J. Am. Geriatr. Soc. 2012, 60, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Jorde, R.; Kubiak, J.; Svartberg, J.; Fuskevåg, O.M.; Figenschau, Y.; Martinaityte, I.; Grimnes, G. Vitamin D supplementation has no effect on cognitive performance after four months in mid-aged and older subjects. J. Neurol. Sci. 2019, 396, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Jia, J.; Zhang, Y.; Miao, R.; Huo, X.; Ma, F. Effects of vitamin D3 supplementation on cognition and blood lipids: A 12-month randomised, double-blind, placebo-controlled trial. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1341–1347. [Google Scholar] [CrossRef]
- Thornalley, P.; Babaei-Jadidi, R.; Al Ali, H.; Rabbani, N.; Antonysunil, A.; Larkin, J.; Ahmed, A.; Rayman, G.; Bodmer, C. High prevalence of low plasma thiamine concentration in diabetes linked to a marker of vascular disease. Diabetologia 2007, 50, 2164–2170. [Google Scholar] [CrossRef]
- Babaei-Jadidi, R.; Karachalias, N.; Kupich, C.; Ahmed, N.; Thornalley, P. High-dose thiamine therapy counters dyslipidaemia in streptozotocin-induced diabetic rats. Diabetologia 2004, 47, 2235–2246. [Google Scholar] [CrossRef]
- Ford, A.H.; Almeida, O.P. Effect of homocysteine lowering treatment on cognitive function: A systematic review and meta-analysis of randomized controlled trials. J. Alzheimer’s Dis. 2012, 29, 133–149. [Google Scholar] [CrossRef]
- McCaddon, A.; Miller, J.W. Assessing the association between homocysteine and cognition: Reflections on Bradford Hill, meta-analyses, and causality. Nutr. Rev. 2015, 73, 723–735. [Google Scholar] [CrossRef]
- Calvaresi, E.; Bryan, J. B vitamins, cognition, and aging: A review. J Gerontol. B-Psychol. 2001, 56, P327–P339. [Google Scholar] [CrossRef] [PubMed]
- Alpert, J.E.; Fava, M. Nutrition and depression: The role of folate. Nutr. Rev. 1997, 55, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Bottiglieri, T. Folate, vitamin B12, and neuropsychiatric disorders. Nutr. Rev. 1996, 54, 382–390. [Google Scholar] [CrossRef]
- Kurian, M.A.; Gissen, P.; Smith, M.; Heales, S.J.; Clayton, P.T. The monoamine neurotransmitter disorders: An expanding range of neurological syndromes. Lancet Neurol. 2011, 10, 721–733. [Google Scholar] [CrossRef]
- Blaise, S.A.; Nédélec, E.; Schroeder, H.; Alberto, J.-M.; Bossenmeyer-Pourié, C.; Guéant, J.-L.; Daval, J.-L. Gestational vitamin B deficiency leads to homocysteine-associated brain apoptosis and alters neurobehavioral development in rats. Am. J. Clin. Pathol. 2007, 170, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Crichton, G.E.; Bryan, J.; Murphy, K.J. Dietary antioxidants, cognitive function and dementia-a systematic review. Plant Food Hum. Nutr. 2013, 68, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Yao-xian, G. Revision of wechsler’s adult intelligence scale in china. Acta Psychol. Sin. 2002, 3, 18. [Google Scholar]
- Egger, M.; Davey-Smith, G.; Altman, D. Systematic reviews in health care: Meta-analysis in context; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Cohen, J. Statistical Power Analyses for the Behavioral Sciences; Laurence Erlbaum Associates. Inc. Publishers: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Mikkelsen, K.; Apostolopoulos, V. B Vitamins and Ageing. In Biochemistry and Cell Biology of Ageing: Part I Biomedical Science; Harris, J.R., Korolchuk, V.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 451–470. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suh, S.W.; Kim, H.S.; Han, J.H.; Bae, J.B.; Oh, D.J.; Han, J.W.; Kim, K.W. Efficacy of Vitamins on Cognitive Function of Non-Demented People: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1168. https://doi.org/10.3390/nu12041168
Suh SW, Kim HS, Han JH, Bae JB, Oh DJ, Han JW, Kim KW. Efficacy of Vitamins on Cognitive Function of Non-Demented People: A Systematic Review and Meta-Analysis. Nutrients. 2020; 12(4):1168. https://doi.org/10.3390/nu12041168
Chicago/Turabian StyleSuh, Seung Wan, Hye Sung Kim, Ji Hyun Han, Jong Bin Bae, Dae Jong Oh, Ji Won Han, and Ki Woong Kim. 2020. "Efficacy of Vitamins on Cognitive Function of Non-Demented People: A Systematic Review and Meta-Analysis" Nutrients 12, no. 4: 1168. https://doi.org/10.3390/nu12041168
APA StyleSuh, S. W., Kim, H. S., Han, J. H., Bae, J. B., Oh, D. J., Han, J. W., & Kim, K. W. (2020). Efficacy of Vitamins on Cognitive Function of Non-Demented People: A Systematic Review and Meta-Analysis. Nutrients, 12(4), 1168. https://doi.org/10.3390/nu12041168




