The Gastroprotective Effect of Small Molecule Oligopeptides Isolated from Walnut (Juglans regia L.) against Ethanol-Induced Gastric Mucosal Injury in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of WOPs Sample
2.2. Chemicals and Reagents
2.3. Animals and Experimental Design
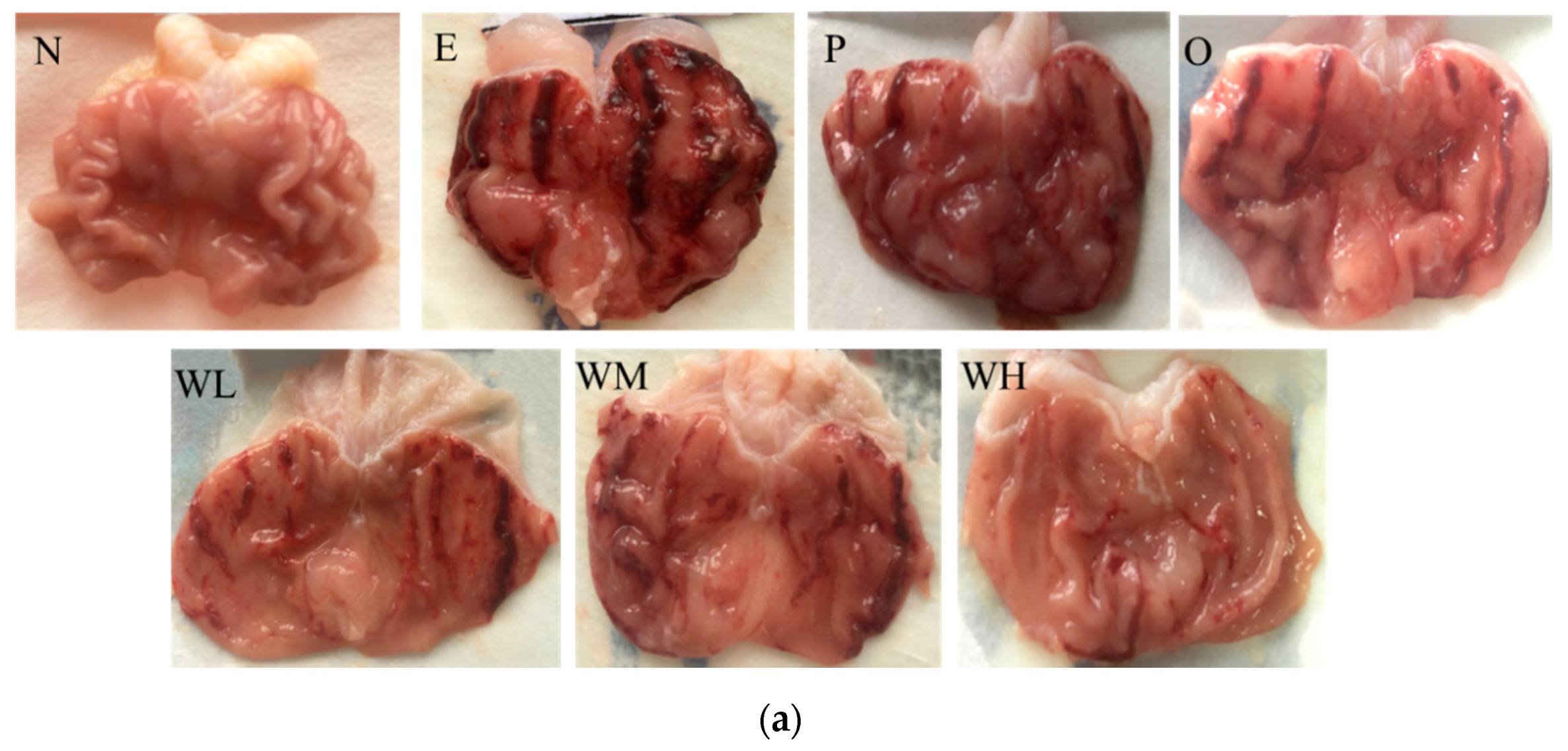
2.4. Microscopic Evaluation of the Gastric Lesions
2.5. Biochemical Assays and Enzyme-Linked Immunobsorbent Assay
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
3.1. Effect of WOPs on Body Weight and Food intake
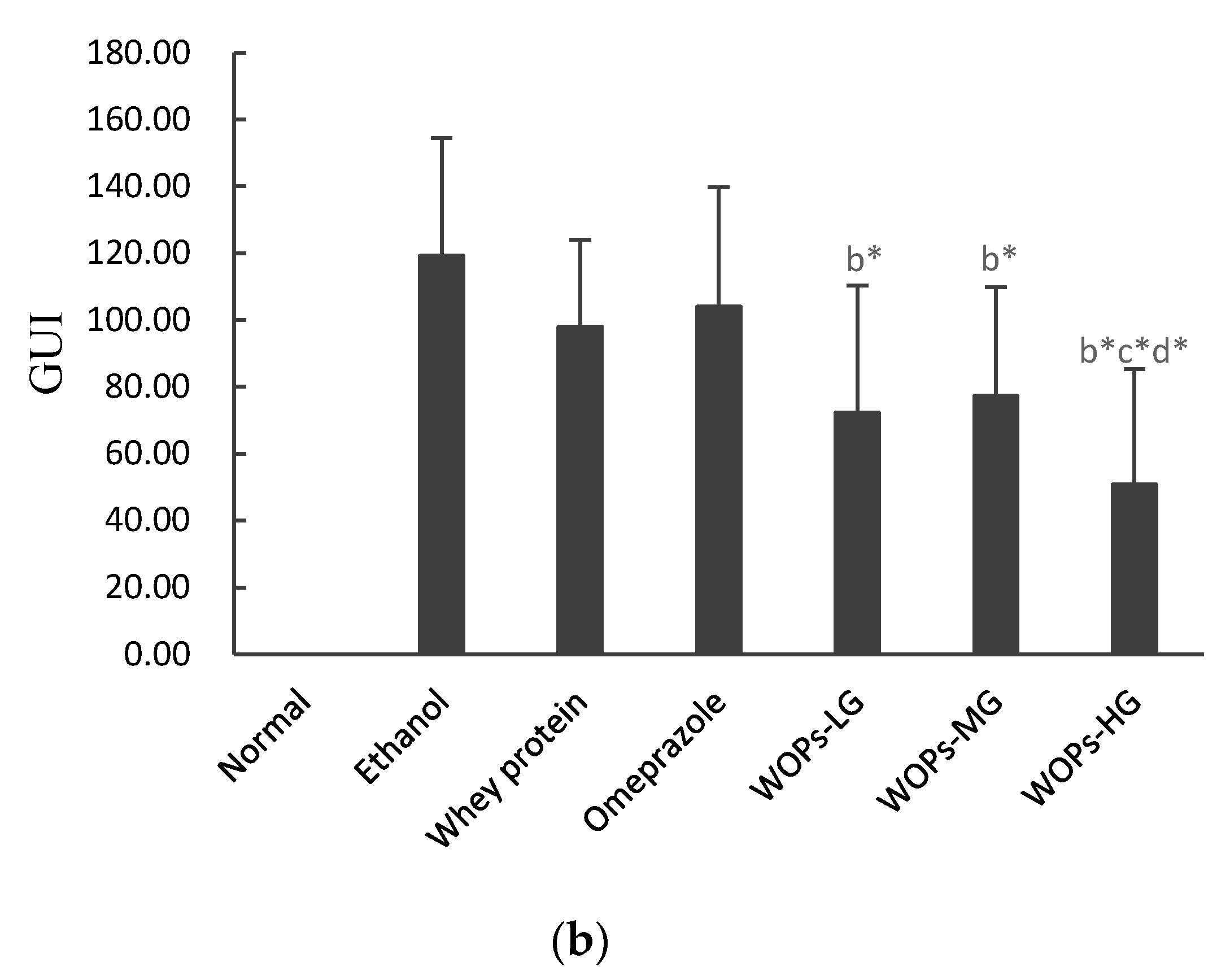
3.2. Effect of WOPs on Gross Evaluation and Gastric Ulcer Index (GUI) of the Gastric Mucosa in Rats
3.3. Effect of WOPs on Gastric Content pH, Pepsinogen, Gastric Mucin Content and Biochemical Analysis
3.4. Effect of WOPs on PGE2, NO, and MPO Levels in Gastric Tissue of Rats
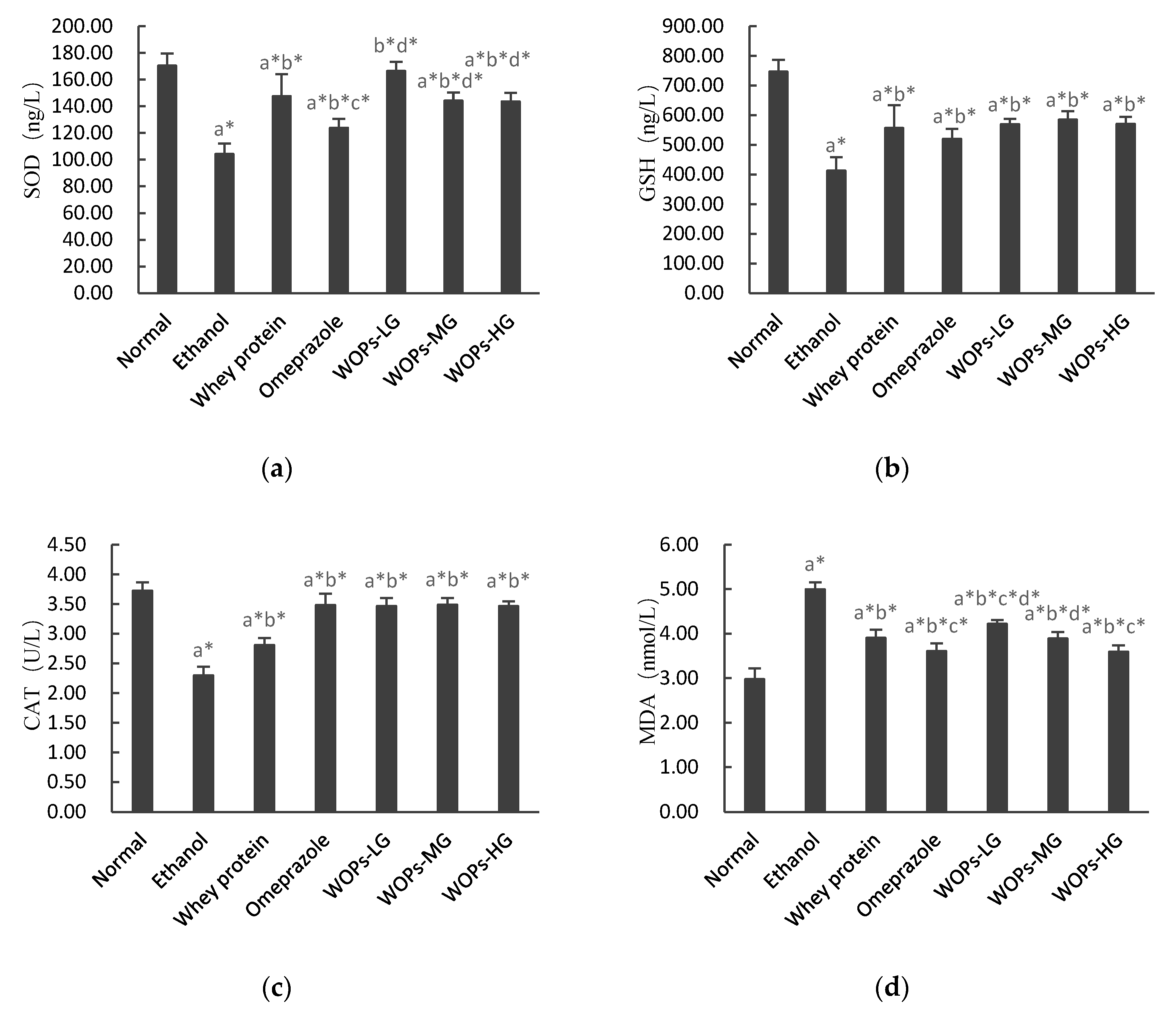
3.5. Effect of WOPs on Ethanol-Induced Oxidative Stress in Gastric Tissue of Rats
3.6. Effect of WOPs on Inflammatory Cytokines in Gastric Tissue of Rats
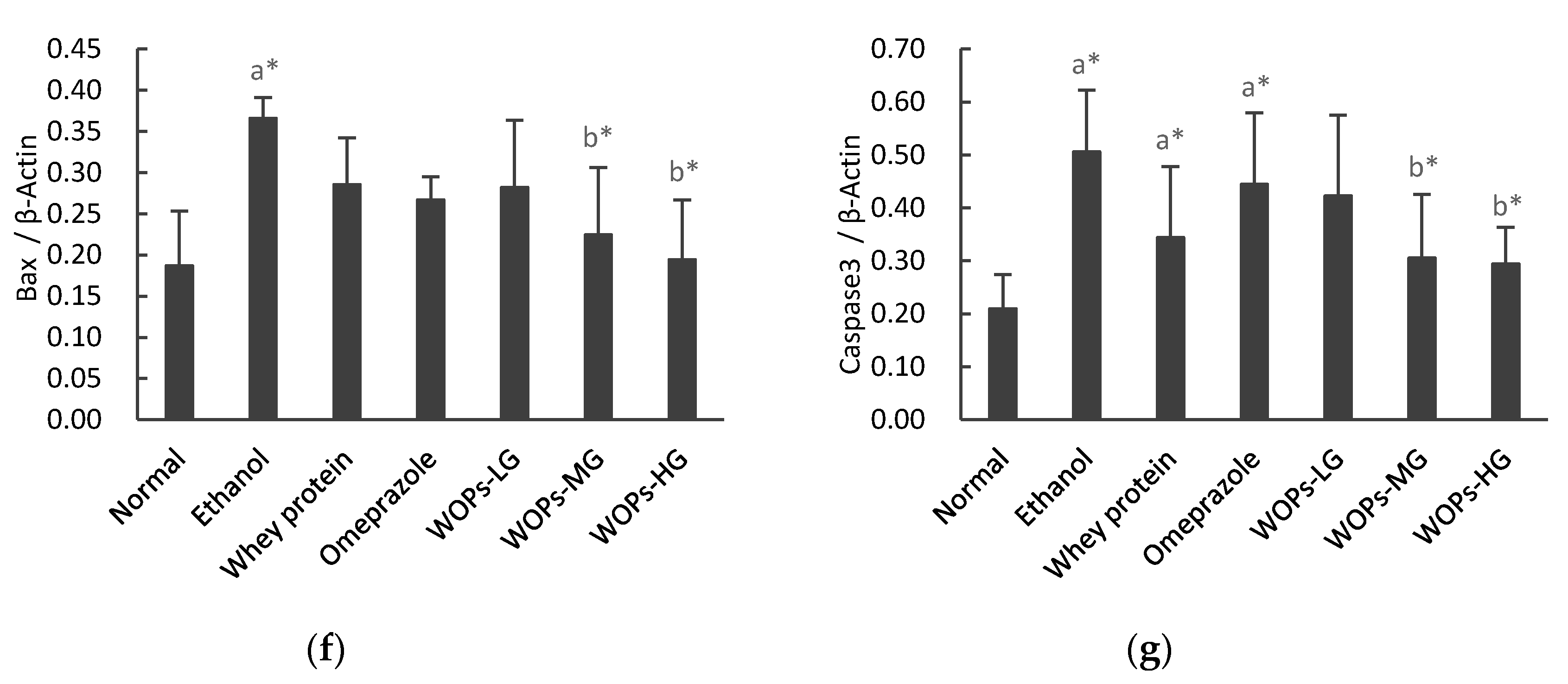
3.7. Effect of WOPs on the NF-κB p65, IκBα, HSP70, Bcl-2, Bax and Caspase-3 Expression in Gastric Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Choi, W.T.; Lauwers, G.Y. Patterns of Gastric Injury: Beyond Helicobacter Pylori. Surg. Pathol. Clin. 2017, 10, 801–822. [Google Scholar] [CrossRef]
- Sidahmed, H.M.; Hashim, N.M.; Abdulla, M.A.; Ali, H.M.; Mohan, S.; Abdelwahab, S.I.; Taha, M.M.; Fai, L.M.; Vadivelu, J. Antisecretory, gastroprotective, antioxidant and anti-Helicobcter pylori activity of zerumbone from Zingiber zerumbet (L.) Smith. PLoS ONE 2015, 10, e0121060. [Google Scholar] [CrossRef] [PubMed]
- Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2018; Available online: https://www.who.int/substance_abuse/publications/global_alcohol_report/en/ (accessed on 17 January 2020).
- Al-Wajeeh, N.S.; Halabi, M.F.; Hajrezaie, M.; Dhiyaaldeen, S.M.; Abdulaziz Bardi, D.; Salama, S.M.; Rouhollahi, E.; Karimian, H.; Abdolmalaki, R.; Azizan, A.H.; et al. The Gastroprotective Effect of Vitex pubescens Leaf Extract against Ethanol-Provoked Gastric Mucosal Damage in Sprague-Dawley Rats. PLoS ONE 2016, 11, e0157431. [Google Scholar] [CrossRef] [PubMed]
- Golbabapour, S.; Hajrezaie, M.; Hassandarvish, P.; Abdul Majid, N.; Hadi, A.H.; Nordin, N.; Abdulla, M.A. Acute toxicity and gastroprotective role of M. pruriens in ethanol-induced gastric mucosal injuries in rats. BioMed Res. Int. 2013, 2013, 974185. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.R.; Goldenring, J.R. Injury, repair, inflammation and metaplasia in the stomach. J. Physiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Raish, M.; Ahmad, A.; Ansari, M.A.; Alkharfy, K.M.; Aljenoobi, F.I.; Jan, B.L.; Al-Mohizea, A.M.; Khan, A.; Ali, N. Momordica charantia polysaccharides ameliorate oxidative stress, inflammation, and apoptosis in ethanol-induced gastritis in mucosa through NF-kB signaling pathway inhibition. Int. J. Biol. Macromol. 2018, 111, 193–199. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Food-derived bioactive peptides—Opportunities for designing future foods. Curr. Pharm. Des. 2003, 9, 1297–1308. [Google Scholar] [CrossRef]
- He, L.X.; Ren, J.W.; Liu, R.; Chen, Q.H.; Zhao, J.; Wu, X.; Zhang, Z.F.; Wang, J.B.; Pettinato, G.; Li, Y. Ginseng (Panax ginseng Meyer) oligopeptides regulate innate and adaptive immune responses in mice via increased macrophage phagocytosis capacity, NK cell activity and Th cells secretion. Food Funct. 2017, 8, 3523–3532. [Google Scholar] [CrossRef]
- He, L.X.; Wang, J.B.; Sun, B.; Zhao, J.; Li, L.; Xu, T.; Li, H.; Sun, J.Q.; Ren, J.W.; Liu, R.; et al. Suppression of TNF-alpha and free radicals reduces systematic inflammatory and metabolic disorders: Radioprotective effects of ginseng oligopeptides on intestinal barrier function and antioxidant defense. J. Nutr. Biochem. 2017, 40, 53–61. [Google Scholar] [CrossRef]
- Li, D.; Ren, J.W.; Zhang, T.; Liu, R.; Wu, L.; Du, Q.; Li, Y. Anti-fatigue effects of small-molecule oligopeptides isolated from Panax quinquefolium L. in mice. Food Funct. 2018, 9, 4266–4273. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Sun, B.; Li, D.; Mao, R.; Li, H.; Li, Y.; Wang, J. Beneficial Effects of Small Molecule Oligopeptides Isolated from Panax ginseng Meyer on Pancreatic Beta-Cell Dysfunction and Death in Diabetic Rats. Nutrients 2017, 9, 1061. [Google Scholar] [CrossRef] [PubMed]
- Castro, G.A.; Carvalho, J.E.; Tinti, S.V.; Possenti, A.; Sgarbieri, V.C. Anti-ulcerogenic effect of a whey protein isolate and collagen hydrolysates against ethanol ulcerative lesions on oral administration to rats. J. Med. Food 2010, 13, 83–90. [Google Scholar] [CrossRef]
- Kan, J.; Hood, M.; Burns, C.; Scholten, J.; Chuang, J.; Tian, F.; Pan, X.; Du, J.; Gui, M. A Novel Combination of Wheat Peptides and Fucoidan Attenuates Ethanol-Induced Gastric Mucosal Damage through Anti-Oxidant, Anti-Inflammatory, and Pro-Survival Mechanisms. Nutrients 2017, 9, 978. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Wang, Z.; Hou, H.; Zhang, Z.; Li, B. Protective Effect of Cod (Gadus macrocephalus) Skin Collagen Peptides on Acetic Acid-Induced Gastric Ulcer in Rats. J. Food Sci. 2016, 81, H1807–H1815. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Yang, H.; Sun, Y.; Niu, J.; Liu, S. Purification and identification of antioxidant peptides from walnut (Juglans regia L.) protein hydrolysates. Peptides 2012, 38, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Huang, D.; Zhai, M.; Yang, L.; Peng, S.; Chen, C.; Feng, X.; Weng, Q.; Zhang, B.; Xu, M. Isolation of a novel bio-peptide from walnut residual protein inducing apoptosis and autophagy on cancer cells. BMC Complement. Altern. Med. 2015, 15, 413. [Google Scholar] [CrossRef]
- Poulose, S.M.; Bielinski, D.F.; Shukitt-Hale, B. Walnut diet reduces accumulation of polyubiquitinated proteins and inflammation in the brain of aged rats. J. Nutr. Biochem. 2013, 24, 912–919. [Google Scholar] [CrossRef]
- Downs, M.L.; Semic-Jusufagic, A.; Simpson, A.; Bartra, J.; Fernandez-Rivas, M.; Rigby, N.M.; Taylor, S.L.; Baumert, J.L.; Mills, E.N. Characterization of low molecular weight allergens from English walnut (Juglans regia). J. Agric. Food Chem. 2014, 62, 11767–11775. [Google Scholar] [CrossRef]
- Kim, D.I.; Kim, K.S. Walnut extract exhibits anti-fatigue action via improvement of exercise tolerance in mice. Lab. Anim. Res. 2013, 29, 190–195. [Google Scholar] [CrossRef]
- Liu, R.; Wu, L.; Du, Q.; Ren, J.W.; Chen, Q.H.; Li, D.; Mao, R.X.; Liu, X.R.; Li, Y. Small Molecule Oligopeptides Isolated from Walnut (Juglans regia L.) and Their Anti-Fatigue Effects in Mice. Molecules 2018, 24, 45. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Liu, R.; He, L.X.; Mao, R.X.; Liu, X.R.; Zhang, T.; Hao, Y.T.; Fan, R.; Xu, M.H.; Li, Y. Radioprotective Effect of Walnut Oligopeptides Against Gamma Radiation-Induced Splenocyte Apoptosis and Intestinal Injury in Mice. Molecules 2019, 24, 1582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.; Mei, N.; Ma, C.; Lou, Z.; Lv, W.; He, G. Protective effects of polysaccharide from Dendrobium nobile against ethanol-induced gastric damage in rats. Int. J. Biol. Macromol. 2018, 107, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.K.; Kang, S.C. Therapeutic potential and mechanism of thymol action against ethanol-induced gastric mucosal injury in rat model. Alcohol (Fayettev N.Y.) 2015, 49, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Al-Sayed, E.; El-Naga, R.N. Protective role of ellagitannins from Eucalyptus citriodora against ethanol-induced gastric ulcer in rats: Impact on oxidative stress, inflammation and calcitonin-gene related peptide. Phytomedicine 2015, 22, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Jeon, W.Y.; Shin, I.S.; Shin, H.K.; Lee, M.Y. Gastroprotective effect of the traditional herbal medicine, Sipjeondaebo-tang water extract, against ethanol-induced gastric mucosal injury. BMC Complement. Altern. Med. 2014, 14, 373. [Google Scholar] [CrossRef]
- Jin, S.E.; Lee, M.Y.; Shin, I.S.; Jeon, W.Y.; Ha, H. Syzygium aromaticum water extract attenuates ethanolinduced gastric injury through antioxidant effects in rats. Mol. Med. Rep. 2016, 14, 361–366. [Google Scholar] [CrossRef]
- Guth, P.H.; Aures, D.; Paulsen, G. Topical aspirin plus HCl gastric lesions in the rat. Cytoprotective effect of prostaglandin, cimetidine, and probanthine. Gastroenterology 1979, 76, 88–93. [Google Scholar] [CrossRef]
- El-Maraghy, S.A.; Rizk, S.M.; Shahin, N.N. Gastroprotective effect of crocin in ethanol-induced gastric injury in rats. Chem. Biol. Interact. 2015, 229, 26–35. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Q.H.; Ren, J.W.; Sun, B.; Cai, X.X.; Li, D.; Mao, R.X.; Wu, X.; Li, Y. Ginseng (Panax ginseng Meyer) Oligopeptides Protect Against Binge Drinking-Induced Liver Damage through Inhibiting Oxidative Stress and Inflammation in Rats. Nutrients 2018, 10, 1665. [Google Scholar] [CrossRef]
- He, L.X.; Zhang, Z.F.; Zhao, J.; Li, L.; Xu, T.; Bin, S.; Ren, J.W.; Liu, R.; Chen, Q.H.; Wang, J.B.; et al. Ginseng oligopeptides protect against irradiation-induced immune dysfunction and intestinal injury. Sci. Rep. 2018, 8, 13916. [Google Scholar] [CrossRef] [PubMed]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Kovac, S.; Shulkes, A.; Baldwin, G.S. Peptide processing and biology in human disease. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.A.; Abdulla, M.A.; Hajrezaie, M.; Bader, A.; Shahzad, N.; Al-Ghamdi, S.S.; Gushash, A.S.; Hasanpourghadi, M. The gastroprotective effects of hydroalcoholic extract of Monolluma quadrangula against ethanol-induced gastric mucosal injuries in Sprague Dawley rats. Drug Des. Dev. Ther. 2016, 10, 93–105. [Google Scholar] [CrossRef]
- Tarnawski, A.S.; Ahluwalia, A.; Jones, M.K.; Brzozowski, T. Expression of nerve growth factor in rat stomach. Implications for interactions between endothelial, neural and epithelial cells. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2016, 67, 879–883. [Google Scholar]
- Said, H.; Kaunitz, J.D. Gastrointestinal defense mechanisms. Curr. Opin. Gastroenterol. 2016, 32, 461–466. [Google Scholar] [CrossRef]
- Yamashita, H.; Kanamori, A.; Kano, C.; Hashimura, H.; Matsumoto, K.; Tsujimae, M.; Yoshizaki, T.; Momose, K.; Obata, D.; Eguchi, T.; et al. The Effects of Switching to Vonoprazan, a Novel Potassium-Competitive Acid Blocker, on Gastric Acidity and Reflux Patterns in Patients with Erosive Esophagitis Refractory to Proton Pump Inhibitors. Digestion 2017, 96, 52–59. [Google Scholar] [CrossRef]
- Laine, L.; Takeuchi, K.; Tarnawski, A. Gastric mucosal defense and cytoprotection: Bench to bedside. Gastroenterology 2008, 135, 41–60. [Google Scholar] [CrossRef]
- Hsu, P.I.; Chen, C.H.; Hsieh, C.S.; Chang, W.C.; Lai, K.H.; Lo, G.H.; Hsu, P.N.; Tsay, F.W.; Chen, Y.S.; Hsiao, M.; et al. Alpha1-antitrypsin precursor in gastric juice is a novel biomarker for gastric cancer and ulcer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 876–883. [Google Scholar] [CrossRef]
- Demitrack, E.S.; Aihara, E.; Kenny, S.; Varro, A.; Montrose, M.H. Inhibitors of acid secretion can benefit gastric wound repair independent of luminal pH effects on the site of damage. Gut 2012, 61, 804–811. [Google Scholar] [CrossRef]
- Brzozowska, I.; Konturek, P.C.; Brzozowski, T.; Konturek, S.J.; Kwiecien, S.; Pajdo, R.; Drozdowicz, D.; Pawlik, M.; Ptak, A.; Hahn, E.G. Role of prostaglandins, nitric oxide, sensory nerves and gastrin in acceleration of ulcer healing by melatonin and its precursor, L-tryptophan. J. Pineal Res. 2002, 32, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Ennulat, D.; Lynch, K.M.; Kimbrough, C.L.; Mirabile, R.C.; Rehm, S. Evaluation of Pepsinogen I as a Biomarker of Drug-induced Gastric Mucosal Injury in Cynomolgus Monkeys. Toxicol. Pathol. 2017, 45, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.Y.; Jeong, C.S. Gastroprotective Activities of Sennoside A and Sennoside B via the Up-Regulation of Prostaglandin E2 and the Inhibition of H(+)/K(+)-ATPase. Biomol. Ther. (Seoul) 2015, 23, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Helmer, K.S.; West, S.D.; Shipley, G.L.; Chang, L.; Cui, Y.; Mailman, D.; Mercer, D.W. Gastric nitric oxide synthase expression during endotoxemia: Implications in mucosal defense in rats. Gastroenterology 2002, 123, 173–186. [Google Scholar] [CrossRef]
- Lucetti, L.T.; Silva, R.O.; Santana, A.P.; de Melo Tavares, B.; Vale, M.L.; Soares, P.M.; de Lima Junior, F.J.; Magalhaes, P.J.; de Queiroz Cunha, F.; de Albuquerque Ribeiro, R.; et al. Nitric Oxide and Hydrogen Sulfide Interact When Modulating Gastric Physiological Functions in Rodents. Dig. Dis. Sci. 2017, 62, 93–104. [Google Scholar] [CrossRef]
- Wallace, J.L.; Ianaro, A.; de Nucci, G. Gaseous Mediators in Gastrointestinal Mucosal Defense and Injury. Dig. Dis. Sci. 2017, 62, 2223–2230. [Google Scholar] [CrossRef]
- Paulrayer, A.; Adithan, A.; Lee, J.H.; Moon, K.H.; Kim, D.G.; Im, S.Y.; Kang, C.W.; Kim, N.S.; Kim, J.H. Aronia melanocarpa (Black Chokeberry) Reduces Ethanol-Induced Gastric Damage via Regulation of HSP-70, NF-kappaB, and MCP-1 Signaling. Int. J. Mol. Sci. 2017, 18, 1195. [Google Scholar] [CrossRef]
- Perez, S.; Talens-Visconti, R.; Rius-Perez, S.; Finamor, I.; Sastre, J. Redox signaling in the gastrointestinal tract. Free Radic. Biol. Med. 2017, 104, 75–103. [Google Scholar] [CrossRef]
- Hsu, D.Z.; Chu, P.Y.; Liu, M.Y. Effect of sesame oil on acidified ethanol-induced gastric mucosal injury in rats. JPEN J. Parenter. Enter. Nutr. 2009, 33, 423–427. [Google Scholar] [CrossRef]
- Jabri, M.A.; Aissani, N.; Tounsi, H.; Sakly, M.; Marzouki, L.; Sebai, H. Protective effect of chamomile (Matricaria recutita L.) decoction extract against alcohol-induced injury in rat gastric mucosa. Pathophysiol. Off. J. Int. Soc. Pathophysiol. 2017, 24, 1–8. [Google Scholar] [CrossRef]
- Tu, P.S.; Tung, Y.T.; Lee, W.T.; Yen, G.C. Protective Effect of Camellia Oil (Camellia oleifera Abel.) against Ethanol-Induced Acute Oxidative Injury of the Gastric Mucosa in Mice. J. Agric. Food Chem. 2017, 65, 4932–4941. [Google Scholar] [CrossRef]
- Diniz, P.B.; Ribeiro, A.R.; Estevam, C.S.; Bani, C.C.; Thomazzi, S.M. Possible mechanisms of action of Caesalpinia pyramidalis against ethanol-induced gastric damage. J. Ethnopharmacol. 2015, 168, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Shibata, W.; Takaishi, S.; Muthupalani, S.; Pritchard, D.M.; Whary, M.T.; Rogers, A.B.; Fox, J.G.; Betz, K.S.; Kaestner, K.H.; Karin, M.; et al. Conditional deletion of IkappaB-kinase-beta accelerates helicobacter-dependent gastric apoptosis, proliferation, and preneoplasia. Gastroenterology 2010, 138, 1022–1034. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wei, R.; Deng, A.; Lei, T. Protective Effects of Ethanolic Extracts from Artichoke, an Edible Herbal Medicine, against Acute Alcohol-Induced Liver Injury in Mice. Nutrients 2017, 9, 1000. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Bao, L.; Wang, N.; Ren, J.; Chen, Q.; Xu, M.; Li, D.; Mao, R.; Li, Y. Dietary nucleotides protect against alcoholic liver injury by attenuating inflammation and regulating gut microbiota in rats. Food Funct. 2016, 7, 2898–2908. [Google Scholar] [CrossRef] [PubMed]



| Severity of Erosion | 1 Point | 2 Points | 3 Points | 4 Points | ||
|---|---|---|---|---|---|---|
| Spot erosion | - | - | - | |||
| Erosion length | <1 mm | 1–2 mm | 2–3 mm | 3–4 mm | >4 mm, segmented scored | |
| Erosion width | >2 mm, score doubled | |||||
| Total score | A sum of partial scores | |||||
| Groups | Body Weight (g) | Food Intake (g) | Food Utilization (%) | |
|---|---|---|---|---|
| Initial | Final | |||
| Normal | 218.63 ± 9.79 | 400.00 ± 47.56 | 712.82 ± 46.74 | 25.37 ± 5.46 |
| Ethanol | 218.23 ± 10.19 | 396.17 ± 31.58 | 733.38 ± 81.68 | 25.28 ± 4.31 |
| Whey protein | 218.88 ± 10.13 | 412.29 ± 49.08 | 722.21 ± 52.95 | 26.68 ± 5.87 |
| Omeprazole | 218.60 ± 12.51 | 403.45 ± 46.70 | 750.68 ± 40.59 | 24.75 ± 5.37 |
| WOPs-LG | 221.75 ± 6.33 | 400.40 ± 46.15 | 748.74 ± 82.38 | 24.54 ± 4.12 |
| WOPs-MG | 220.14 ± 8.58 | 398.68 ± 33.54 | 739.33 ± 51.50 | 25.52 ± 2.43 |
| WOPs-HG | 221.15 ± 7.06 | 425.73 ± 55.07 | 787.00 ± 64.67 a*b*c* | 27.08 ± 3.32 |
| Groups | pH | PG1 (μg/L) | PG2 (μg/L) | PGR | Mucin (ng/L) | ALT (U/L) | AST (U/L) |
|---|---|---|---|---|---|---|---|
| Normal | 2.42 ± 0.77 | 5.09 ± 0.35 | 2.89 ± 0.13 | 1.77 ± 0.18 | 4.42 ± 0.23 | 60.83 ± 17.37 | 211.50 ± 57.15 |
| Ethanol | 5.15 ± 0.95 a* | 8.74 ± 0.43 a* | 4.90 ± 0.17 a* | 1.79 ± 0.08 | 2.47 ± 0.16 a* | 83.75 ± 15.74 a* | 300.80 ± 56.03 a* |
| Whey protein | 4.08 ± 1.52 | 6.10 ± 0.32 a*b* | 4.20 ± 0.24 a*b* | 1.46 ± 0.09 b* | 3.39 ± 0.14 a*b* | 68.11 ± 15.28 b* | 269.20 ± 58.54 a* |
| Omeprazole | 6.54 ± 1.17 a*b*c* | 5.81 ± 0.25 a*b* | 4.27 ± 0.21 a*b* | 1.36 ± 0.09 a*b* | 3.59 ± 0.17 a*b*c* | 71.00 ± 19.03 | 262.00 ± 44.47 a* |
| WOPs-LG | 4.16 ± 1.26 d* | 7.46 ± 0.18 a*b*c*d* | 4.40 ± 0.20 a*b*c* | 1.71 ± 0.09 c*d* | 3.41 ± 0.17 a*b* | 68.45 ± 8.70 b* | 274.78 ± 53.67 a* |
| WOPs-MG | 3.14 ± 1.58 d* | 6.83 ± 0.36 a*b*c*d* | 4.00 ± 0.18 a*b*c*d* | 1.71 ± 0.11 c*d* | 3.29 ± 0.15 a*b*d* | 66.50 ± 12.78 b* | 283.00 ± 33.36 a* |
| WOPs-HG | 2.75 ± 0.44 b*d* | 6.97 ± 0.35 a*b*c*d* | 3.87 ± 0.25 a*b*c*d* | 1.80 ± 0.16 c*d* | 3.22 ± 0.13 a*b*c*d* | 64.60 ± 10.16 b* | 247.38 ± 29.21 b* |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Hao, Y.-T.; Zhu, N.; Liu, X.-R.; Kang, J.-W.; Mao, R.-X.; Hou, C.; Li, Y. The Gastroprotective Effect of Small Molecule Oligopeptides Isolated from Walnut (Juglans regia L.) against Ethanol-Induced Gastric Mucosal Injury in Rats. Nutrients 2020, 12, 1138. https://doi.org/10.3390/nu12041138
Liu R, Hao Y-T, Zhu N, Liu X-R, Kang J-W, Mao R-X, Hou C, Li Y. The Gastroprotective Effect of Small Molecule Oligopeptides Isolated from Walnut (Juglans regia L.) against Ethanol-Induced Gastric Mucosal Injury in Rats. Nutrients. 2020; 12(4):1138. https://doi.org/10.3390/nu12041138
Chicago/Turabian StyleLiu, Rui, Yun-Tao Hao, Na Zhu, Xin-Ran Liu, Jia-Wei Kang, Rui-Xue Mao, Chao Hou, and Yong Li. 2020. "The Gastroprotective Effect of Small Molecule Oligopeptides Isolated from Walnut (Juglans regia L.) against Ethanol-Induced Gastric Mucosal Injury in Rats" Nutrients 12, no. 4: 1138. https://doi.org/10.3390/nu12041138
APA StyleLiu, R., Hao, Y.-T., Zhu, N., Liu, X.-R., Kang, J.-W., Mao, R.-X., Hou, C., & Li, Y. (2020). The Gastroprotective Effect of Small Molecule Oligopeptides Isolated from Walnut (Juglans regia L.) against Ethanol-Induced Gastric Mucosal Injury in Rats. Nutrients, 12(4), 1138. https://doi.org/10.3390/nu12041138





