Flaxseed (Linum Usitatissimum L.) Supplementation in Patients Undergoing Lipoprotein Apheresis for Severe Hyperlipidemia—A Pilot Study
Abstract
1. Introduction
2. Methods
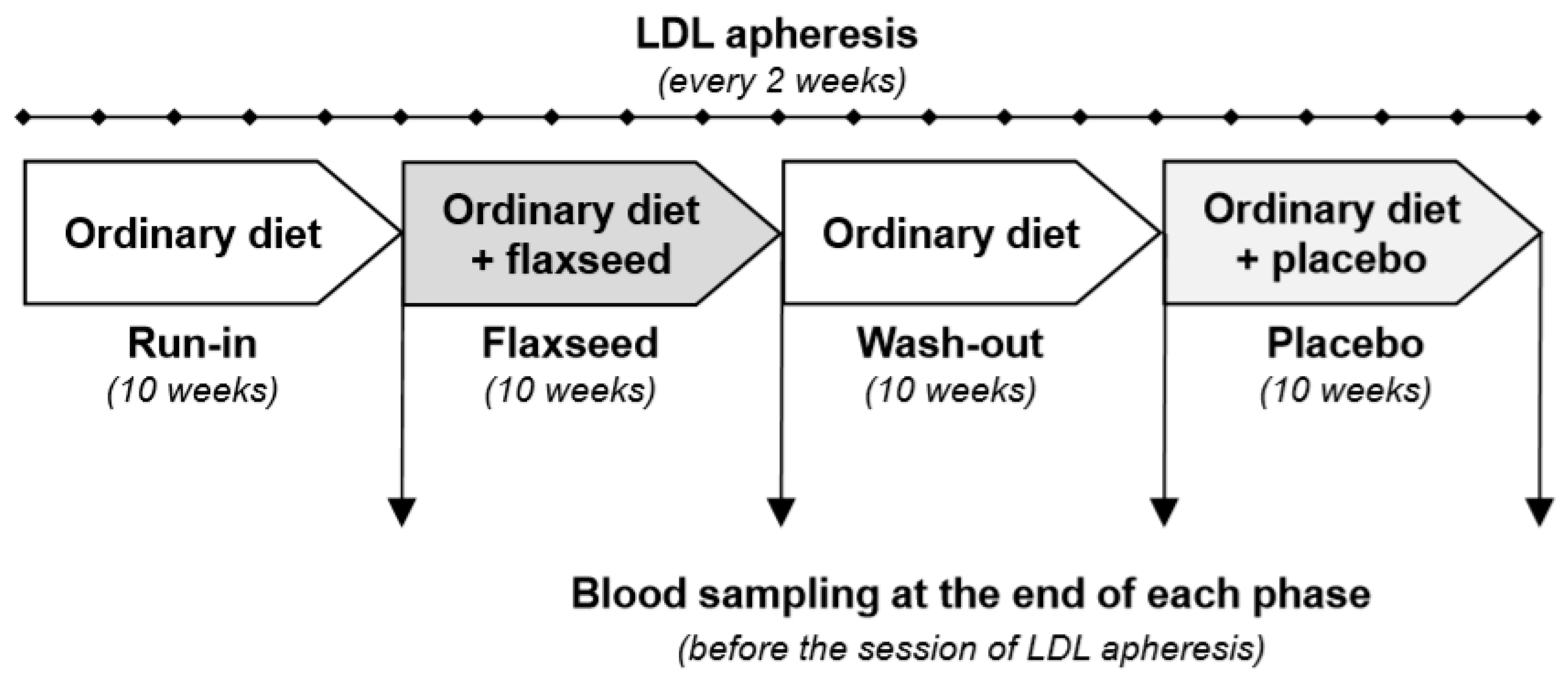
2.1. Study Design
2.2. Study Population
- Statin intolerance and severe hypercholesterolemia with LDL-C > 130 mg/dL, despite treatment for >12 months with maximally tolerated doses and documented atherosclerosis;
- Severe familial hypercholesterolemia with LDL-C > 130 mg/dL despite treatment for >12 months with maximally tolerated doses, documented atherosclerosis and coexisting elevated Lp(a) >60 mg/dl;
- Severe hyperlipoproteinemia (a) with Lp(a) level >100 mg/dL in patients with documented atherosclerosis.
2.3. Flaxseed Supplementation and Study Protocol
2.4. Statistical Analysis
3. Results
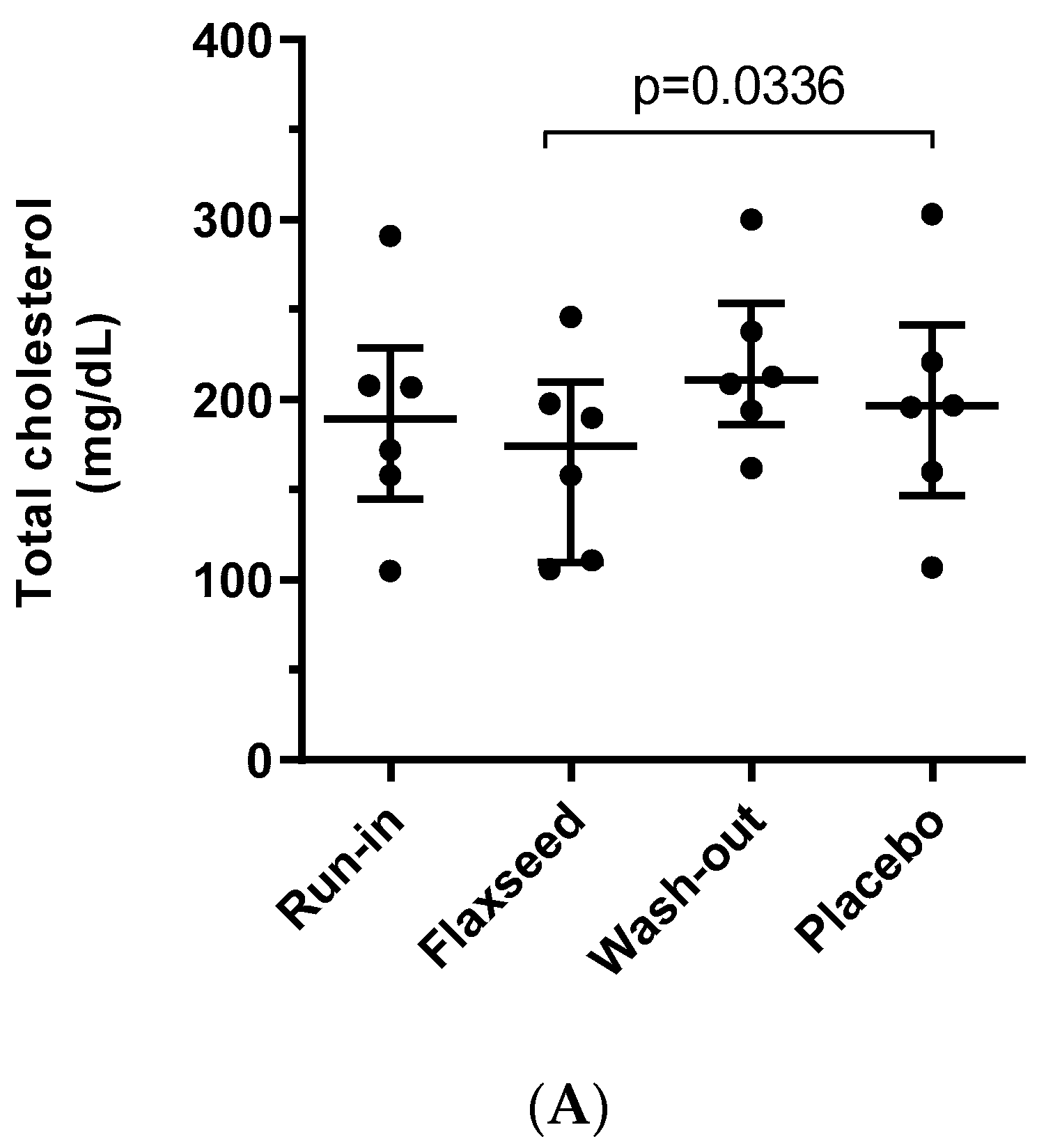
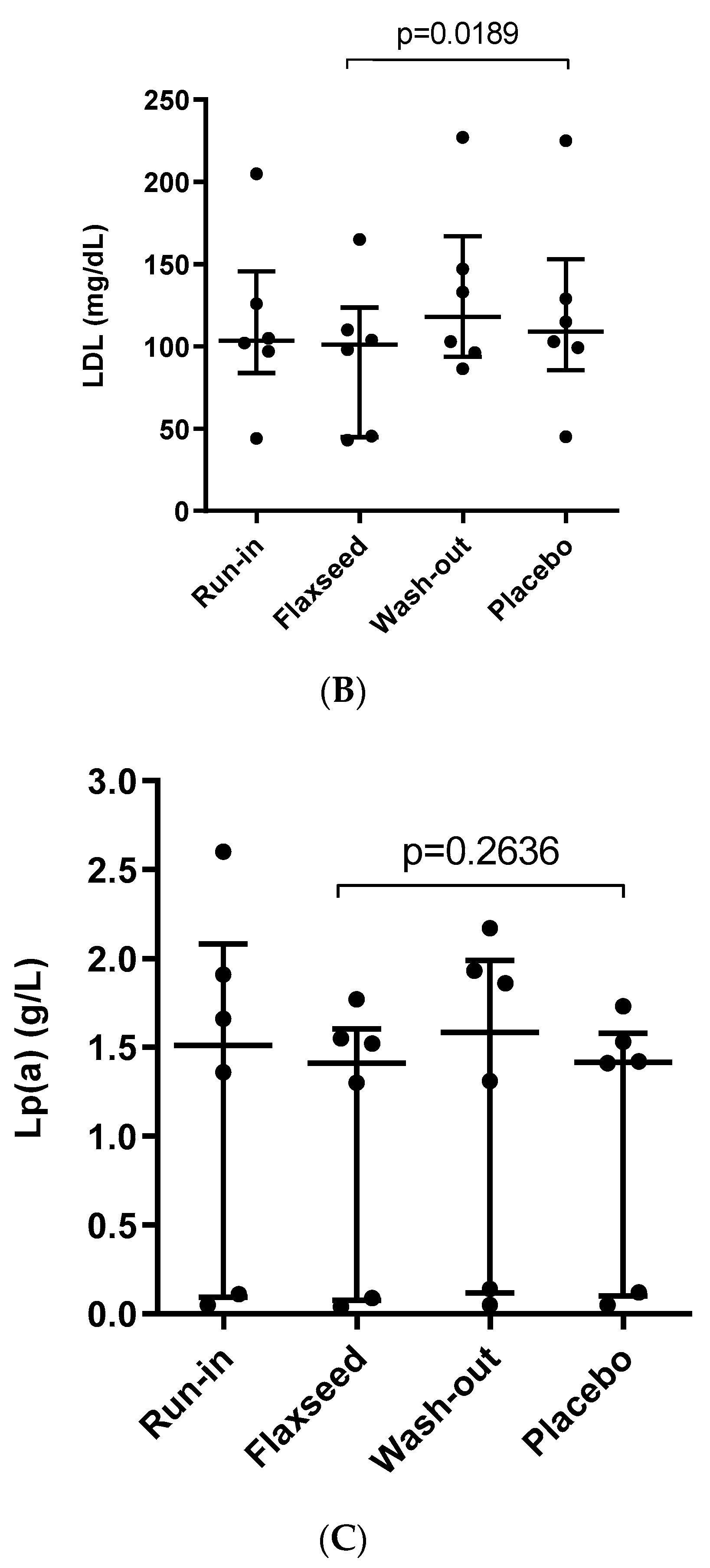
3.1. Effect of Flaxseed on Total Cholesterol, LDL and Lp(a) Levels
3.2. Effect of Flaxseed on Parameters of Inflammation
3.3. Other Parameters
3.4. Detailed Lipid Parameters before and after Lipoprotein Apheresis Sessions
3.5. Side Effects and Tolerability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Austin, M.A.; Hutter, C.M.; Zimmern, R.L.; Humphries, S.E. Genetic causes of monogenic heterozygous familial hypercholesterolemia: A HuGE prevalence review. Am. J. Epidemiol. 2004, 160, 407–420. [Google Scholar] [CrossRef]
- de Beer, V.J.; Merkus, D.; Bender, S.B. Familial hypercholesterolemia impairs exercise-induced systemic vasodilation due to reduced NO bioavailability. J. Appl. Physiol. 2013, 115, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, T.A.; Ito, M.K.; Maki, K.C. National lipid association recommendations for patient-centered management of dyslipidemia: Part 1-full report. J. Clin. Lipidol. 2015, 9, 129–169. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Chapman, M.J.; Ray, K. Lipoprotein(a) as a cardiovascular risk factor: Current status. Eur. Heart J. 2010, 31, 2844–2853. [Google Scholar] [CrossRef] [PubMed]
- Vuorio, A.; Watts, G.F.; Schneider, W.J.; Tsimikas, S.; Kovanen, P.T. Familial hypercholesterolemia and elevated lipoprotein(a): Double heritable risk and new therapeutic opportunities. J. Intern. Med. 2020, 287, 2–18. [Google Scholar] [CrossRef]
- Gossios, T.; Zografou, I.; Simoulidou, V.; Pirpassopoulou, A.; Christou, K.; Karagiannis, A. Multimodal Treatment of Homozygous Familial Hypercholesterolemia. Curr. Pharm. Des. 2018, 24, 3616–3621. [Google Scholar] [CrossRef] [PubMed]
- Tsimikas, S.; Karwatowska-Prokopczuk, E.; Gouni-Berthold, I. Lipoprotein(a) Reduction in Persons with Cardiovascular Disease. N. Engl. J. Med. 2020, 382, 244–255. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Visioli, F. Polyphenol-based nutraceuticals for the prevention and treatment of cardiovascular disease: Review of human evidence. Phytomedicine 2016, 23, 1145–1174. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, W.; Spinetti, T. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 2013, 38, 1154–1163. [Google Scholar] [CrossRef]
- Parikh, M.; Netticadan, T.; Pierce, G.N. Flaxseed: Its bioactive components and their cardiovascular benefits. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H146–H159. [Google Scholar] [CrossRef]
- Perez-Martinez, P.; Katsiki, N.; Mikhailidis, D.P. The Role of n-3 Fatty Acids in Cardiovascular Disease: Back to the Future. Angiology 2020, 71, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Wiktorowska-Owczarek, A.; Berezińska, M.; Nowak, J.Z. PUFAs: Structures, Metabolism and Functions. Adv. Clin. Exp. Med. 2015, 24, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, S.K.; Psota, T.L.; Harris, W.S.; Kris-Etherton, P.M. n-3 fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. Am. J. Clin. Nutr. 2006, 83, 1526S–1535S. [Google Scholar] [CrossRef] [PubMed]
- Ras, R.T.; Geleijnse, J.M.; Trautwein, E.A. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: A meta-analysis of randomised controlled studies. Br. J. Nutr. 2014, 112, 214–219. [Google Scholar] [CrossRef]
- Moreau, R.A.; Nyström, L.; Whitaker, B.D. Phytosterols and their derivatives: Structural diversity, distribution, metabolism, analysis, and health-promoting uses. Prog. Lipid Res. 2018, 70, 35–61. [Google Scholar] [CrossRef]
- Vilahur, G.; Ben-Aicha, S.; Diaz-Riera, E.; Badimon, L.; Padró, T. Phytosterols and Inflammation. Curr. Med. Chem. 2019, 26, 6724–6734. [Google Scholar] [CrossRef]
- Law, M.R. Plant sterol and stanol margarines and health. West J. Med. 2000, 173, 43–47. [Google Scholar] [CrossRef]
- Mickiewicz, A.; Chmara, M.; Futema, M. Efficacy of clinical diagnostic criteria for familial hypercholesterolemia genetic testing in Poland. Atherosclerosis 2016, 249, 52–58. [Google Scholar] [CrossRef]
- Dittrich, M.; Jahreis, G.; Bothor, K. Benefits of foods supplemented with vegetable oils rich in α-linolenic, stearidonic or docosahexaenoic acid in hypertriglyceridemic subjects: A double-blind, randomized, controlled trail. Eur. J. Nutr. 2015, 54, 881–893. [Google Scholar] [CrossRef]
- Pan, A.; Yu, D.; Demark-Wahnefried, W.; Franco, O.H.; Lin, X. Meta-analysis of the effects of flaxseed interventions on blood lipids. Am. J. Clin. Nutr. 2009, 90, 288–297. [Google Scholar] [CrossRef]
- Wong, H.; Chahal, N.; Manlhiot, C.; Niedra, E.; McCrindle, B.W. Flaxseed in pediatric hyperlipidemia: A placebo-controlled, blinded, randomized clinical trial of dietary flaxseed supplementation for children and adolescents with hypercholesterolemia. JAMA Pediatr. 2013, 167, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Hadi, A.; Askarpour, M.; Salamat, S.; Ghaedi, E.; Symonds, M.E.; Miraghajani, M. Effect of flaxseed supplementation on lipid profile: An updated systematic review and dose-response meta-analysis of sixty-two randomized controlled trials. Pharmacol. Res. 2020, 152, 104622. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Ali, S.; Khan, F. Partial Reversal of Obesity-Induced Insulin Resistance Owing to Anti-Inflammatory Immunomodulatory Potential of Flaxseed Oil. Immunol. Investig. 2015, 44, 451–469. [Google Scholar] [CrossRef] [PubMed]
- Dupasquier, C.M.; Dibrov, E.; Kneesh, A.L. Dietary flaxseed inhibits atherosclerosis in the LDL receptor-deficient mouse in part through antiproliferative and anti-inflammatory actions. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H2394–H2402. [Google Scholar] [CrossRef] [PubMed]
- Bassett, C.M.; Rodriguez-Leyva, D.; Pierce, G.N. Experimental and clinical research findings on the cardiovascular benefits of consuming flaxseed. Appl. Physiol. Nutr. Metab. 2009, 34, 965–974. [Google Scholar] [CrossRef]
- Marx, N.; Duez, H.; Fruchart, J.C.; Staels, B. Peroxisome proliferator-activated receptors and atherogenesis: Regulators of gene expression in vascular cells. Circ. Res. 2004, 94, 1168–1178. [Google Scholar] [CrossRef]
- Sahebkar, A.; Katsiki, N.; Ward, N.; Reiner, Ž. Flaxseed Supplementation Reduces Plasma Lipoprotein(a) Levels: A Meta-Analysis. Altern. Ther. Health Med. 2019. [Google Scholar]
- Henkin, Y.; Shai, I. Dietary treatment of hypercholesterolemia: Can we predict long-term success? J. Am. Coll. Nutr. 2003, 22, 555–561. [Google Scholar] [CrossRef]
- Fumeron, F.; Bard, J.M.; Lecerf, J.M. Interindividual variability in the cholesterol-lowering effect of supplementation with plant sterols or stanols. Nutr. Rev. 2017, 75, 134–145. [Google Scholar] [CrossRef]
- Askarpour, M.; Karimi, M.; Hadi, A. Effect of flaxseed supplementation on markers of inflammation and endothelial function: A systematic review and meta-analysis. Cytokine 2020, 126, 154922. [Google Scholar] [CrossRef]
- de Oliveira, P.A.; Kovacs, C.; Moreira, P.; Magnoni, D.; Saleh, M.H.; Faintuch, J. Unsaturated Fatty Acids Improve Atherosclerosis Markers in Obese and Overweight Non-diabetic Elderly Patients. Obes. Surg. 2017, 27, 2663–2671. [Google Scholar] [CrossRef] [PubMed]
- Skulas-Ray, A.C.; Kris-Etherton, P.M.; Harris, W.S.; Vanden Heuvel, J.P.; Wagner, P.R.; West, S.G. Dose-response effects of omega-3 fatty acids on triglycerides, inflammation, and endothelial function in healthy persons with moderate hypertriglyceridemia. Am. J. Clin. Nutr. 2011, 93, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, L.; Gajulapuri, A.; Frumento, P. Interleukin 6 trans-signalling and risk of future cardiovascular events. Cardiovasc. Res. 2019, 115, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Riethmueller, S.; Ehlers, J.C.; Lokau, J. Cleavage Site Localization Differentially Controls Interleukin-6 Receptor Proteolysis by ADAM10 and ADAM17. Sci. Rep. 2016, 6, 2555. [Google Scholar] [CrossRef] [PubMed]
- Speck, N.; Brandsch, C.; Schmidt, N. The Antiatherogenic Effect of Fish Oil in Male Mice Is Associated with a Diminished Release of Endothelial ADAM17 and ADAM10 Substrates. J. Nutr. 2015, 145, 1218–1226. [Google Scholar] [CrossRef]
| Sex, M/F | 4/2 |
| Age, years | 55.5 (48.0–79.0) |
| BMI, kg/m2 | 29.5 (22.0–32.0) |
| Underlying condition: | |
| Isolated hyper-Lp(a) | 2 |
| Familial hypercholesterolemia (confirmed) | 1 |
| Clinical phenotype of familial hypercholesterolemia | 3 |
| Statin intolerance | 2 |
| Ever smoked, Y/N | 3/3 |
| Coronary artery disease, Y/N | 5/1 |
| Stroke, Y/N | 0/6 |
| Diabetes, Y/N | 0/6 |
| Hypertension, Y/N | 3/3 |
| Time on lipoprotein apheresis, months | 26.5 (19.0–38.0) |
| Lipid profile before commencement of lipoprotein apheresis: | |
| Lp(a), g/L Reference range | 1.1 (0.06–3.1) 0.02–0.74 |
| Total cholesterol, mg/dL Reference range: | 239.5 (191.0–367.0) 115–190 |
| LDL, mg/dL Reference range: | 152.8 (118.0–152.5) <100 |
| HDL, mg/dL Reference range: | 49.0 (39.0–77.0) F > 45; M > 40 |
| Triglycerides, mg/dL Reference range: | 294.5 (187.0–735.0) <150 |
| ApoA1, g/L Reference range: | 1.6 (1.3–1.8) 1.25–2.15 |
| ApoB, g/L Reference range: | 1.1 (0.7–2.0) 0.55–1.25 |
| ApoB/ApoA1 Reference range: | 0.7 (0.4–1.2) 0.3–0.9 |
| Additional medication: | |
| Statins, Y/N | 4/2 |
| Ezetimibe, Y/N | 5/1 |
| Fenofibrate, Y/N | 1/5 |
| Composition | Flaxseed Biscuit | Placebo Biscuit |
|---|---|---|
| Energy, kcal | 274.2 | 230.0 |
| Protein, g | 7.0 | 3.0 |
| Carbohydrates, g | 19.6 | 30.4 |
| Total fat, g | 19.2 | 11.0 |
| • Saturated FA, g | 7.0 | 7.0 |
| • Monounsaturated FA, g | 4.2 | 3.0 |
| • Polyunsaturated FA, g | 6.0 | 0.4 |
| • Cholesterol, mg | 33.2 | 33.2 |
| • Omega-6 FA, g | 1.4 | 0.4 |
| • Omega-3 FA, g | 4.8 | 0.08 |
| Fibers, g | 1.0 | 0.6 |
| Parameter | After | p-Value Flaxseed vs. Placebo | |||
|---|---|---|---|---|---|
| Run-in | Flaxseed | Wash-out | Placebo | ||
| IL 6 (pg/mL) | 11.0 (7.7–37.0) | 12.7 (8.3–19.3) | 11.4 (7.5–35.7) | 10.2 (7.7–55.0) | p = 0.371 |
| sIL-6R (pg/mL) | 53.1 (45.0–75.1) | 51.3 (41.3–80.3) | 51.9 (38.7–93.0) | 65.9 (42.7–81.0) | p = 0.057 |
| CRP (µg/mL) | 0.46 (0.30–2.93) | 0.41 (0.28–0.88) | 0.54 (0.34–1.50) | 0.53 (0.32–1.10) | p = 0.179 |
| TNFα (pg/mL) | 0.56 (0.39–0.73) | 0.57 (0.31–1.00) | 0.40 (0.35–1.05) | 0.54 (0.28–1.04) | p = 0.654 |
| sgp130 (ng/mL) | 490.0(326.8–1287.0) | 563.0 (304.0–1920.0) | 509.2 (434.5–1067.0) | 507.9 (445.9–579.7) | p = 0.179 |
| Parameter | After | p-Value Flaxseed vs. Placebo | |||
|---|---|---|---|---|---|
| Run-in | Flaxseed | Wash-out | Placebo | ||
| HDL (mg/dL) | 46.5 (32.0–68.0) | 44.0 (31.0–94.0) | 54.4 (31.0–79.0) | 43.5 (38.0–77.0) | p = 0.314 |
| TG (mg/dL) | 153.5 (61.0–413.0) | 118.0 (87.0–352.0) | 137.5 (89.0–457.0) | 123.5 (61.0–244.0) | p = 0.911 |
| ApoA1 (g/L) | 1.35 (1.16–1.86) | 1.49 (1.17–1.91) | 1.77 (1.23–1.90) | 1.97 (1.24–2.01) | p = 0.911 |
| ApoB (g/L) | 0.99 (0.54–1.94) | 1.04 (0.55–1.42) | 0.87 (0.56–1.62) | 1.2 (0.58–1.69) | p = 0.117 |
| ApoB/ApoA1 | 0.65 (0.42–1.49) | 0.68 (0.28–0.99) | 0.68 (0.42–1.14) | 0.64 (0.41–1.27) | p = 0.771 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanikowska, D.; Korybalska, K.; Mickiewicz, A.; Rutkowski, R.; Kuchta, A.; Sato, M.; Kreft, E.; Fijałkowski, M.; Gruchała, M.; Jankowski, M.; et al. Flaxseed (Linum Usitatissimum L.) Supplementation in Patients Undergoing Lipoprotein Apheresis for Severe Hyperlipidemia—A Pilot Study. Nutrients 2020, 12, 1137. https://doi.org/10.3390/nu12041137
Kanikowska D, Korybalska K, Mickiewicz A, Rutkowski R, Kuchta A, Sato M, Kreft E, Fijałkowski M, Gruchała M, Jankowski M, et al. Flaxseed (Linum Usitatissimum L.) Supplementation in Patients Undergoing Lipoprotein Apheresis for Severe Hyperlipidemia—A Pilot Study. Nutrients. 2020; 12(4):1137. https://doi.org/10.3390/nu12041137
Chicago/Turabian StyleKanikowska, Dominika, Katarzyna Korybalska, Agnieszka Mickiewicz, Rafał Rutkowski, Agnieszka Kuchta, Maki Sato, Ewelina Kreft, Marcin Fijałkowski, Marcin Gruchała, Maciej Jankowski, and et al. 2020. "Flaxseed (Linum Usitatissimum L.) Supplementation in Patients Undergoing Lipoprotein Apheresis for Severe Hyperlipidemia—A Pilot Study" Nutrients 12, no. 4: 1137. https://doi.org/10.3390/nu12041137
APA StyleKanikowska, D., Korybalska, K., Mickiewicz, A., Rutkowski, R., Kuchta, A., Sato, M., Kreft, E., Fijałkowski, M., Gruchała, M., Jankowski, M., Bręborowicz, A., & Witowski, J. (2020). Flaxseed (Linum Usitatissimum L.) Supplementation in Patients Undergoing Lipoprotein Apheresis for Severe Hyperlipidemia—A Pilot Study. Nutrients, 12(4), 1137. https://doi.org/10.3390/nu12041137





