Effects of Cabbage-Apple Juice Fermented by Lactobacillus plantarum EM on Lipid Profile Improvement and Obesity Amelioration in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Fermented Cabbage-Apple Juice
2.2. Sample Analyses
2.3. Animals and Experimental Design
2.4. Blood and Tissue Sample Processing
2.5. Serum Biomarkers and Hepatic and Adipose Tissue Lipids
2.6. Serum Biochemical Parameters
2.7. Hepatic RNA Extraction and Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Analysis
2.8. Histopathological Analysis of Hepatic Tissue and Adipocytes in the Epididymal Adipose Tissue
2.9. Statistical Analysis
3. Results
3.1. pH, Acidity, Nutrient Components, Organic Acid and Free Sugar Compositions, Total Polyphenol, and Total Glucosinolates Contents of Juice Samples
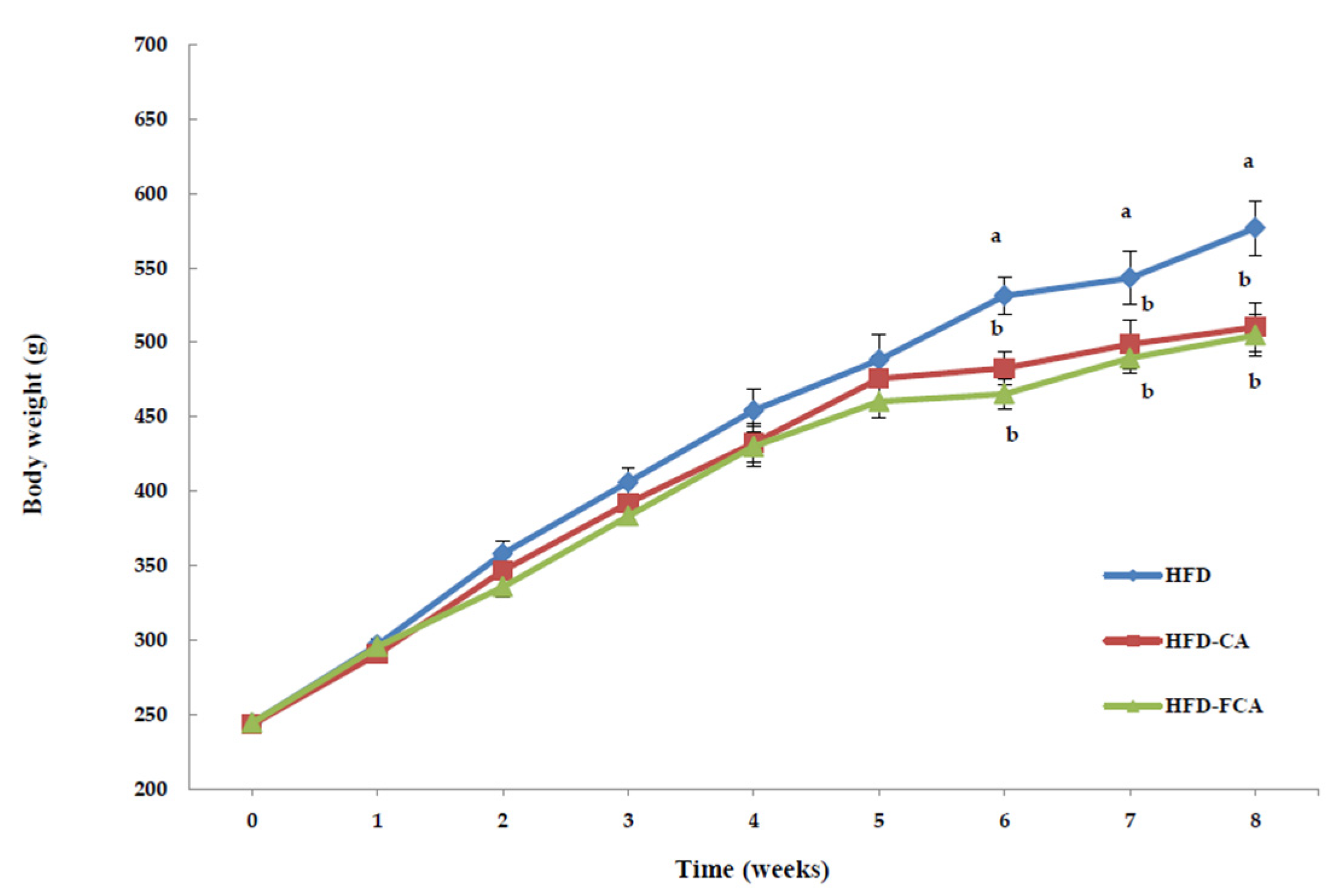
3.2. Body Weight Gain and Food Intake
3.3. Liver and White Fat Pad Weights
3.4. Biochemical Indicators of Hepatic Function
3.5. Serum Lipid Levels
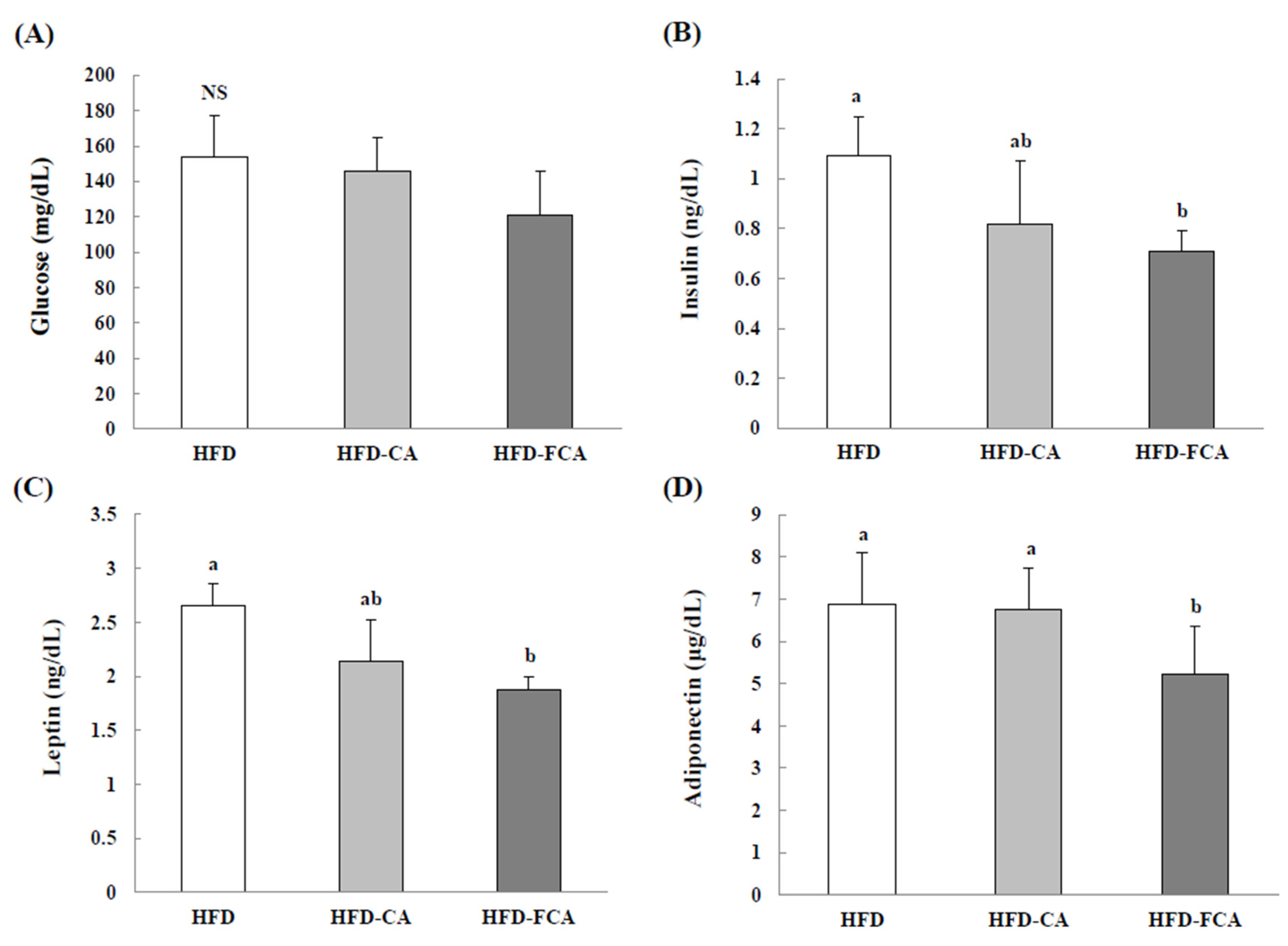
3.6. Serum Insulin, Glucose, Leptin, and Adiponectin Levels
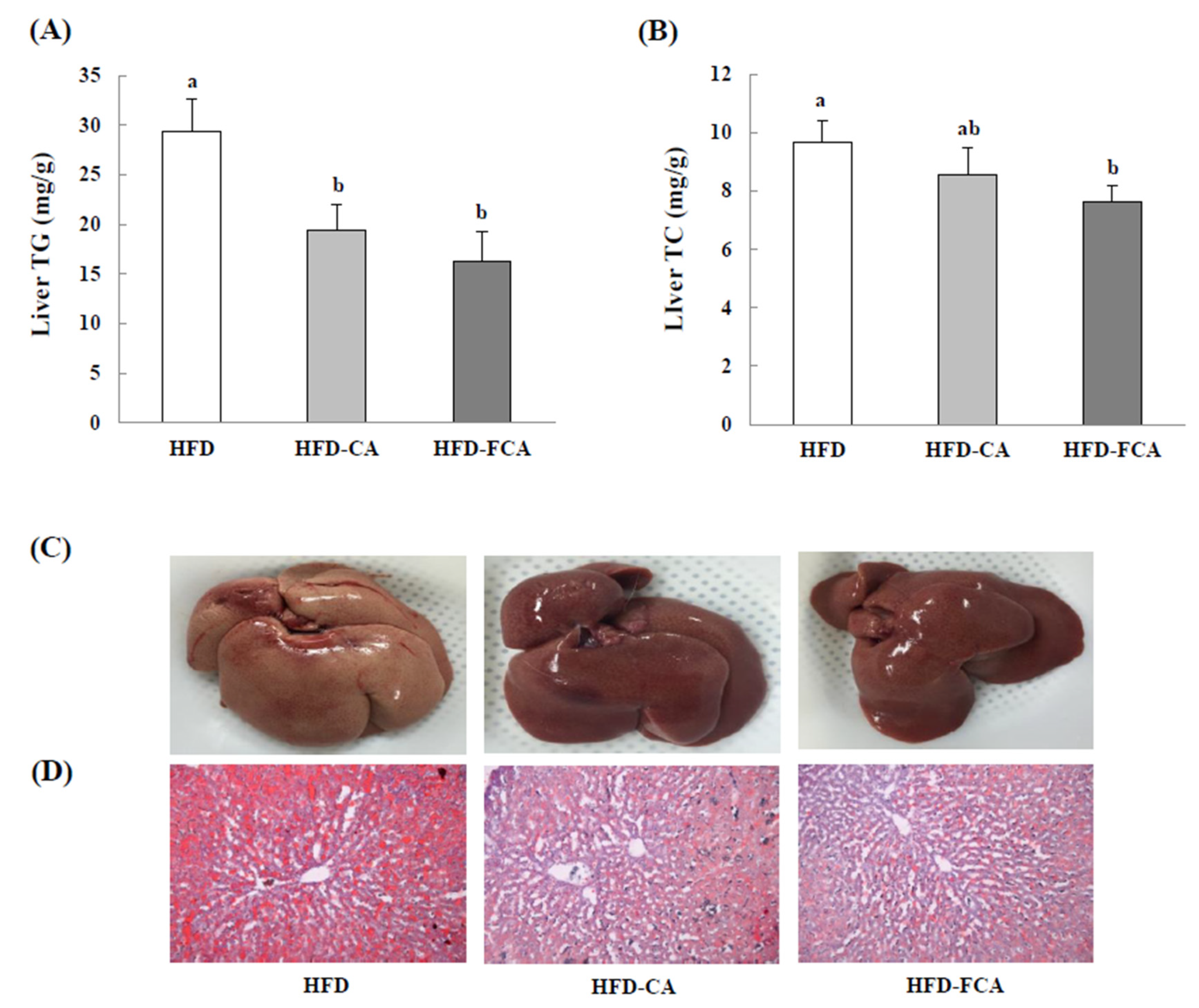
3.7. Hepatic Lipid Levels and Histopathological Changes
3.8. mRNA Expression of an Enzyme Related to Lipid Synthesis in the Liver
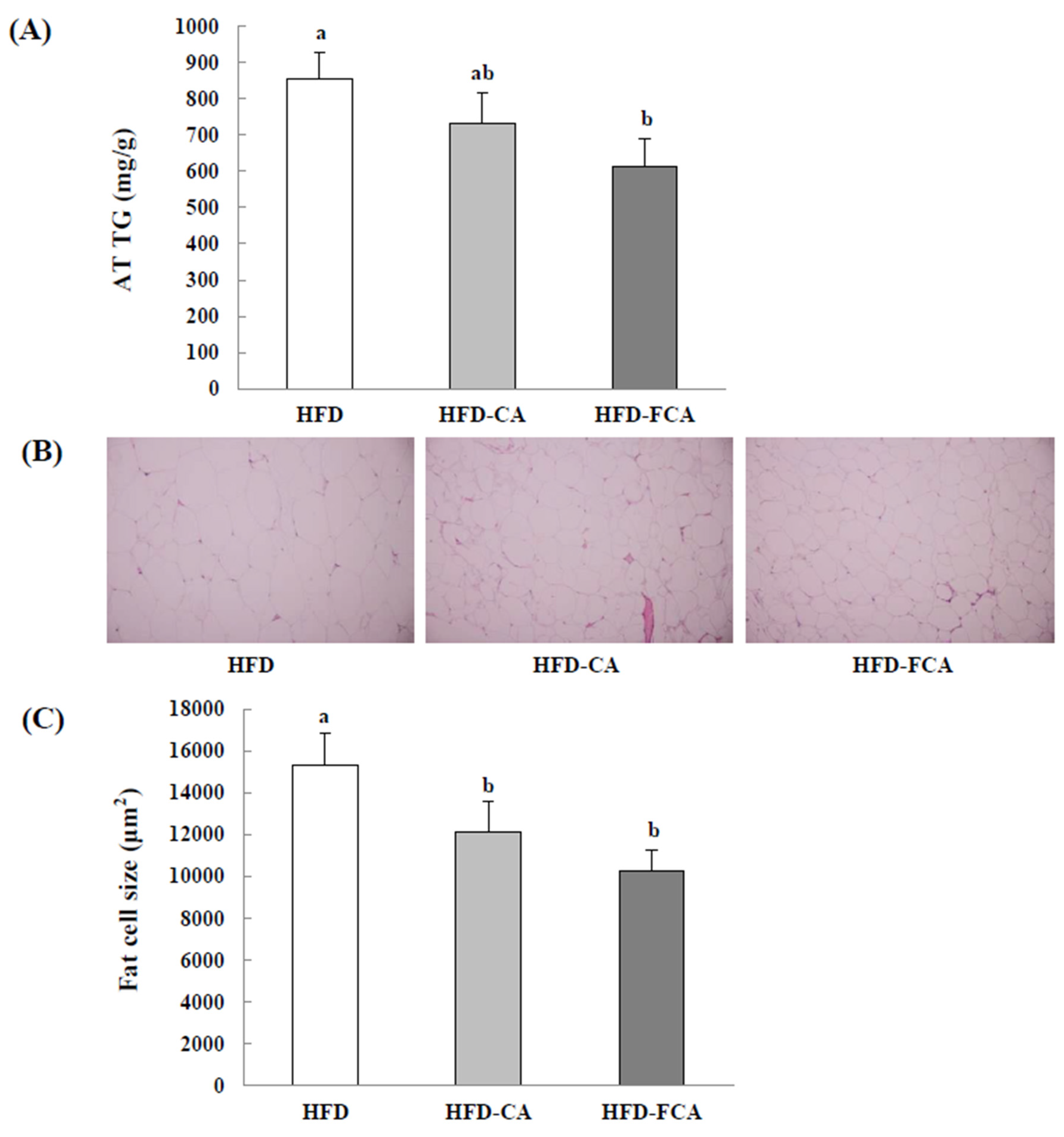
3.9. Epididymal Adipose Tissue TG Contents and Histopathological Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boheing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef]
- Rimm, E.B.; Ascherio, A.; Giovannucci, E.; Spiegelman, D.; Stampfer, M.J.; Willett, W.C. Vegetable, fruit, and cereal fiber intake and risk of coronary heart disease among men. JAMA 1996, 275, 447–451. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.; Gray, L.J.; Troughton, J.; Khunti, K.; Davies, M.J. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: Systematic review and meta-analysis. BMJ 2010, 341, c4229. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.C.; Joshipura, K.J.; Jiang, R.; Hu, F.B.; Hunter, D.; Smith-Warner, S.A.; Colditz, G.A.; Rosner, B.; Spiegelman, D.; Willett, W.C. Fruit and vegetable intake and risk of major chronic disease. J. Natl. Cancer Inst. 2004, 96, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Koryachkina, S.Y.; Ladnova, O.L.; Godunov, O.A.; Kholodova, E.N.; Lazareva, T.N. The study of physiological effect of fruit and vegetable powders in animal experiment. Vopr. Pitan. 2016, 85, 48–56. [Google Scholar] [PubMed]
- Peluso, I.; Raguzzini, A.; Catasta, G.; Cammisotto, V.; Perrone, A.; Tomino, C.; Toti, E.; Serafini, M. Effects of high consumption of vegetables on clinical, immunological, and antioxidant markers in subjects at risk of cardiovascular diseases. Oxid. Med. Cell Longev. 2018, 5417165. [Google Scholar] [CrossRef]
- Lichtenthäler, R.; Marx, F. Total oxidant scavenging capacities of common European fruit and vegetable juices. J. Agric. Food Chem. 2005, 53, 103–110. [Google Scholar] [CrossRef]
- Li, Z.; Teng, J.; Lyu, Y.; Hu, X.; Zhao, Y.; Wang, M. Enhanced antioxidant activity for apple juice fermented with Lactobacillus plantarum ATCC14917. Molecules Dec. 2019, 24, 51. [Google Scholar] [CrossRef]
- Trošt, K.; Ulaszewska, M.M.; Stanstrup, J.; Albanese, D.; De Filippo, C.; Tuohy, K.M.; Natella, F.; Scaccini, C.; Mattivi, F. Host: Microbiome co-metabolic processing of dietary polyphenols—An acute, single blinded, cross-over study with different doses of apple polyphenols in healthy subjects. Food Res. Int. 2018, 112, 108–128. [Google Scholar] [CrossRef]
- Zielinski, A.A.; Zardo, D.M.; Alberti, A.; Bortolini, D.G.; Benvenutti, L.; Demiate, I.M.; Nogueira, A. Effect of cryoconcentration process on phenolic compounds and antioxidant activity in apple juice. J. Sci. Food Agric. 2019, 99, 2786–2792. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Rastqar, A.; Keshvari, M. Weight loss associated with consumption of apples: A review. J. Am. Coll. Nutr. 2018, 37, 627–639. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, C.E.; Nicklas, T.A.; Fulgoni, V.L. Consumption of apples is associated with a better diet quality and reduced risk of obesity in children: National Health and Nutrition Examination Survey (NHANES) 2003–2010. Nutr. J. 2015, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, X.; Gong, G.; Li, G.; Li, C. Consumption of fruit, but not vegetable, may reduce risk of gastric cancer: Results from a meta-analysis of cohort studies. Eur. J. Cancer 2014, 50, 1498–1509. [Google Scholar] [CrossRef]
- Samout, N.; Bouzenna, H.; Dhibi, S.; Ncib, S.; ElFeki, A.; Hfaiedh, N. Therapeutic effect of apple pectin in obese rats. Biomed. Pharmacother. 2016, 83, 1233–1238. [Google Scholar] [CrossRef]
- Anubhuti, S.H.; Ashok, S.H.; Prashant, Y.; Dhiraj, S. Isothiocyanates in Brassica: Potential anticancer agents. Asian Pac. J. Cancer Prev. 2016, 17, 4507–4510. [Google Scholar]
- Shim, K.H.; Sung, N.K.; Kang, K.S.; Ahn, C.W.; Seo, K.I. Analysis of glucosinolates and the change of contents during processing and storage in Cruciferous vegetables. J. Korean Soc. Food Sci. Nutr. 1992, 21, 43–48. [Google Scholar]
- Rodríguez-Cantú, L.N.; Gutiérrez-Uribe, J.A.; Arriola-Vucovich, J.; Díaz-De La Garza, R.I.; Fahey, J.W.; Serna-Saldivar, S.O. Broccoli (Brassica oleracea var. italica) sprouts and extracts rich in glucosinolates and isothiocyanates affect cholesterol metabolism and genes involved in lipid homeostasis in hamsters. J. Agric. Food Chem. 2011, 59, 1095–1103. [Google Scholar]
- Jahangir, M.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Health-affecting compounds in Brassicaceae. Comp. Rev. Food Sci. Food Saf. 2009, 8, 31–43. [Google Scholar] [CrossRef]
- Gessler, N.N.; Bezzubov, A.A.; Podlepa, E.M.; Bykhovski, V.Y. S-methylmethionine (vitamin U) metabolism in plants. Appl. Biochem. Micro. 1991, 27, 275–280. [Google Scholar]
- Komatsu, W.; Miura, Y.; Yagasaki, K. Suppression of hypercholesterolemia in hepatoma-bearing rats by cabbage extract and its component, S-methyl-L-cysteine sulfoxide. Lipids 1998, 33, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Elvira-Torales, L.I.; García-Alonso, J.; Periago-Castón, M.J. Nutritional importance of carotenoids and their effect on liver health: A review. Antioxidants (Basel) 2019, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Sahin, K.; Bilen, H.; Bahcecioglu, I.B.; Bilir, B.; Ashraf, S.; Halazun, K.J.; Kucuk, O. Carotenoids and non-alcoholic fatty liver disease. Hepatobiliary Surg Nutr. 2015, 4, 161–171. [Google Scholar]
- Hubert, J.; Berger, M.; Mepveu, F.; Paul, F.; Dayd, J. Effects of fermentation on the phytochemical composition and antioxidant properties of soy germ. Food Chem. 2008, 109, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Foo, H.L.; Loh, T.C.; Lim, Y.S.; Shukriyah, M.H.; Kufli, C.N.; Law, F.L. Effects of fermented fruits on growth performance, shedding of Enterobacteriaceae and lactic acid bacteria and plasma cholesterol in rats. Pakistan J. Nutr. 2003, 2, 228–233. [Google Scholar]
- Li, C.; Ding, Q.; Nie, S.P.; Zhang, Y.S.; Xiong, T.; Xie, M.Y. Carrot juice fermented with Lactobacillus plantarum NCU116 ameliorates type 2 diabetes in rats. J. Agri. Food Chem. Dec. 2014, 62, 11884–11891. [Google Scholar] [CrossRef] [PubMed]
- Mauro, C.S.I.; Guergoletto, K.B.; Garcia, S. Development of blueberry and carrot juice blend fermented by Lactobacillus reuteri LR92. Beverages 2016, 2, 37. [Google Scholar] [CrossRef]
- Chu, H.; Kim, J. Anti-obesity effect of Fructus pyri Pyrifoliae extract fermented by lactic-acid bacteria on rats. Appl. Microsc. 2018, 48, 62–72. [Google Scholar] [CrossRef]
- Takemura, N.; Okubo, T.; Sonoyama, K. Lactobacillus plantarum strain No. 14 reduces adipocyte size in mice fed high-fat diet. Exp. Biol. Med (Maywood) 2010, 235, 849–856. [Google Scholar] [CrossRef]
- Park, D.Y.; Ahn, Y.T.; Huh, C.S.; Jeon, S.M.; Choi, M.S. The inhibitory effect of Lactobacillus plantarum KY1032 cell extract on the adipogenesis of 3T3-L1 cells. J. Med. Food 2011, 14, 670–675. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Szaefer, H.; Bartoszek, A.; Baer-Dubowska, W. Modulation of rat hepatic and kidney phase II enzymes by cabbage juices: Comparison with the effects of indole-3-carbinol and phenethyl isothiocyanate. Br. J. Nutr. 2011, 105, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.A.; Chang, H.C. Cholesterol-lowering effects of a putative probiotic strain Lactobacillus plantarum EM isolated from kimchi. LWT Food Sci. Technol. 2015, 62, 210–217. [Google Scholar] [CrossRef]
- Heydemann, A. An overview of murine high fat diet as a model for type 2 diabetes mellitus. J. Diabetes Res. 2016, 2902351. [Google Scholar] [CrossRef] [PubMed]
- A.O.A.C. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemist: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Sturm, K.; Koron, D.; Stampar, F. The composition of fruit of different strawberry varieties depending on maturity stage. Food Chem. 2003, 83, 417–422. [Google Scholar] [CrossRef]
- Richmond, M.L.; Brandao, S.C.; Gray, J.I.; Markakis, P.; Stine, C.M. Analysis of simple sugars and sorbitol in fruit by high-performance liquid chromatography. J. Agric. Food Chem. 1981, 29, 4–7. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Ravebtos, R.M. Analysis of total phenols and others oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- International Standard Organization. Rapeseed: Determination of Glucosinolates Content-Part Ⅰ: Method Using High Performance Liquid Chromatography; ISO 9167-1. 1992; International Standard Organization: Geneva, Switzerland, 1992; pp. 1–9. [Google Scholar]
- Folch, J.; Lees, M.; Sloane-Stanley, G. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Biggs, H.G.; Erikson, T.M.; Moorehead, W.R. A mannual colorimetric assay of triglyceride in serum. Clin. Chem. 1975, 21, 437. [Google Scholar] [CrossRef]
- Zlatkis, A.; Zak, B. Study of a new cholesterol reagent. Anal. Biochem. 1969, 29, 143–148. [Google Scholar] [CrossRef]
- Jiang, T.; Gao, X.; Wu, C.; Tian, F.; Lei, Q.; Bi, J.; Xie, B.; Wang, H.Y.; Chen, S.; Wang, X. Apple-derived pectin modulates gut microbiota, improves gut barrier function, and attenuates metabolic endotoxemia in rats with diet-induced obesity. Nutrients 2016, 8, 126. [Google Scholar] [CrossRef]
- Mounien, L.; Tourniaire, F.; Landrier, J.F. Anti-obesity effect of carotenoids: Direct impact on adipose tissue and adipose-tissue-driven indirect effects. Nutrients 2019, 11, 1562. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.H.; Kim, B.; Mun, E.G.; Cha, Y.S.; Yu, O.K. Effects of fermented blueberry liquid in high-fat diet-induced obese C57BL/6J mice. J. Nutr. Health 2017, 50, 543–551. [Google Scholar] [CrossRef]
- Parvez, S.; Malik, K.A.; Ah Kang, S.; Kim, H.Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.J.; Soh, J.R.; Yu, J.J.; Shon, H.S.; Cha, Y.S.; Oh, S.H. Intracellular lipid accumulation inhibitory effect of Weissella koreensis OK1-6 isolated from Kimchi on differentiating adipocyte. J. Appl. Microbiol. 2012, 113, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Borges, G.; Lean, M.E.; Roberts, S.A.; Crozier, A. 2013, Bioavailability of dietary (poly)phenols: A study with ileostomists to discriminate between absorption in small and large intestine. Food Funct. 2013, 4, 754–762. [Google Scholar] [CrossRef]
- Trinh, H.T.; Han, S.J.; Kim, S.W.; Lee, Y.C.; Kim, D.H. Bifidus fermentation increases hypolipidemic and hypoglyccemic effects of red ginseng. J. Miocrobiol. Biotechnol. 2007, 17, 1127–1133. [Google Scholar]
- Valero-Cases, E.; Nuncio-Jáuregui, N.; Frutos, M.J. Influence of fermentation with different lactic acid bacteria and in vitro digestion on the biotransformation of phenolic compounds in fermented pomegranate juices. J. Agric. Food Chem. 2017, 65, 6488–6496. [Google Scholar] [CrossRef]
- Kim, J.Y.; Nolte, L.A.; Hansen, P.A.; Han, D.H.; Ferguson, K.; Thompson, P.A.; Holloszy, J.O. High-fat diet-induced muscle insulin resistance: Relationship to visceral fat mass. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R2057–R2065. [Google Scholar] [CrossRef]
- Ha, S.K.; Chae, C. Inducible nitric oxide distribution in the fatty liver of a mouse with high fat diet-induced obesity. Exp, Anim. 2010, 59, 595–604. [Google Scholar] [CrossRef][Green Version]
- Wu, Y.G.; Xia, L.L.; Lin, H.; Zhou, D.; Qian, H.; Lin, S.T. Prevention of early liver injury by breviscapine in streptozotocin-induced diabetic rats. Planta Med. 2007, 73, 433–438. [Google Scholar] [CrossRef]
- Despres, J.P. Abdominal obesity as important component of insulin-resistant syndrome. Nutrition 1993, 19, 452–459. [Google Scholar]
- Kunitomi, M.; Wada, J.; Takahashi, K.; Tsuchiyama, Y.; Mimura, Y.; Hida, K.; Miyatake, N.; Fujii, M.; Kira, S.; Shikata, K.; et al. Relationship between reduced serum IGF-I levels and accumulation of visceral fat in Japanese men. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.J. Pharmacological treatment of obesity. J. Korean Endocrine. Soc. 2008, 23, 223–233. [Google Scholar] [CrossRef][Green Version]
- Masumoto, S.; Terao, A.; Yamamoto, Y.; Mukai, T.; Miura, T.; Shoji, T. Non-absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes. Sci. Rep. 2016, 6, 31208. [Google Scholar] [CrossRef]
- Sugiyama, H.; Akazome, Y.; Shoji, T.; Yamaguchi, A.; Yasue, M.; Kanda, T.; Ohtake, Y. Oligomeric procyanidins in apple polyphenol are main active components for inhibition of pancreatic lipase and triglyceride absorption. J. Agric. Food Chem. 2007, 55, 4604–4609. [Google Scholar] [CrossRef]
- Plaa, G.L.; Charbonneau, M. Detection and evaluation of chemically induced liver injury. In Principles and Methods of Toxicology; Hayes, A.W., Ed.; Raven Press: New York, NY, USA, 1994; pp. 839–870. [Google Scholar]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminase. Am. J. Clin. Pathol. 1957, 2891, 56–63. [Google Scholar] [CrossRef]
- Corinne, H.R.; Emma, S.W. Basic Nutrition and Diettherapy, 5th ed.; Macmillan Pub. Co.: New York, NY, USA, 1984; pp. 37–44. [Google Scholar]
- Lim, S.S.; Kim, M.H.; Lee, J.H. Effect of Artemisia princeps var orientalis and Circium japonicum var ussuriense on liver function body lipid and bile acid of hyperlipidemic rat. Korean J. Nutr. 1997, 30, 797–802. [Google Scholar]
- Kim, H.K. The effects of protecting liver and improving liver function on cabbage extract. JCCT 2019, 5, 389–395. [Google Scholar]
- Jurisic, V.; Radenkovic, S.; Konjevic, G. The actual role of LDH as tumor marker, biochemical and clinical aspects. Adv. Exp. Med. Biol. 2015, 867, 115–124. [Google Scholar]
- Ratter, J.M.; Rooijackers, H.M.M.; Hooiveld, G.J.; Hijmans, A.G.M.; De Galan, B.E.; Tack, C.J.; Stienstra, R. In vitro and in vivo effects of lactate on metabolism and cytokine production of human primary PBMCs and monocytes. Front Immunol. 2018, 12, 2564. [Google Scholar] [CrossRef]
- Davignon, J.; Cohn, J.S. Triglyceride: A risk factor for coronary heart disease. Atherosclerosis 1996, 124, S57–S64. [Google Scholar] [CrossRef]
- Yugarani, T.; Tan, B.K.; Teh, M.; Das, N.P. Effects of polyphenolic natural products on the lipid profiles of rats fed high fat diets. Lipids 1992, 27, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen elderly study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef]
- Wang, L.; Fumoto, T.; Masumoto, S.; Shoji, T.; Miura, T.; Naraoka, M.; Matsuda, N.; Imaizumi, T.; Ohkuma, H. Regression of atherosclerosis with apple procyanidins by activating the ATP-binding cassette subfamily A member 1 in a rabbit model. Atherosclerosis 2017, 258, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Satoh, A.; Numazawa, S.; Takahashi, E. Effects of cabbage leaf protein concentrate on the serum and liver lipid concentrations in rats. J. Nutr. Sci. Vitaminol. (Tokyo) 1997, 43, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Korenblat, K.M.; Fabbrini, E.; Mohammed, B.S.; Klein, S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology 2008, 134, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Fain, J.N. Release of inflammatory mediators by human adipose tissue is enhanced in obesity and primarily by the nonfats cells: A review. Mediators Inflamm. 2010, 513948. [Google Scholar] [CrossRef]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L.; et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Berneis, K.K.; Krauss, R.M. Metabolic origins and clinical significance of LDL heterogeneity. J. Lipid Res. 2002, 43, 1363–1379. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T. Adiponectin and adiponectin receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef] [PubMed]


| CA Juice | FCA Juice | |
|---|---|---|
| pH | 4.08 ± 0.02 *** | 3.63 ± 0.03 |
| Acidity (%) | 1.26 ± 0.04 ** | 1.58 ± 0.04 |
| Proximate composition (g/100 mL) | ||
| Carbohydrate | 10.10 ± 0.09 | 9.21 ± 0.07 |
| Crude fat | 3.72 ± 0.03 *** | 1.52 ± 0.01 |
| Crude protein | 0.70 ± 0.02 ** | 0.61 ± 0.01 |
| Moisture | 89.45 ± 0.23 * | 90.31 ± 0.45 |
| Ash | 0.62 ± 0.02 | 0.61 ± 0.01 |
| Total dietary fiber | 0.91 ± 0.01 *** | 1.12 ± 0.03 |
| Organic acid (g/100 mL) | ||
| Citric acid | 0.04 ± 0.00 | ND |
| Malic acid | 0.56 ± 0.01 *** | 0.26 ± 0.00 |
| Fumaric acid | ND | ND |
| Acetic acid | 0.32 ± 0.00 *** | 0.42 ± 0.00 |
| Lactic acid | ND | 1.45 ± 0.01 |
| Total organic acid | 1.03 ± 0.01 *** | 2.13 ± 0.01 |
| Free sugars (g/100 mL) | ||
| Sucrose | 1.44 ± 0.00 *** | 1.30 ± 0.30 |
| Glucose | 3.22 ± 0.01 *** | 2.17 ± 0.00 |
| Xylose | 0.05 ± 0.00 | ND |
| Galactose | ND | 0.07 ± 0.00 |
| Fructose | 5.07 ± 0.01 *** | 4.86 ± 0.01 |
| Sorbitol | 0.20 ± 0.00 *** | 0.20 ± 0.00 |
| Total free sugar | 9.98 ± 0.01 *** | 8.59 ± 0.01 |
| Total polyphenol (mg TAE/100 mL) | 39.88 ± 2.68 | 42.36 ± 3.21 |
| Total glucosinolates (mg/100 mL) | 402.01 ± 20.32 | 365.55 ± 22.46 |
| Group | Body Weight (g) | Food Intake (g/day) | |||
|---|---|---|---|---|---|
| Initial Weight (g) | Final Weight (g) | Total Weight Gain (g) | Body Weight Gain (g/day) | ||
| HFD | 244.19 ± 2.29 | 576.90 ± 18.29 a | 332.64 ± 12.36 a | 5.94 ± 0.79 a | 22.72 ± 2.63 NS |
| HFD-CA | 243.19 ± 2.01 | 510.14 ± 15.89 b | 265.49 ± 11.13 b | 4.74 ± 0.47 b | 22.02 ± 1.87 |
| HFD-FCA | 244.25 ± 2.38 | 504.67 ± 14.33 b | 260.45 ± 9.88 b | 4.65 ± 0.38 b | 22.86 ± 2.25 |
| Group | Liver | White Fat Pads | ||||
|---|---|---|---|---|---|---|
| Epididymal Fat Pads | Mesenteric Fat Pads | Retroperitoneal Fat Pads | Perinenal Fat Pads | Total White Fat Pads | ||
| (g/100 g Body Weight) | ||||||
| HFD | 5.33 ± 0.58 a | 1.99 ± 0.33 a | 1.14 ± 0.57 a | 2.43 ± 0.62 a | 0.75 ± 0.16 NS | 6.31 ± 0.47 a |
| HFD-CA | 4.60 ± 0.30 b | 1.79 ± 0.42 b | 1.03 ± 0.18 ab | 2.36 ± 0.59 a | 0.71 ± 0.14 | 5.87 ± 0.65 b |
| HFD-FCA | 4.46 ± 0.28 b | 1.69 ± 0.62 b | 0.94 ± 0.20 b | 2.08 ± 0.40 b | 0.70 ± 0.07 | 5.32 ± 0.51 b |
| HFD | HFD-CA | HFD-FCA | |
|---|---|---|---|
| Triglyceride (mg/dL) | 101.63 ± 11.97 a | 81.95 ± 8.16 b | 73.79 ± 8.22 b |
| Total cholesterol (mg/dL) | 123.51 ± 10.33 a | 99.88 ± 11.92 b | 90.60 ± 10.58 b |
| LDL/VLDL cholesterol (mg/dL) | 92.68 ± 9.94 a | 80.05 ± 7.02 ab | 71.19 ± 8.25 b |
| HDL-cholesterol (mg/dL) | 30.75 ± 5.97 b | 34.13 ± 4.12 b | 44.75 ± 4.53 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Son, H.-K.; Chang, H.-C.; Lee, J.-J. Effects of Cabbage-Apple Juice Fermented by Lactobacillus plantarum EM on Lipid Profile Improvement and Obesity Amelioration in Rats. Nutrients 2020, 12, 1135. https://doi.org/10.3390/nu12041135
Park S, Son H-K, Chang H-C, Lee J-J. Effects of Cabbage-Apple Juice Fermented by Lactobacillus plantarum EM on Lipid Profile Improvement and Obesity Amelioration in Rats. Nutrients. 2020; 12(4):1135. https://doi.org/10.3390/nu12041135
Chicago/Turabian StylePark, Sihoon, Hee-Kyoung Son, Hae-Choon Chang, and Jae-Joon Lee. 2020. "Effects of Cabbage-Apple Juice Fermented by Lactobacillus plantarum EM on Lipid Profile Improvement and Obesity Amelioration in Rats" Nutrients 12, no. 4: 1135. https://doi.org/10.3390/nu12041135
APA StylePark, S., Son, H.-K., Chang, H.-C., & Lee, J.-J. (2020). Effects of Cabbage-Apple Juice Fermented by Lactobacillus plantarum EM on Lipid Profile Improvement and Obesity Amelioration in Rats. Nutrients, 12(4), 1135. https://doi.org/10.3390/nu12041135






