Short-Communication: Ingestion of a Nucleotide-Rich Mixed Meal Increases Serum Uric Acid Concentrations but Does Not Affect Postprandial Blood Glucose or Serum Insulin Responses in Young Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Medical Screening
2.2. Experimental Protocol
2.3. Plasma and Serum Collection and Analyses
2.4. Statistical Analyses
3. Results
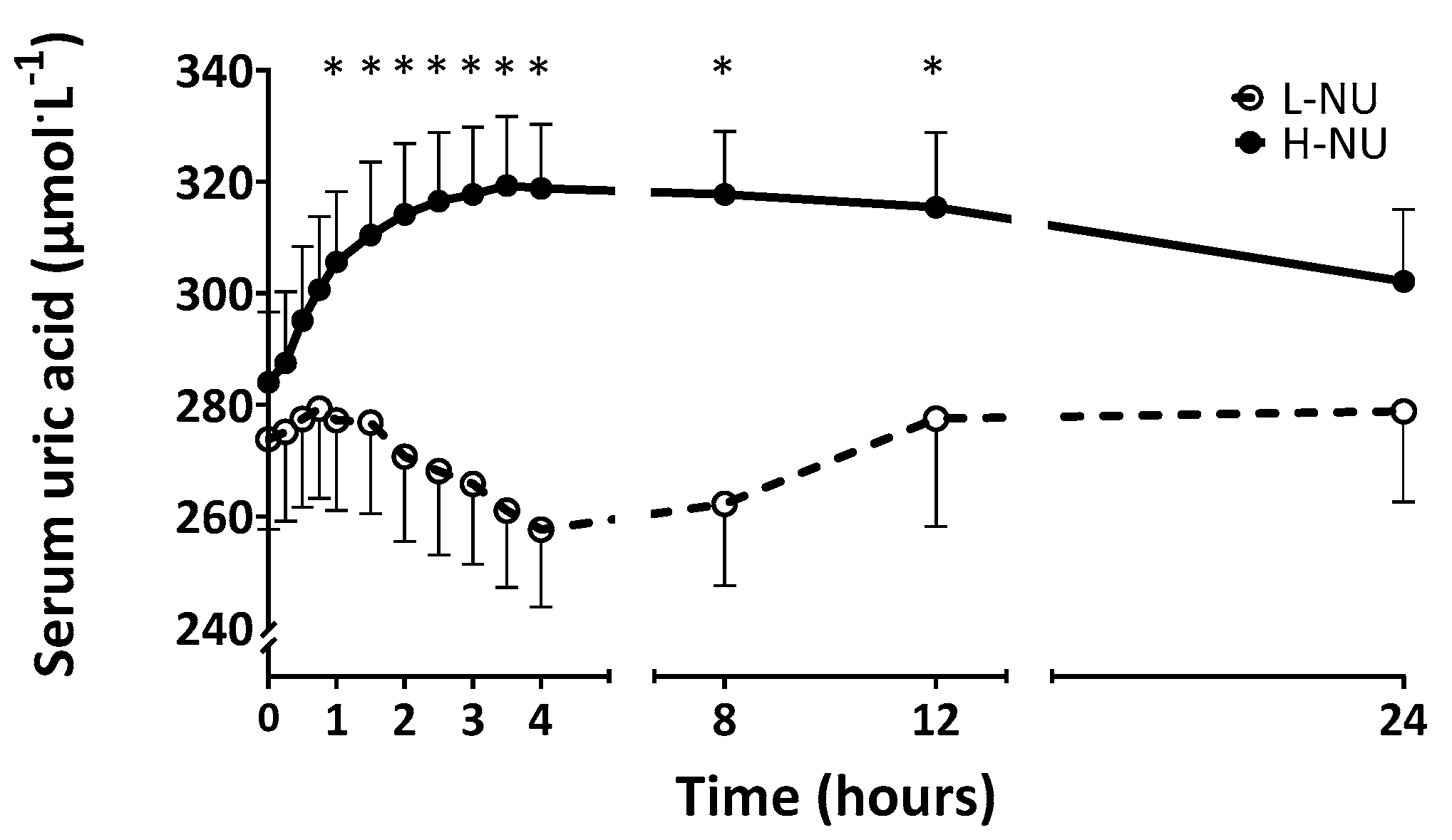
3.1. Serum Uric Acid
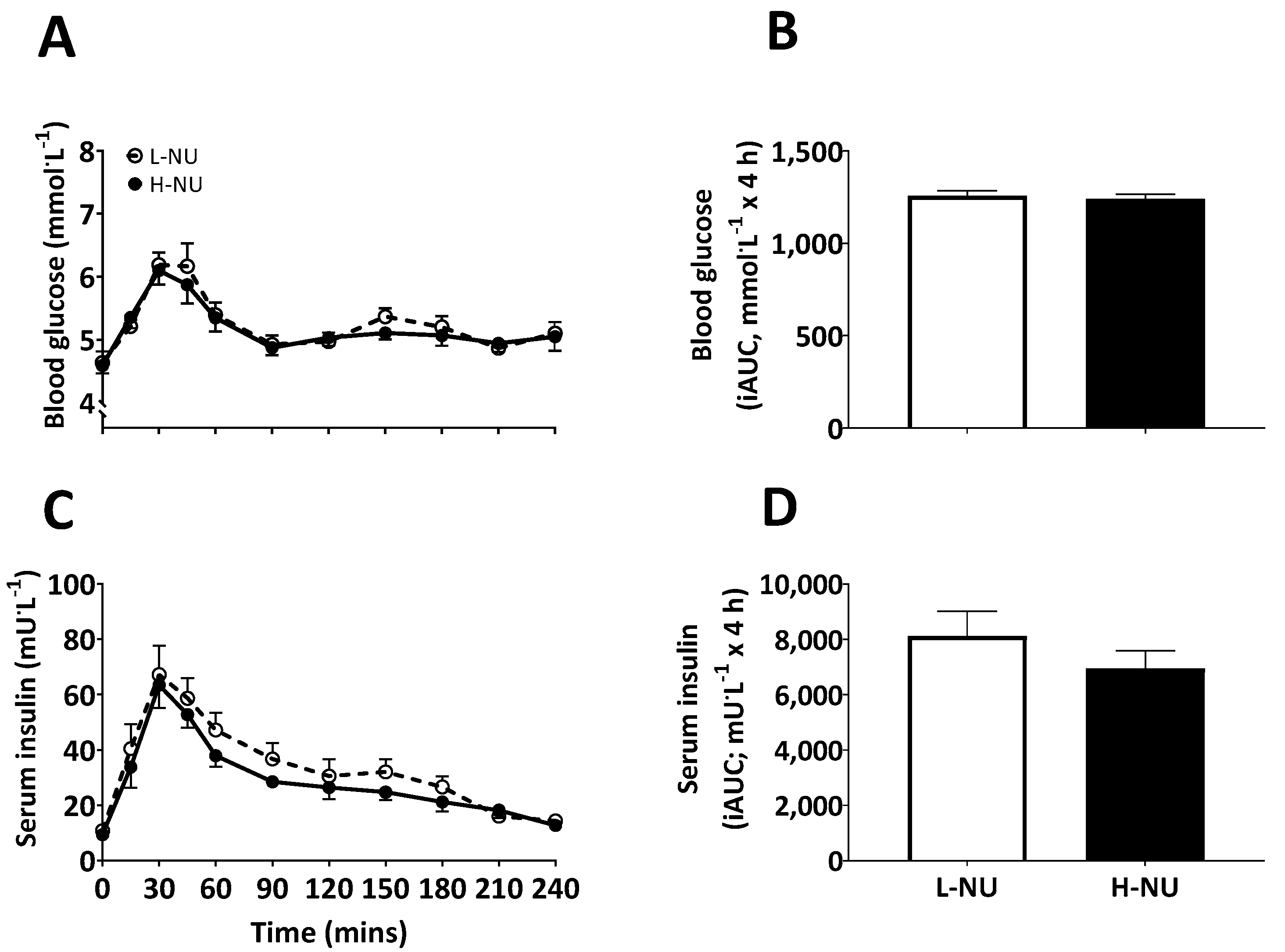
3.2. Blood Glucose and Serum Insulin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Waslien, C.I.; Calloway, D.H.; Margen, S. Uric acid production of men fed graded amounts of egg protein and yeast nucleic acid. Am. J. Clin. Nutr. 1968, 21, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Edozien, J.; Udo, U.; Young, V.; Scrimshaw, N. Effects of high levels of yeast feeding on uric acid metabolism of young men. Nature 1970, 228, 180. [Google Scholar] [CrossRef] [PubMed]
- Waslien, C.I.; Calloway, D.H.; Margen, S.; Costa, F. Uric acid levels in men fed algae and yeast as protein sources. J. Food Sci. 1970, 35, 294–298. [Google Scholar] [CrossRef]
- Seegmiller, J.; Grayzel, A.I.; Laster, L.; Liddle, L. Uric acid production in gout. J. Clin. Investig. 1961, 40, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Kang, D.-H.; Feig, D.; Kivlighn, S.; Kanellis, J.; Watanabe, S.; Tuttle, K.R.; Rodriguez-Iturbe, B.; Herrera-Acosta, J.; Mazzali, M. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension 2003, 41, 1183–1190. [Google Scholar] [CrossRef]
- Cibičková, L.; Langová, K.; Vaverková, H.; Kubíčková, V.; Karásek, D. Correlation of Uric Acid Levels and Parameters of Metabolic Syndrome. Physiol. Res. 2017, 66, 481. [Google Scholar] [CrossRef]
- Dehghan, A.; Van Hoek, M.; Sijbrands, E.J.; Hofman, A.; Witteman, J.C. High serum uric acid as a novel risk factor for type 2 diabetes. Diabetes Care 2008, 31, 361–362. [Google Scholar] [CrossRef]
- British Nutrition Foundation. Exploring whether the 2 g/day RNA limit from single cell protein novel foods set by the FAO/WHO Protein Advisory Group in the 1970s still has relevance today. 2016. [Google Scholar]
- Calloway, D. Safety of single-cell proteins-as evaluated by human feeding trials at the University of California, Berkeley. In Proceedings of the 16th Meeting of the FAO/WHO/UNICEF Protein Advisory Group, Geneva, Switzerland, 8–11 September 1969; pp. 8–11. [Google Scholar]
- Finnigan, T. Mycoprotein: Origins, production and properties. In Handbook of Food Proteins; Elsevier: Cambridge, UK, 2011; pp. 335–352. [Google Scholar]
- Dunlop, M.V.; Kilroe, S.P.; Bowtell, J.L.; Finnigan, T.J.; Salmon, D.L.; Wall, B.T. Mycoprotein represents a bioavailable and insulinotropic non-animal-derived dietary protein source: A dose–response study. Br. J. Nutr. 2017, 118, 673–685. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Clinical Knowledge Summary—Gout. Available online: https://cks.nice.org.uk/gout (accessed on 26 February 2020).
- Choi, H.K.; Atkinson, K.; Karlson, E.W.; Willett, W.; Curhan, G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N. Engl. J. Med. 2004, 350, 1093–1103. [Google Scholar] [CrossRef]
- McGuire, E.; Helderman, J.; Tobin, J.; Andres, R.; Berman, M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J. Appl. Physiol. 1976, 41, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Jensen, T.; Solak, Y.; Le, M.; Roncal-Jimenez, C.; Rivard, C.; Lanaspa, M.A.; Nakagawa, T.; Johnson, R.J. Uric acid in metabolic syndrome: From an innocent bystander to a central player. Eur. J. Intern. Med. 2016, 29, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Desideri, G.; Castaldo, G.; Lombardi, A.; Mussap, M.; Testa, A.; Pontremoli, R.; Punzi, L.; Borghi, C. Is it time to revise the normal range of serum uric acid levels. Eur. Rev. Med. Pharm. Sci. 2014, 18, 1295–1306. [Google Scholar]
- Toyoki, D.; Shibata, S.; Kuribayashi-Okuma, E.; Xu, N.; Ishizawa, K.; Hosoyamada, M.; Uchida, S. Insulin stimulates uric acid reabsorption via regulating urate transporter 1 and ATP-binding cassette subfamily G member 2. Am. J. Physiol. Ren. Physiol. 2017, 313, F826–F834. [Google Scholar] [CrossRef] [PubMed]
- Garrel, D.; Verdy, M.; PetitClerc, C.; Martin, C.; Brulé, D.; Hamet, P. Milk- and soy-protein ingestion: Acute effect on serum uric acid concentration. Am. J. Clin. Nutr. 1991, 53, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Dalbeth, N.; Wong, S.; Gamble, G.; Horne, A.; Mason, B.; Pool, B.; Fairbanks, L.; McQueen, F.; Cornish, J.; Reid, I.; et al. Acute effect of milk on serum urate concentrations: A randomised controlled crossover trial. Ann. Rheum. Dis. 2010, 69, 1677–1682. [Google Scholar] [CrossRef]
- Villegas, R.; Xiang, Y.-B.; Elasy, T.; Xu, W.-H.; Cai, H.; Cai, Q.; Linton, M.; Fazio, S.; Zheng, W.; Shu, X.-O. Purine-rich foods, protein intake, and the prevalence of hyperuricemia: The Shanghai Men’s Health Study. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 409–416. [Google Scholar] [CrossRef]
- Zykova, S.; Storhaug, H.; Toft, I.; Chadban, S.; Jenssen, T.; White, S. Cross-sectional analysis of nutrition and serum uric acid in two Caucasian cohorts: The AusDiab Study and the Tromsø study. Nutr. J. 2015, 14, 14–49. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.-J.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.; Marek, G.; Duranay, M. Uric Acid induces hepatic steatosis by generation of mitochondrial oxidative stress potential role in fructose-dependent and-independent fatty liver. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar] [CrossRef]
- Yu, M.-A.; Sánchez-Lozada, L.G.; Johnson, R.J.; Kang, D.-H. Oxidative stress with an activation of the renin–angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J. Hypertens. 2010, 28, 1234–1242. [Google Scholar] [CrossRef]
- Sautin, Y.Y.; Nakagawa, T.; Zharikov, S.; Johnson, R.J. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am. J. Physiol. Cell Physiol. 2007, 293, C584–C596. [Google Scholar] [CrossRef] [PubMed]
- Corry, D.B.; Eslami, P.; Yamamoto, K.; Nyby, M.D.; Makino, H.; Tuck, M.L. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin–angiotensin system. J. Hypertens. 2008, 26, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Roncal-Jimenez, C.A.; Lanaspa, M.A.; Rivard, C.J.; Nakagawa, T.; Sanchez-Lozada, L.G.; Jalal, D.; Andres-Hernando, A.; Tanabe, K.; Madero, M.; Li, N. Sucrose induces fatty liver and pancreatic inflammation in male breeder rats independent of excess energy intake. Metabolism 2011, 60, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-J.; Shin, H.-S.; Choi, H.S.; Park, J.-W.; Jo, I.; Oh, E.-S.; Lee, K.-Y.; Lee, B.-H.; Johnson, R.J.; Kang, D.-H. Uric acid induces fat accumulation via generation of endoplasmic reticulum stress and SREBP-1c activation in hepatocytes. Lab. Investig. 2014, 94, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
| Sex | 6 female/4 male |
| Age (years) | 25 ± 1 |
| Height (cm) | 171 ± 3 |
| Body mass (kg) | 72 ± 4 |
| Body mass index (kg/m2) | 24.4 ± 1.0 |
| Body fat (% of body mass) | 22 ± 4 |
| Lean mass (kg) | 57 ± 5 |
| Systolic blood pressure (mmHg) | 117 ± 5 |
| Diastolic blood pressure (mmHg) | 68 ± 2 |
| L-NU | H-NU | |
| Energy and macronutrients | ||
| Energy (kJ) | 2519 | 2519 |
| Energy (kcal) | 602 | 602 |
| Protein (g) | 28 | 28 |
| Carbohydrate (g) | 58 | 58 |
| Fat (g) | 26 | 26 |
| Nucleotides (in dry mycoprotein) | ||
| CMP (g/%) | - | 0.12/0.62 |
| UMP (g/%) | 0.05/0.26 | 0.08/0.44 |
| GMP (g/%) | 0.04/0.21 | 0.05/0.28 |
| TMP (g/%) | 0.09/0.46 | 0.57/3.00 |
| CDP (g/%) | 0.04/0.19 | 0.16/0.84 |
| UDP (g/%) | 0.01/0.07 | 0.06/0.34 |
| CTP (g/%) | 0.09/0.47 | 0.60/3.18 |
| ADP (g/%) | 0.00/0.02 | 0.02/0.08 |
| TTP (g/%) | - | 0.01/0.04 |
| ITP (g/%) | 0.06/0.29 | - |
| ATP (g/%) | - | 0.00/0.01 |
| Total nucleotides (g/%) | 0.38/1.96 | 1.70/8.83 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, M.O.C.; Monteyne, A.J.; Kamalanathan, I.D.; Najdanovic-Visak, V.; Finnigan, T.J.A.; Stephens, F.B.; Wall, B.T. Short-Communication: Ingestion of a Nucleotide-Rich Mixed Meal Increases Serum Uric Acid Concentrations but Does Not Affect Postprandial Blood Glucose or Serum Insulin Responses in Young Adults. Nutrients 2020, 12, 1115. https://doi.org/10.3390/nu12041115
Coelho MOC, Monteyne AJ, Kamalanathan ID, Najdanovic-Visak V, Finnigan TJA, Stephens FB, Wall BT. Short-Communication: Ingestion of a Nucleotide-Rich Mixed Meal Increases Serum Uric Acid Concentrations but Does Not Affect Postprandial Blood Glucose or Serum Insulin Responses in Young Adults. Nutrients. 2020; 12(4):1115. https://doi.org/10.3390/nu12041115
Chicago/Turabian StyleCoelho, Mariana O. C., Alistair J. Monteyne, Ishara D. Kamalanathan, Vesna Najdanovic-Visak, Tim J. A. Finnigan, Francis B. Stephens, and Benjamin T. Wall. 2020. "Short-Communication: Ingestion of a Nucleotide-Rich Mixed Meal Increases Serum Uric Acid Concentrations but Does Not Affect Postprandial Blood Glucose or Serum Insulin Responses in Young Adults" Nutrients 12, no. 4: 1115. https://doi.org/10.3390/nu12041115
APA StyleCoelho, M. O. C., Monteyne, A. J., Kamalanathan, I. D., Najdanovic-Visak, V., Finnigan, T. J. A., Stephens, F. B., & Wall, B. T. (2020). Short-Communication: Ingestion of a Nucleotide-Rich Mixed Meal Increases Serum Uric Acid Concentrations but Does Not Affect Postprandial Blood Glucose or Serum Insulin Responses in Young Adults. Nutrients, 12(4), 1115. https://doi.org/10.3390/nu12041115






