Treatment with Modified Extracts of the Microalga Planktochlorella nurekis Attenuates the Development of Stress-Induced Senescence in Human Skin Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Extract Preparation and Treatment
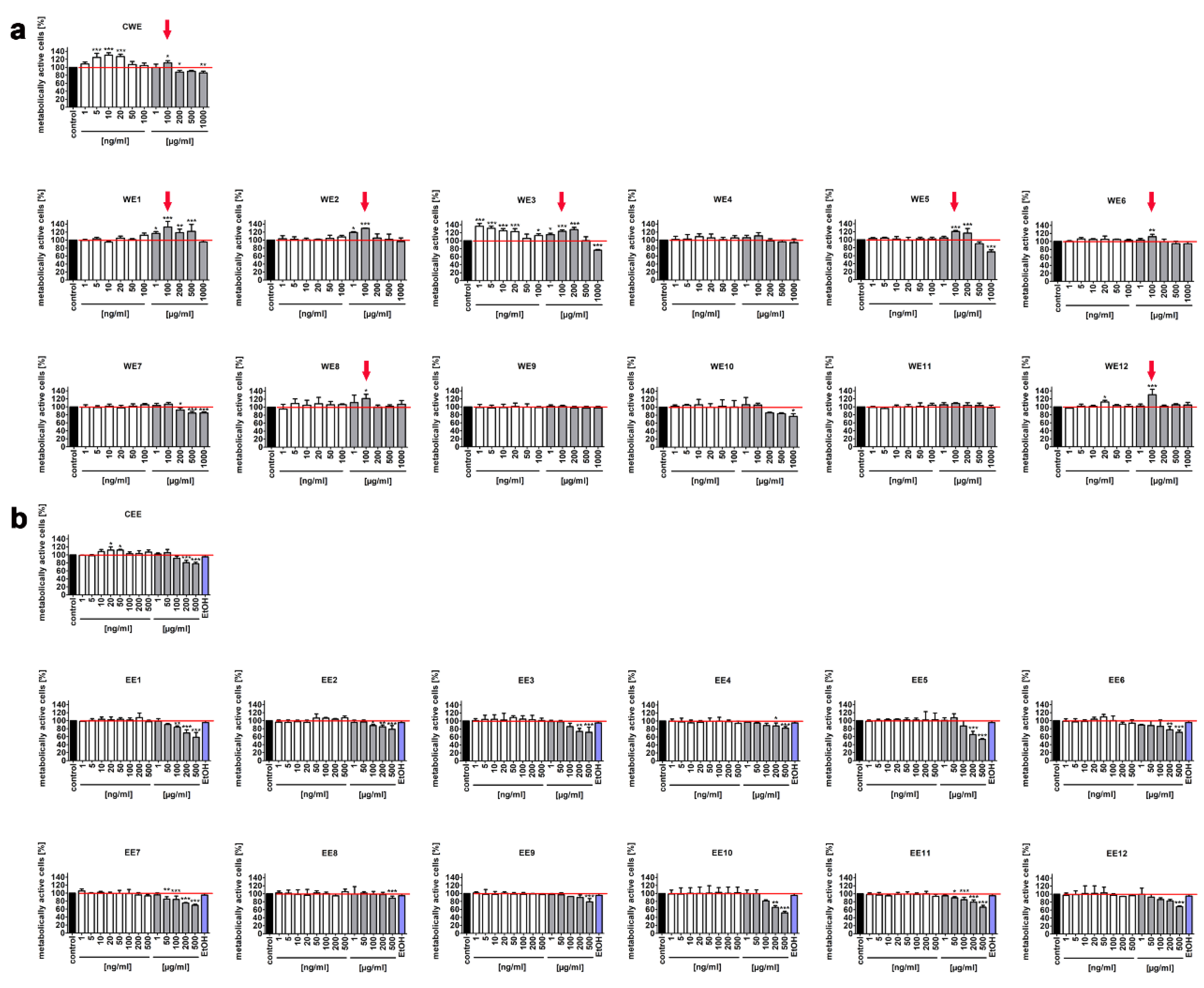
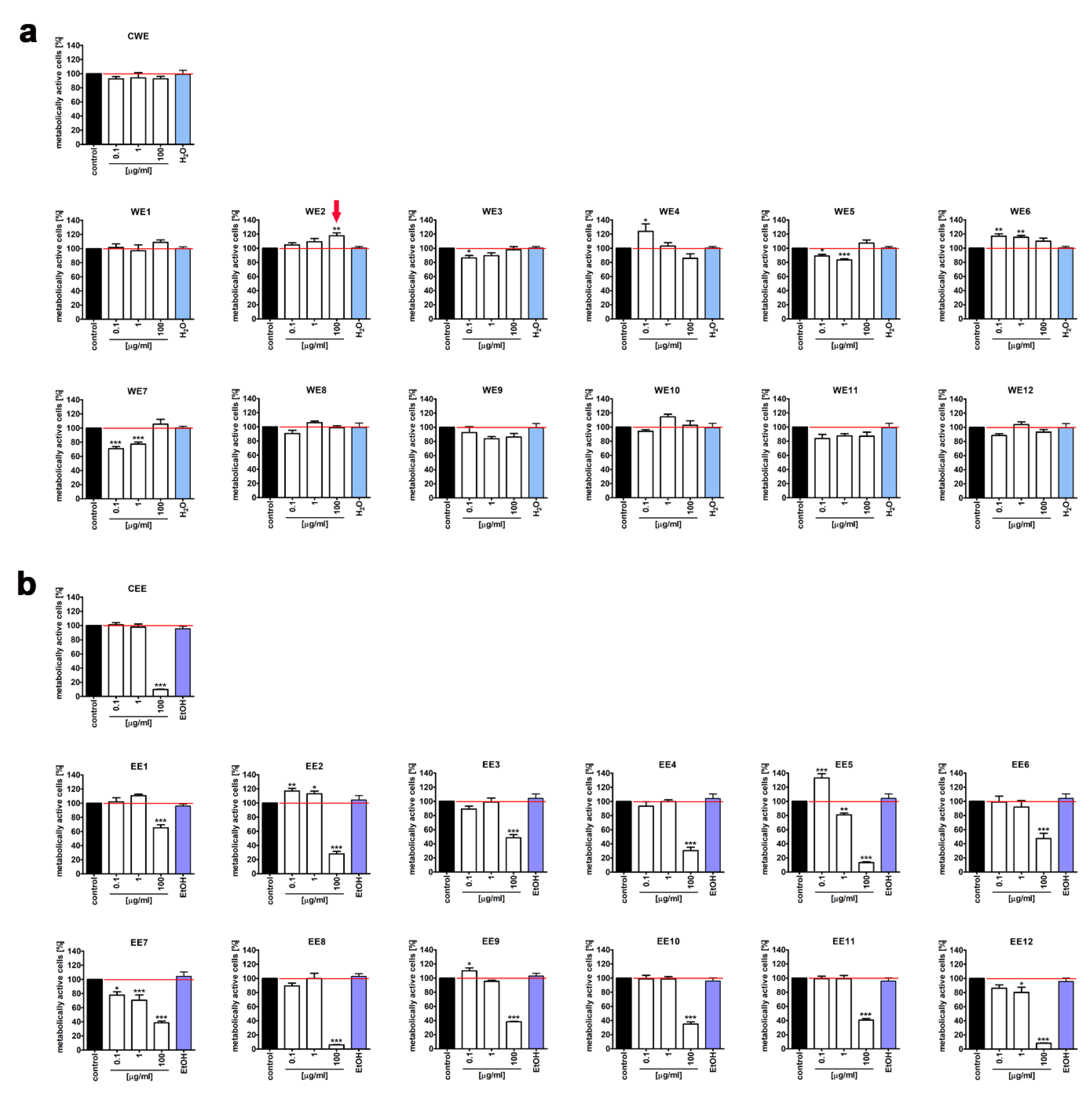
2.2. MTT Assay
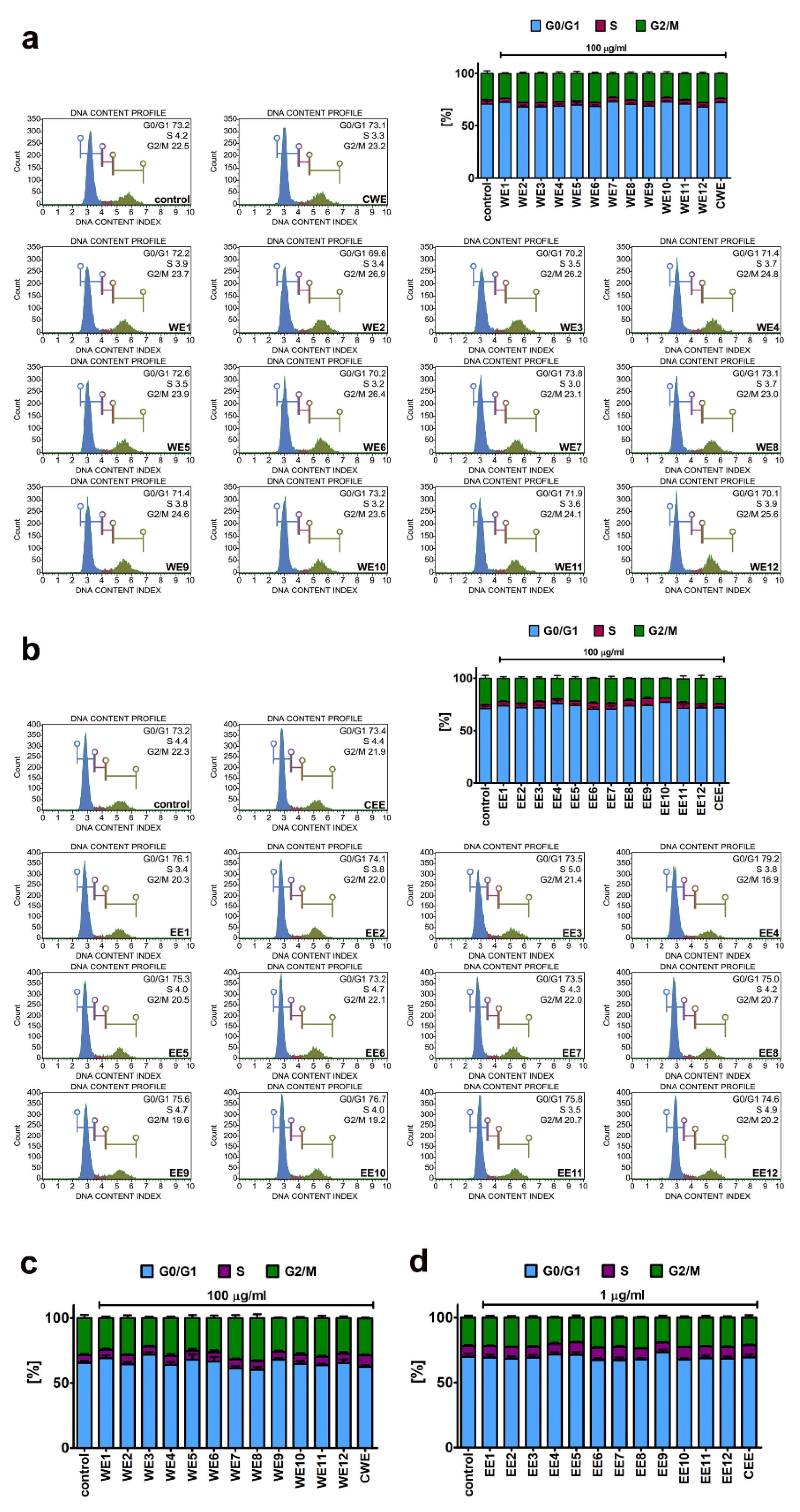
2.3. Cell Cycle
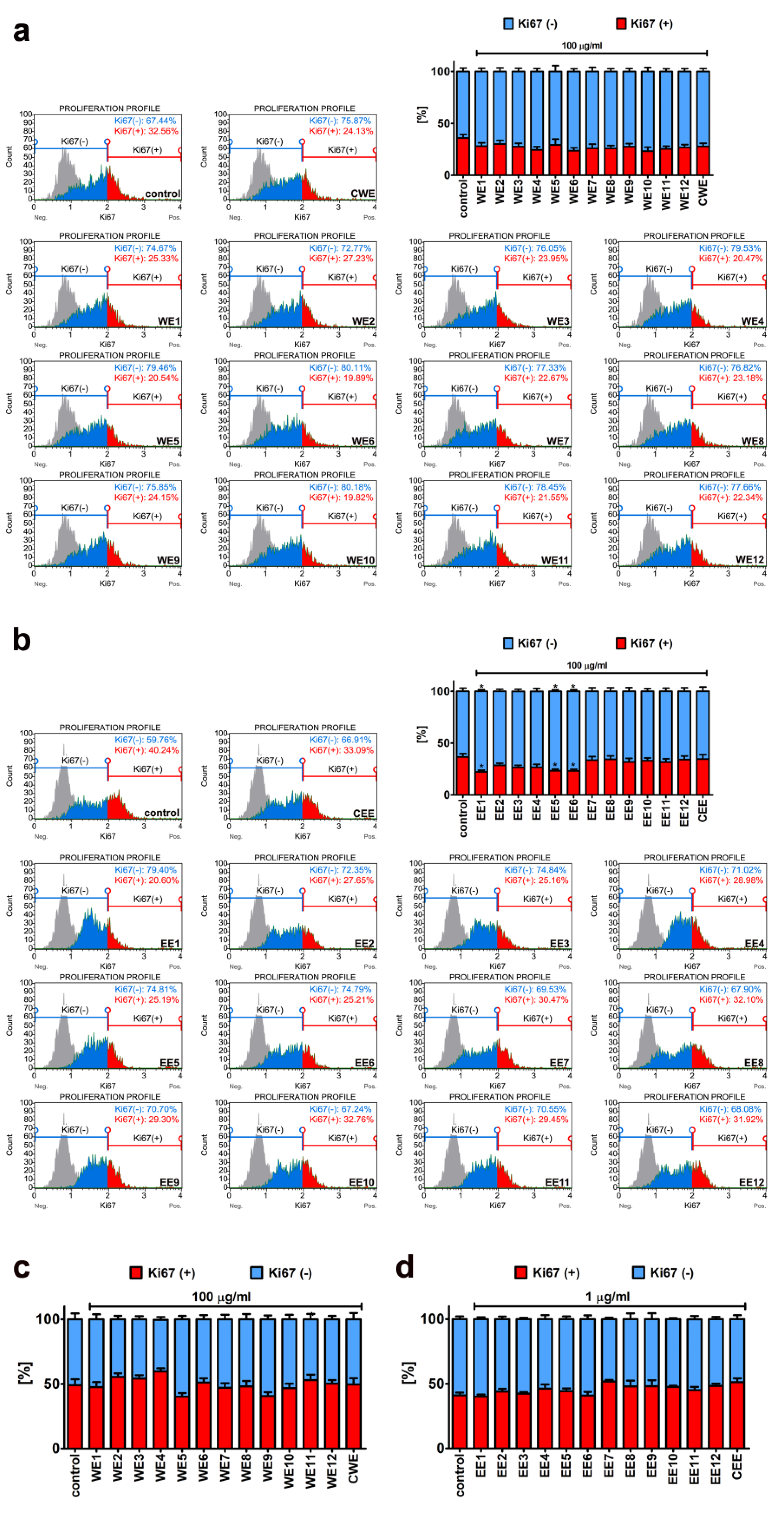
2.4. Cell Proliferation
2.5. Mitogenic Activity
2.6. Wound Healing Assay
2.7. Apoptosis versus Necrosis
2.8. Superoxide and Nitric Oxide Levels
2.9. Senescence-Associated Beta-Galactosidase Activity
2.10. Preliminary Analysis of Anticancer Activity
2.11. Statistical Analysis
3. Results and Discussion
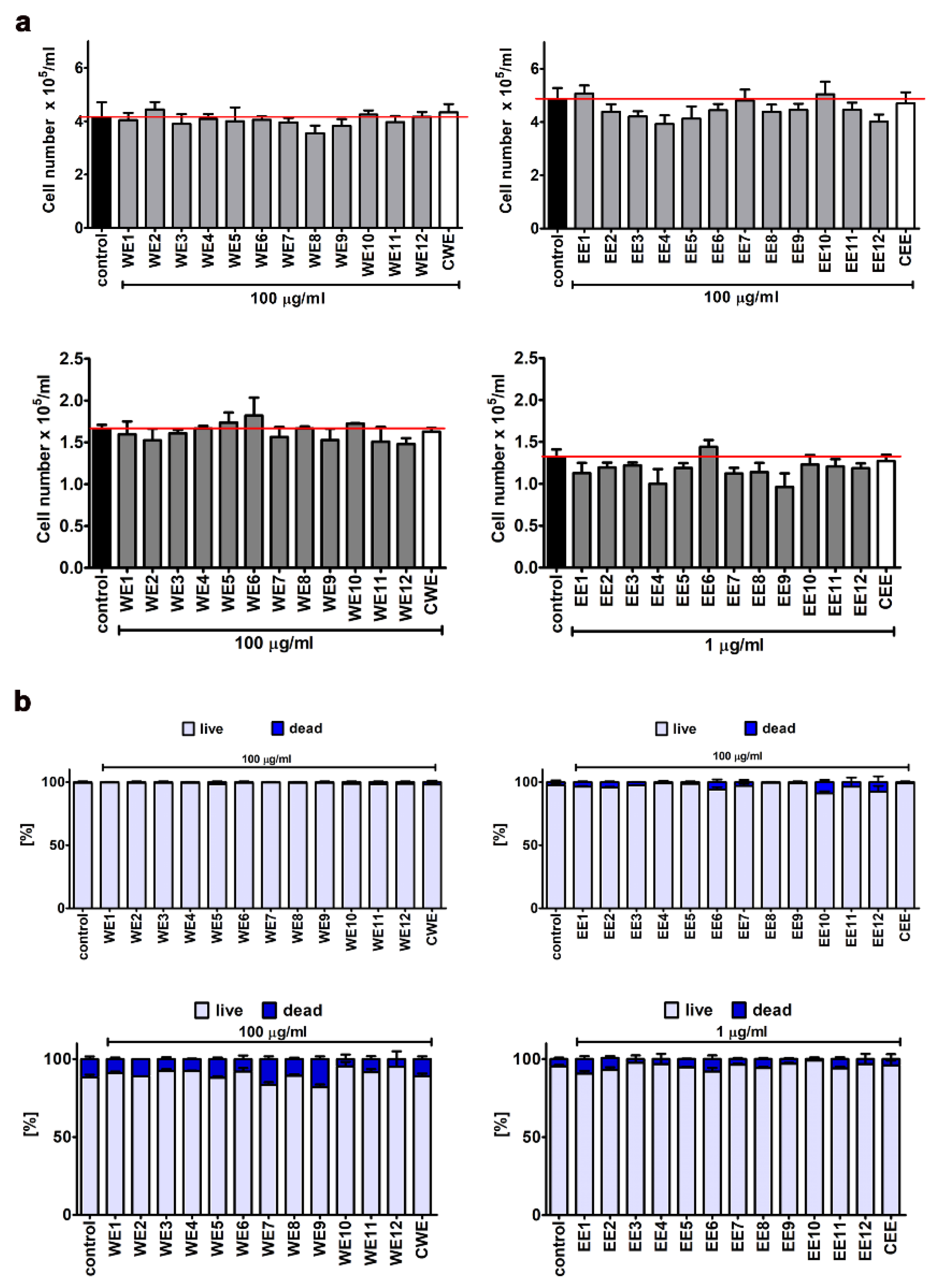
3.1. The Effect of Water and Ethanolic Extracts of the Microalga Planktochlorella Nurekis on Cell Cycle Progression and Proliferation of Human Skin Cells
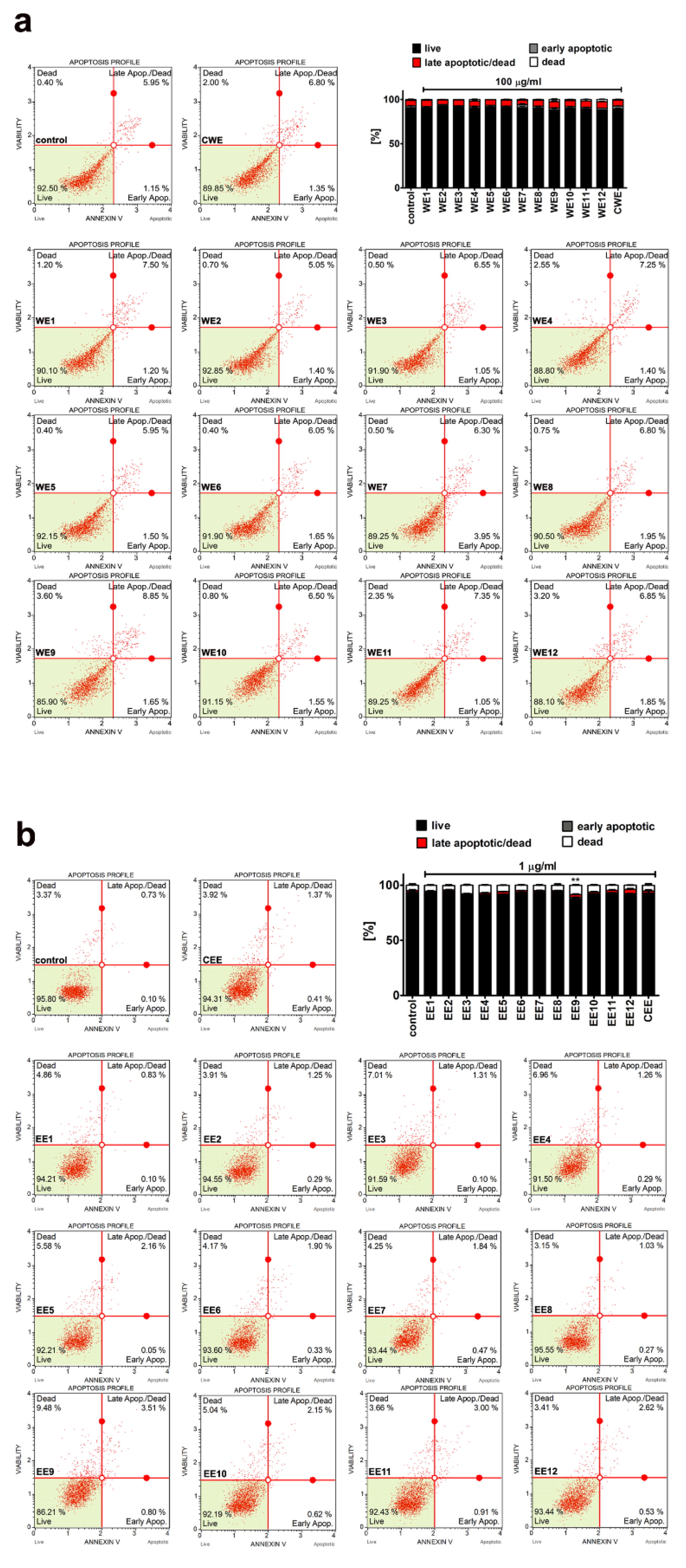
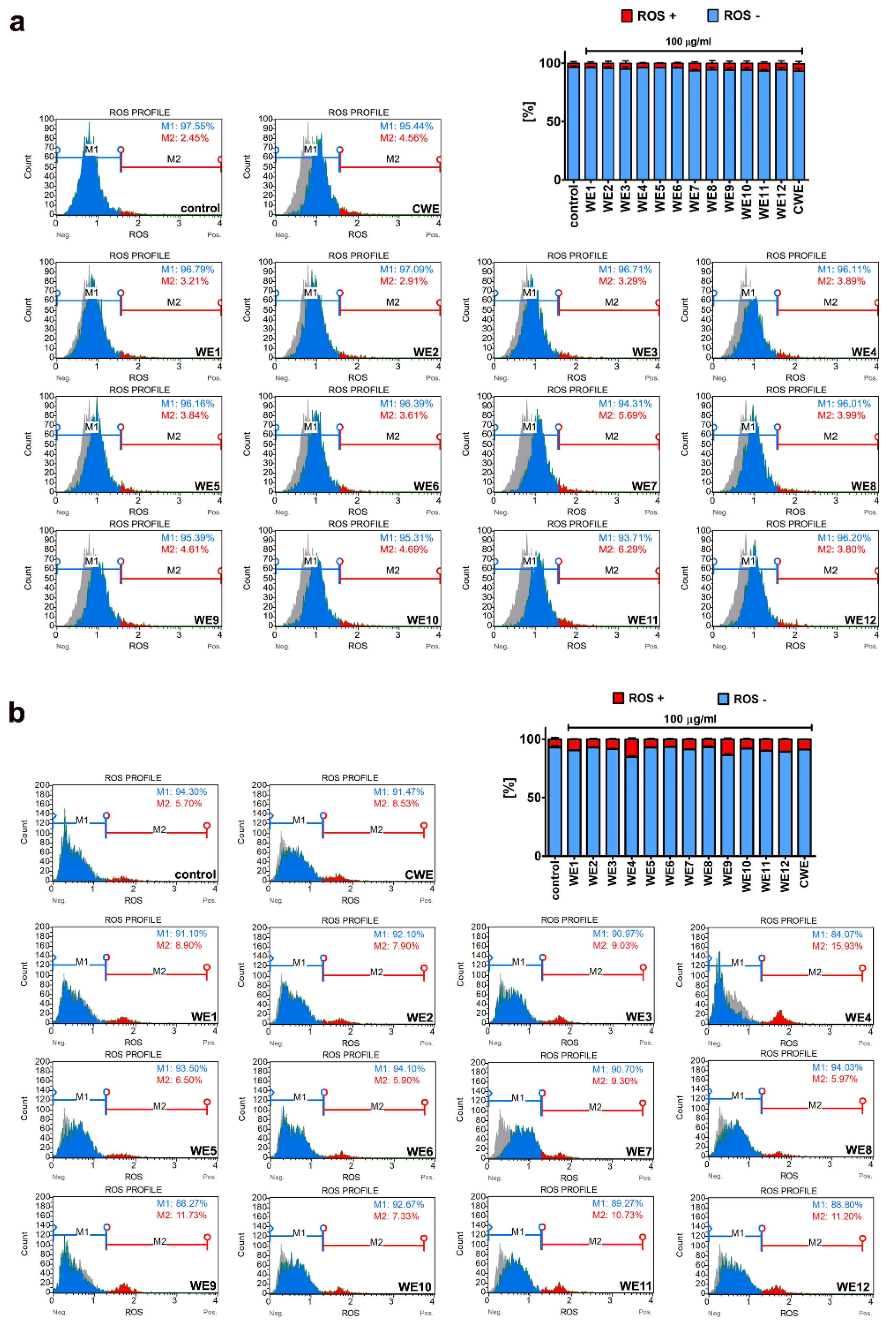
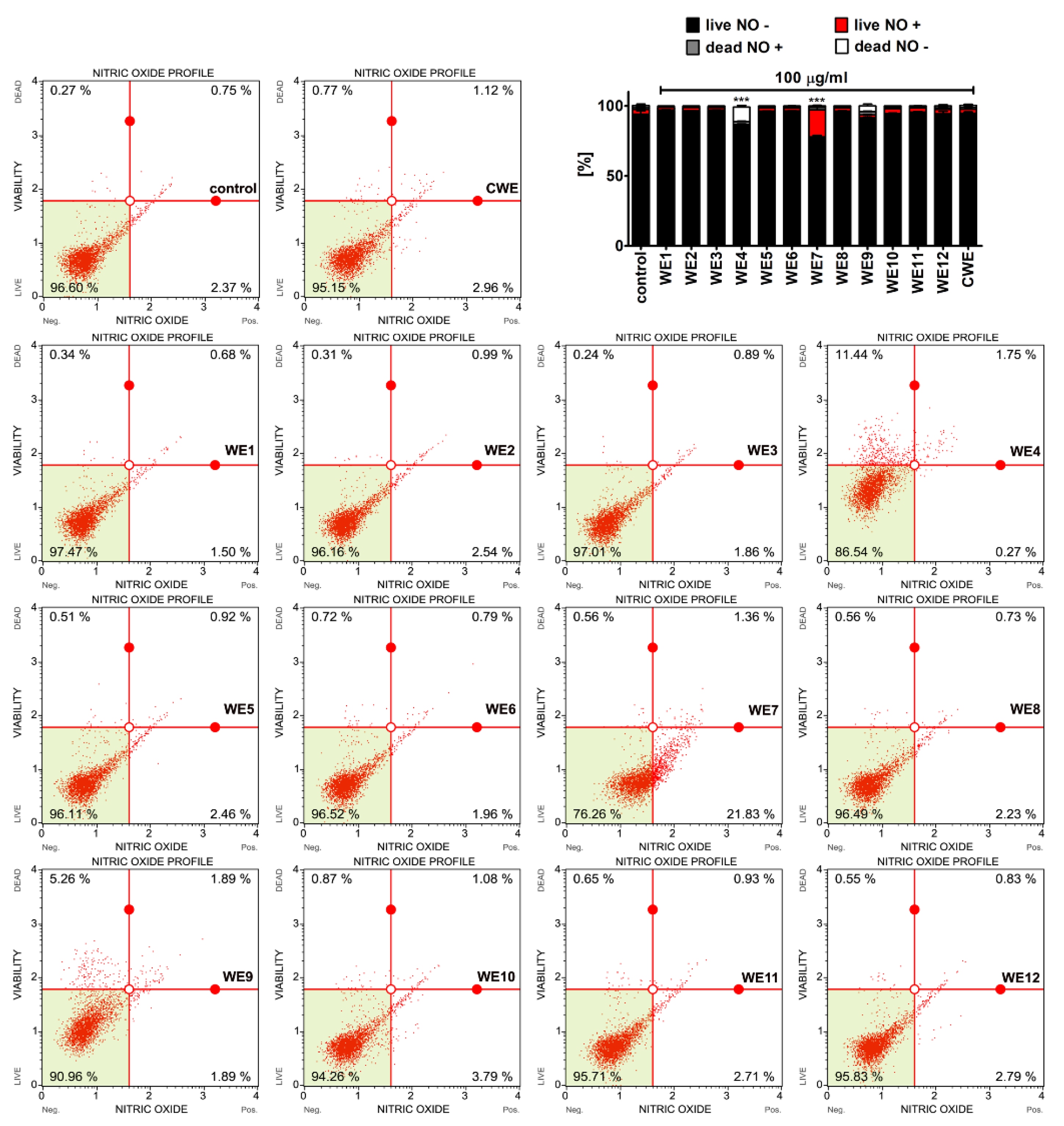
3.2. Extract-Mediated Cytotoxicity, Oxidative and Nitrosative Stress
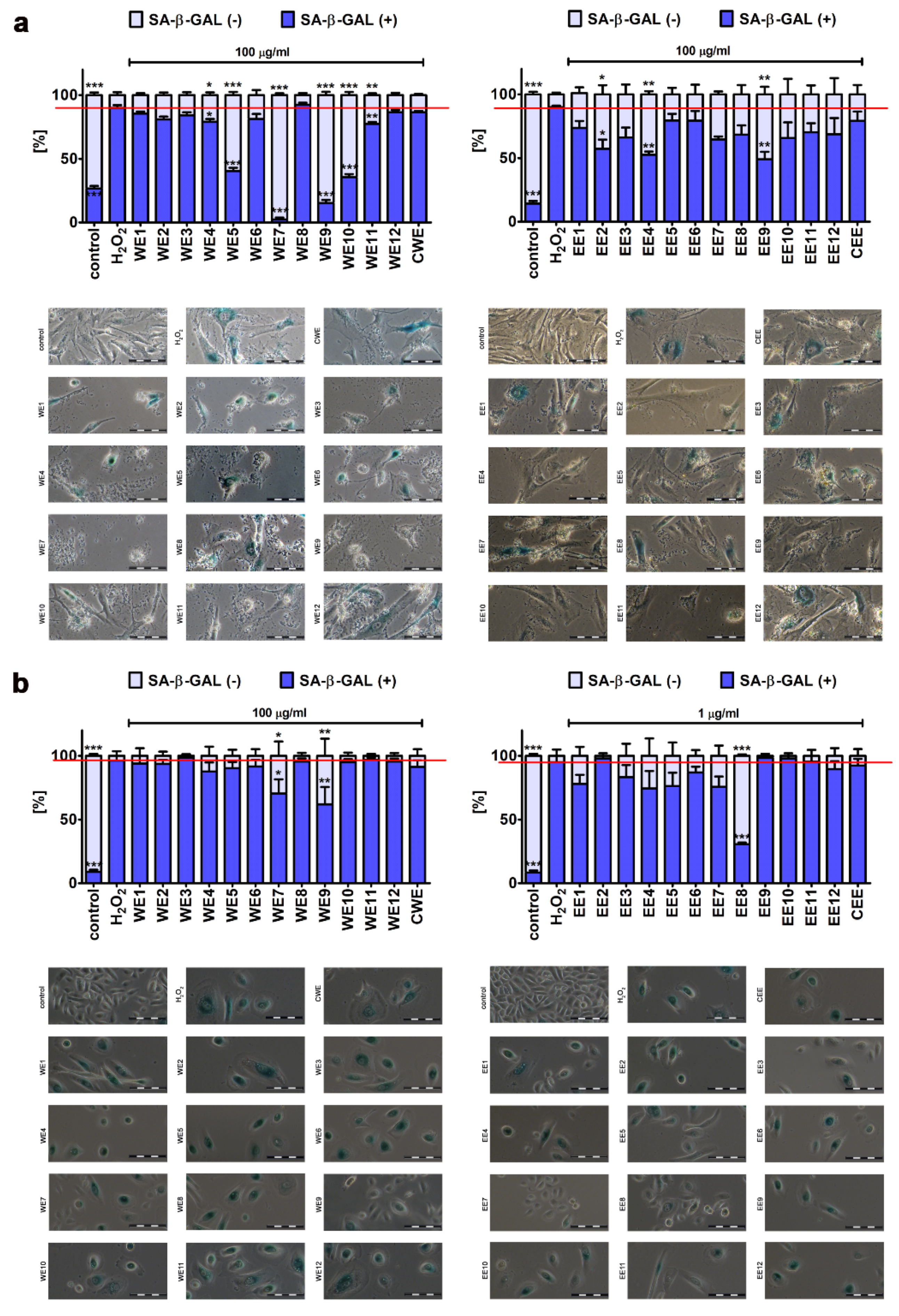
3.3. Attenuation of the Development of Stress-Induced Senescence in Skin Cells by Microalgal Extracts
3.4. Preliminary Analysis of Extract-Mediated Anticancer Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kim, K.E.; Cho, D.; Park, H.J. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental stressors on skin aging. Mechanistic insights. Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Soeur, J.; Belaidi, J.P.; Chollet, C.; Denat, L.; Dimitrov, A.; Jones, C.; Perez, P.; Zanini, M.; Zobiri, O.; Mezzache, S.; et al. Photo-pollution stress in skin: Traces of pollutants (PAH and particulate matter) impair redox homeostasis in keratinocytes exposed to UVA1. J. Dermatol. Sci. 2017, 86, 162–169. [Google Scholar] [CrossRef]
- Naylor, E.C.; Watson, R.E.; Sherratt, M.J. Molecular aspects of skin ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Duan, E. Fighting against skin aging: The way from bench to bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.D.; Chen, C.C.; Huynh, P.; Chang, J.S. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef]
- Wang, H.D.; Li, X.C.; Lee, D.J.; Chang, J.S. Potential biomedical applications of marine algae. Bioresour. Technol. 2017, 244, 1407–1415. [Google Scholar] [CrossRef]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Urikura, I.; Sugawara, T.; Hirata, T. Protective effect of fucoxanthin against UVB-induced skin photoaging in hairless mice. Biosci. Biotechnol. Biochem. 2011, 75, 757–760. [Google Scholar] [CrossRef]
- Kim, M.S.; Oh, G.H.; Kim, M.J.; Hwang, J.K. Fucosterol inhibits matrix metalloproteinase expression and promotes type-1 procollagen production in UVB-induced HaCaT cells. Photochem. Photobiol. 2013, 89, 911–918. [Google Scholar] [CrossRef]
- Ryu, J.; Park, S.J.; Kim, I.H.; Choi, Y.H.; Nam, T.J. Protective effect of porphyra-334 on UVA-induced photoaging in human skin fibroblasts. Int. J. Mol. Med. 2014, 34, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Balboa, E.M.; Li, Y.X.; Ahn, B.N.; Eom, S.H.; Dominguez, H.; Jimenez, C.; Rodriguez, J. Photodamage attenuation effect by a tetraprenyltoluquinol chromane meroterpenoid isolated from Sargassum muticum. J. Photochem. Photobiol. B 2015, 148, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.N.; Oyler, G.A.; Wilkinson, L.; Betenbaugh, M.J. A green light for engineered algae: Redirecting metabolism to fuel a biotechnology revolution. Curr. Opin. Biotechnol. 2008, 19, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Škaloud, P.; Němcová, Y.; Pytela, J.; Bogdanov, N.I.; Bock, C.; Pickinpaugh, S.H. Planktochlorella nurekis gen. et sp. nov. (Trebouxiophyceae, Chlorophyta), a novel coccoid green alga carrying significant biotechnological potential. Fottea 2014, 14, 53–62. [Google Scholar] [CrossRef]
- Szpyrka, E.; Broda, D.; Oklejewicz, B.; Podbielska, M.; Slowik-Borowiec, M.; Jagusztyn, B.; Chrzanowski, G.; Kus-Liskiewicz, M.; Duda, M.; Zuczek, J.; et al. A non-vector approach to increase lipid levels in the microalga Planktochlorella nurekis. Molecules 2020, 25, 270. [Google Scholar] [CrossRef]
- Lewinska, A.; Adamczyk-Grochala, J.; Kwasniewicz, E.; Deregowska, A.; Semik, E.; Zabek, T.; Wnuk, M. Reduced levels of methyltransferase DNMT2 sensitize human fibroblasts to oxidative stress and DNA damage that is accompanied by changes in proliferation-related miRNA expression. Redox Biol. 2018, 14, 20–34. [Google Scholar] [CrossRef]
- Lewinska, A.; Bocian, A.; Petrilla, V.; Adamczyk-Grochala, J.; Szymura, K.; Hendzel, W.; Kaleniuk, E.; Hus, K.K.; Petrillova, M.; Wnuk, M. Snake venoms promote stress-induced senescence in human fibroblasts. J. Cell. Physiol. 2019, 234, 6147–6160. [Google Scholar] [CrossRef]
- Lewinska, A.; Adamczyk-Grochala, J.; Bloniarz, D.; Olszowka, J.; Kulpa-Greszta, M.; Litwinienko, G.; Tomaszewska, A.; Wnuk, M.; Pazik, R. AMPK-mediated senolytic and senostatic activity of quercetin surface functionalized Fe3O4 nanoparticles during oxidant-induced senescence in human fibroblasts. Redox Biol. 2020, 28, 101337. [Google Scholar] [CrossRef]
- Chon, J.W.; Sung, J.H.; Hwang, E.J.; Park, Y.K. Chlorella methanol extract reduces lipid accumulation in and increases the number of apoptotic 3T3-L1 cells. Ann. N. Y. Acad. Sci. 2009, 1171, 183–189. [Google Scholar] [CrossRef]
- Song, S.H.; Kim, I.H.; Nam, T.J. Effect of a hot water extract of Chlorella vulgaris on proliferation of IEC-6 cells. Int. J. Mol. Med. 2012, 29, 741–746. [Google Scholar] [PubMed]
- Ilavenil, S.; Kim, D.H.; Vijayakumar, M.; Srigopalram, S.; Roh, S.G.; Arasu, M.V.; Lee, J.S.; Choi, K.C. Potential role of marine algae extract on 3T3-L1 cell proliferation and differentiation: An in vitro approach. Biol. Res. 2016, 49, 38. [Google Scholar] [CrossRef] [PubMed]
- Himuro, S.; Ueno, S.; Noguchi, N.; Uchikawa, T.; Kanno, T.; Yasutake, A. Safety evaluation of Chlorella sorokiniana strain CK-22 based on an in vitro cytotoxicity assay and a 13-week subchronic toxicity trial in rats. Food Chem. Toxicol. 2017, 106, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, F.; Durani, L.W.; Makpol, S. Chlorella vulgaris modulates the expression of senescence-associated genes in replicative senescence of human diploid fibroblasts. Mol. Biol. Rep. 2020, 47, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Grether-Beck, S.; Muhlberg, K.; Brenden, H.; Felsner, I.; Brynjolfsdottir, A.; Einarsson, S.; Krutmann, J. Bioactive molecules from the blue lagoon: In vitro and in vivo assessment of silica mud and microalgae extracts for their effects on skin barrier function and prevention of skin ageing. Exp. Dermatol. 2008, 17, 771–779. [Google Scholar] [CrossRef]
- Gunes, S.; Tamburaci, S.; Dalay, M.C.; Deliloglu Gurhan, I. In vitro evaluation of Spirulina platensis extract incorporated skin cream with its wound healing and antioxidant activities. Pharm. Biol. 2017, 55, 1824–1832. [Google Scholar] [CrossRef]
- Saberbaghi, T.; Abbasian, F.; Mohd Yusof, Y.A.; Makpol, S. Modulation of cell cycle profile by Chlorella vulgaris prevents replicative senescence of human diploid fibroblasts. Evid. Based Complement. Altern. Med. 2013, 2013, 780504. [Google Scholar] [CrossRef]
- Jeong, S.J.; Choi, J.W.; Lee, M.K.; Choi, Y.H.; Nam, T.J. Spirulina crude protein promotes the migration and proliferation in IEC-6 cells by activating EGFR/MAPK signaling pathway. Mar. Drugs 2019, 17, 205. [Google Scholar] [CrossRef]
- Zailan, N.; Abdul Rashid, A.H.; Das, S.; Abdul Mokti, N.A.; Hassan Basri, J.; Teoh, S.L.; Wan Ngah, W.Z.; Mohd Yusof, Y.A. Comparison of Chlorella vulgaris dressing and sodium alginate dressing: An experimental study in rats. Clin. Ter. 2010, 161, 515–521. [Google Scholar]
- Hidalgo-Lucas, S.; Bisson, J.F.; Duffaud, A.; Nejdi, A.; Guerin-Deremaux, L.; Baert, B.; Saniez-Degrave, M.H.; Rozan, P. Benefits of oral and topical administration of roquette Chlorella sp. On skin inflammation and wound healing in mice. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2014, 13, 93–102. [Google Scholar] [CrossRef]
- Bari, E.; Arciola, C.R.; Vigani, B.; Crivelli, B.; Moro, P.; Marrubini, G.; Sorrenti, M.; Catenacci, L.; Bruni, G.; Chlapanidas, T.; et al. In vitro effectiveness of microspheres based on silk sericin and Chlorella vulgaris or Arthrospira platensis for wound healing applications. Materials 2017, 10, 983. [Google Scholar] [CrossRef] [PubMed]
- Syarina, P.N.; Karthivashan, G.; Abas, F.; Arulselvan, P.; Fakurazi, S. Wound healing potential of Spirulina platensis extracts on human dermal fibroblast cells. EXCLI J. 2015, 14, 385–393. [Google Scholar] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef]
- Russell, E.G.; Cotter, T.G. New insight into the role of reactive oxygen species (ROS) in cellular signal-transduction processes. Int. Rev. Cell Mol. Biol. 2015, 319, 221–254. [Google Scholar]
- Labunskyy, V.M.; Gladyshev, V.N. Role of reactive oxygen species-mediated signaling in aging. Antioxid. Redox Signal. 2013, 19, 1362–1372. [Google Scholar] [CrossRef]
- Chen, K.; Feng, H.; Zhang, M.; Wang, X. Nitric oxide alleviates oxidative damage in the green alga Chlorella pyrenoidosa caused by UV-B radiation. Folia Microbiol. 2003, 48, 389–393. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, L.; Mallick, N. Antioxidative role of nitric oxide on copper toxicity to a chlorophycean alga, Chlorella. Ecotoxicol. Environ. Saf. 2004, 59, 223–227. [Google Scholar] [CrossRef]
- De Magalhaes, J.P.; Passos, J.F. Stress, cell senescence and organismal ageing. Mech. Ageing Dev. 2018, 170, 2–9. [Google Scholar] [CrossRef]
- Naylor, R.M.; Baker, D.J.; van Deursen, J.M. Senescent cells: A novel therapeutic target for aging and age-related diseases. Clin. Pharmacol. Ther. 2013, 93, 105–116. [Google Scholar] [CrossRef]
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.M.; Marquess, D.; Dananberg, J.; van Deursen, J.M. Senescent cells: An emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O. B vitamins and the brain: Mechanisms, dose and efficacy—A review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Makpol, S.; Yaacob, N.; Zainuddin, A.; Yusof, Y.A.; Ngah, W.Z. Chlorella vulgaris modulates hydrogen peroxide-induced DNA damage and telomere shortening of human fibroblasts derived from different aged individuals. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Makpol, S.; Yeoh, T.W.; Ruslam, F.A.; Arifin, K.T.; Yusof, Y.A. Comparative effect of Piper betle, Chlorella vulgaris and tocotrienol-rich fraction on antioxidant enzymes activity in cellular ageing of human diploid fibroblasts. BMC Complement. Altern. Med. 2013, 13, 210. [Google Scholar] [CrossRef] [PubMed]
- Zainul Azlan, N.; Mohd Yusof, Y.A.; Alias, E.; Makpol, S. Chlorella vulgaris improves the regenerative capacity of young and senescent myoblasts and promotes muscle regeneration. Oxid. Med. Cell. Longev. 2019, 2019, 3520789. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Liou, S.F.; Chen, S.J.; Shih, M.F. Protective effects of Chlorella-derived peptide on UVB-induced production of MMP-1 and degradation of procollagen genes in human skin fibroblasts. Regul. Toxicol. Pharmacol. 2011, 60, 112–119. [Google Scholar] [CrossRef]
- Shih, M.F.; Cherng, J.Y. Protective effects of Chlorella-derived peptide against UVC-induced cytotoxicity through inhibition of caspase-3 activity and reduction of the expression of phosphorylated FADD and cleaved PARP-1 in skin fibroblasts. Molecules 2012, 17, 9116–9128. [Google Scholar] [CrossRef]
- Lee, J.J.; Kim, K.B.; Heo, J.; Cho, D.H.; Kim, H.S.; Han, S.H.; Ahn, K.J.; An, I.S.; An, S.; Bae, S. Protective effect of Arthrospira platensis extracts against ultraviolet B-induced cellular senescence through inhibition of DNA damage and matrix metalloproteinase-1 expression in human dermal fibroblasts. J. Photochem. Photobiol. B 2017, 173, 196–203. [Google Scholar] [CrossRef]
- Chung, J.-G.; Peng, H.-Y.; Chu, Y.-C.; Hsieh, Y.-M.; Wang, S.-D.; Chou, S.-T. Anti-invasion and apoptosis induction of Chlorella (Chlorella sorokiniana) in Hep G2 human hepatocellular carcinoma cells. J. Funct. Foods 2012, 4, 302–310. [Google Scholar] [CrossRef]
- Lin, P.Y.; Tsai, C.T.; Chuang, W.L.; Chao, Y.H.; Pan, I.H.; Chen, Y.K.; Lin, C.C.; Wang, B.Y. Chlorella sorokiniana induces mitochondrial-mediated apoptosis in human non-small cell lung cancer cells and inhibits xenograft tumor growth in vivo. BMC Complement. Altern. Med. 2017, 17, 88. [Google Scholar] [CrossRef]
- Zhang, Z.D.; Liang, K.; Li, K.; Wang, G.Q.; Zhang, K.W.; Cai, L.; Zhai, S.T.; Chou, K.C. Chlorella vulgaris induces apoptosis of human non-small cell lung carcinoma (NSCLC) cells. Med. Chem. 2017, 13, 560–568. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczyk-Grochala, J.; Wnuk, M.; Duda, M.; Zuczek, J.; Lewinska, A. Treatment with Modified Extracts of the Microalga Planktochlorella nurekis Attenuates the Development of Stress-Induced Senescence in Human Skin Cells. Nutrients 2020, 12, 1005. https://doi.org/10.3390/nu12041005
Adamczyk-Grochala J, Wnuk M, Duda M, Zuczek J, Lewinska A. Treatment with Modified Extracts of the Microalga Planktochlorella nurekis Attenuates the Development of Stress-Induced Senescence in Human Skin Cells. Nutrients. 2020; 12(4):1005. https://doi.org/10.3390/nu12041005
Chicago/Turabian StyleAdamczyk-Grochala, Jagoda, Maciej Wnuk, Magdalena Duda, Janusz Zuczek, and Anna Lewinska. 2020. "Treatment with Modified Extracts of the Microalga Planktochlorella nurekis Attenuates the Development of Stress-Induced Senescence in Human Skin Cells" Nutrients 12, no. 4: 1005. https://doi.org/10.3390/nu12041005
APA StyleAdamczyk-Grochala, J., Wnuk, M., Duda, M., Zuczek, J., & Lewinska, A. (2020). Treatment with Modified Extracts of the Microalga Planktochlorella nurekis Attenuates the Development of Stress-Induced Senescence in Human Skin Cells. Nutrients, 12(4), 1005. https://doi.org/10.3390/nu12041005





