Effects of 12-Week Ingestion of Yogurt Containing Lactobacillus plantarum OLL2712 on Glucose Metabolism and Chronic Inflammation in Prediabetic Adults: A Randomized Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Test Foods
2.4. Randomization and Blinding
2.5. Interventions
2.6. Diet Records
2.7. Measurements
2.8. Safety Assessment
2.9. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Effect of OLL2712 Yogurt on Glucose Metabolism-Related Parameters
3.3. Effect of OLL2712 Yogurt on Chronic Inflammation
3.4. Subgroup Analyses
3.5. Safety Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Zeng, L.; He, Q.; Wang, W.; Lei, J.; Zou, X. Effect of probiotics on glucose and lipid metabolism in type 2 diabetes mellitus: A meta-analysis of 12 randomized controlled trials. Med. Sci. Monit. 2017, 23, 3044–3053. [Google Scholar] [CrossRef] [PubMed]
- Barengolts, E.; Smith, E.D.; Reutrakul, S.; Tonucci, L.; Anothaisintawee, T. The effect of probiotic yogurt on glycemic control in type 2 diabetes or obesity: A meta-analysis of nine randomized controlled trials. Nutrients 2019, 11, 671. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Brit. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef]
- Pittas, A.; Lau, J.; Hu, F.; Dawson-Hughes, B. The role of Vitamin D and calcium in type 2 diabetes: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2007, 92, 2017–2029. [Google Scholar] [CrossRef]
- Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 2013, 369, 145–154. [Google Scholar] [CrossRef]
- Torres, S.; Fabersani, E.; Marquez, A.; Gauffin-Cano, P. Adipose tissue inflammation and metabolic syndrome. The proactive role of probiotics. Eur. J. Nutr. 2019, 58, 27–43. [Google Scholar] [CrossRef]
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–919. [Google Scholar] [CrossRef]
- Andreasen, A.S.; Larsen, N.; Pedersen-Skovsgaard, T.; Berg, R.M.; Møller, K.; Svendsen, K.D.; Jakobsen, M.; Pedersen, B.K. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br. J. Nutr. 2010, 104, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Hirose, Y.; Yamamoto, Y.; Yoshikai, Y.; Murosaki, S. Daily intake of heat-killed Lactobacillus plantarum L-137 improves inflammation and lipid metabolism in overweight healthy adults: A randomized-controlled trial. Eur. J. Nutr. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Gøbel, R.J.; Larsen, N.; Jakobsen, M.; Mølgaard, C.; Michaelsen, K.F. Probiotics to adolescents with obesity: Effects on inflammation and metabolic syndrome. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Toshimitsu, T.; Mochizuki, J.; Ikegami, S.; Itou, H. Identification of a Lactobacillus plantarum strain that ameliorates chronic inflammation and metabolic disorders in obese and type 2 diabetic mice. J. Dairy Sci. 2016, 99, 933–946. [Google Scholar] [CrossRef]
- Sakai, T.; Taki, T.; Nakamoto, A.; Shuto, E.; Tsutsumi, R.; Toshimitsu, T.; Makino, S.; Ikegami, S. Lactobacillus plantarum OLL2712 regulates glucose metabolism in C57BL/6 mice fed a high-fat diet. J. Nutr. Sci. Vitaminol. 2013, 59, 144–147. [Google Scholar] [CrossRef]
- Toshimitsu, T.; Ozaki, S.; Mochizuki, J.; Furuichi, K.; Asami, Y. Effects of Lactobacillus plantarum strain OLL2712 culture conditions on the anti-inflammatory activities for murine immune cells and obese and type 2 diabetic mice. Appl. Environ. Microbiol. 2017, 83, 03001–03016. [Google Scholar] [CrossRef]
- Toshimitsu, T.; Gotou, A.; Furuichi, K.; Hachimura, S.; Asami, Y. Effects of 12-wk Lactobacillus plantarum OLL2712 treatment on glucose metabolism and chronic inflammation in prediabetic individuals: A single-arm pilot study. Nutrition 2019, 58, 175–180. [Google Scholar] [CrossRef]
- Wolever, T.M. Yogurt is a low-glycemic index food. J. Nutr. 2017, 147, 1462S–1467S. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; the CONSORT Group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Ann. Intern. Med. 2010, 152, 726–732. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Fujita, H.; Hosono, A.; Shibata, K.; Tsujimura, S.; Oka, K.; Okamoto, N.; Kamiya, M.; Kondo, F.; Wakabayashi, R.; Ichikawa, M.; et al. Physical activity earlier in life is inversely associated with insulin resistance among adults in Japan. J. Epidemiol. 2019, 29, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Takeda, E.; Yamanaka-Okumura, H.; Taketani, Y.; Inagaki, N.; Hosokawa, M.; Shide, K.; Maegawa, H.; Kondo, K.; Kawasaki, E.; Shinozaki, S.; et al. Effect of nutritional counseling and long term isomaltulose based liquid formula (MHN-01) intake on metabolic syndrome. J. Clin. Biochem. Nutr. 2015, 57, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Suzuki, H.; Baba, T.; Nakamura, K.; Watanabe, T. Seasonal variation of glycemic control in type 2 diabetic patients. Diabetes Care 2001, 24, 1503. [Google Scholar] [CrossRef] [PubMed]
- Kamezaki, F.; Sonoda, S.; Tomotsune, Y.; Yunaka, H.; Otsuji, Y. Seasonal variation in metabolic syndrome prevalence. Hypertens. Res. 2010, 33, 568–572. [Google Scholar] [CrossRef]
- Higgins, T.; Saw, S.; Sikaris, K.; Wiley, C.L.; Cembrowski, G.C.; Lyon, A.W.; Khajuria, A.; Tran, D. Seasonal variation in hemoglobin A1c: Is it the same in both hemispheres? J. Diabetes Sci. Technol. 2009, 3, 668–671. [Google Scholar] [CrossRef]
- Pereira, M.T.; Lira, D.; Bacelar, C.; Oliveira, J.C.; de Carvalho, A.C. Seasonal variation of haemoglobin A1c in a Portuguese adult population. Arch. Endocrinol. Metab. 2015, 59, 231–235. [Google Scholar] [CrossRef]
- Chen, M.; Sun, Q.; Giovannucci, E.; Mozaffarian, D.; Manson, J.E.; Willett, W.C.; Hu, F.B. Dairy consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. BMC Med. 2014, 12, 215. [Google Scholar] [CrossRef]
- Watanabe, D.; Kuranuki, S.; Sunto, A.; Matsumoto, N.; Nakamura, T. Daily yogurt consumption improves glucose metabolism and insulin sensitivity in young nondiabetic Japanese subjects with type-2 diabetes risk alleles. Nutrients 2018, 10, 1834. [Google Scholar] [CrossRef]
- Guo, J.; Givens, D.I.; Astrup, A.; Bakker, S.J.L.; Goossens, G.H.; Kratz, M.; Marette, A.; Pijl, H.; Soedamah-Muthu, S.S. The Impact of Dairy Products in the Development of Type 2 Diabetes: Where Does the Evidence Stand in 2019? Adv. Nutr. 2019, 10, 1066–1075. [Google Scholar] [CrossRef]
- Gijsbers, L.; Ding, E.L.; Malik, V.S.; de Goede, J.; Geleijnse, J.M.; Soedamah-Muthu, S.S. Consumption of dairy foods and diabetes incidence: A dose-response meta-analysis of observational studies. Am. J. Clin. Nutr. 2016, 103, 1111–1124. [Google Scholar] [CrossRef]
- Usui, Y.; Kimura, Y.; Satoh, T.; Takemura, N.; Ouchi, Y.; Ohmiya, H.; Kobayashi, K.; Suzuki, H.; Koyama, S.; Hagiwara, S.; et al. Effects of long-term intake of a yogurt fermented with Lactobacillus delbrueckii subsp bulgaricus 2038 and Streptococcus thermophilus 1131 on mice. Int. Immunol. 2018, 30, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Wen, L. and Duffy, A. Factors Influencing the Gut Microbiota, Inflammation, and Type 2 Diabetes. J. Nutr. 2017, 147, 1468S–1475S. [Google Scholar] [CrossRef] [PubMed]
- Fändriks, L. Roles of the gut in the metabolic syndrome: An overview. J. Intern. Med. 2017, 281, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; Ohshiba, Y.; Mogami, O. Novel dipeptidyl peptidase-4-inhibiting peptide derived from β-lactoglobulin. J. Pharmacol. Sci. 2011, 117, 63–66. [Google Scholar] [CrossRef]
- Avignon, A.; Radauceanu, A.; Monnier, L. Nonfasting plasma glucose is a better marker of diabetic control than fasting plasma glucose in type 2 diabetes. Diabetes Care 1997, 20, 1822–1826. [Google Scholar] [CrossRef]
- Monnier, L.; Lapinski, H.; Colette, C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: Variations with increasing levels of HbA(1c). Diabetes Care 2003, 26, 881–885. [Google Scholar] [CrossRef]
- Monnier, L.; Colettem, C.; Owens, D. Postprandial and basal glucose in type 2 diabetes: Assessment and respective impacts. Diabetes Technol. Ther. 2011, 13, S25–S32. [Google Scholar] [CrossRef]
- Loughrey, B.V.; McGinty, A.; Young, I.S.; McCance, D.R.; Powell, L.A. Increased circulating CC chemokine levels in the metabolic syndrome are reduced by low-dose atorvastatin treatment: Evidence from a randomized controlled trial. Clin. Endocrinol. 2013, 79, 800–806. [Google Scholar] [CrossRef]
- Tisato, V.; Toffoli, B.; Monasta, L.; Bernardi, S.; Candido, R.; Zauli, G.; Secchiero, P. Patients affected by metabolic syndrome show decreased levels of circulating platelet derived growth factor (PDGF)-BB. Clin. Nutr. 2013, 32, 259–264. [Google Scholar] [CrossRef]
- Yu, R.; Kim, C.S.; Kang, J.H. Inflammatory components of adipose tissue as target for treatment of metabolic syndrome. Forum Nutr. 2009, 61, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Shoelson, S.E.; Herrero, L.; Naaz, A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007, 132, 2169–2180. [Google Scholar] [CrossRef] [PubMed]
- Winer, S.; Chan, Y.; Paltser, G.; Truong, D.; Tsui, H.; Bahrami, J. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 2009, 15, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Bouter, K.E.; van Raalte, D.H.; Groen, A.K.; Nieuwdorp, M. Role of the Gut Microbiome in the Pathogenesis of Obesity and Obesity-Related Metabolic Dysfunction. Gastroenterology 2017, 152, 1671–1678. [Google Scholar] [CrossRef]
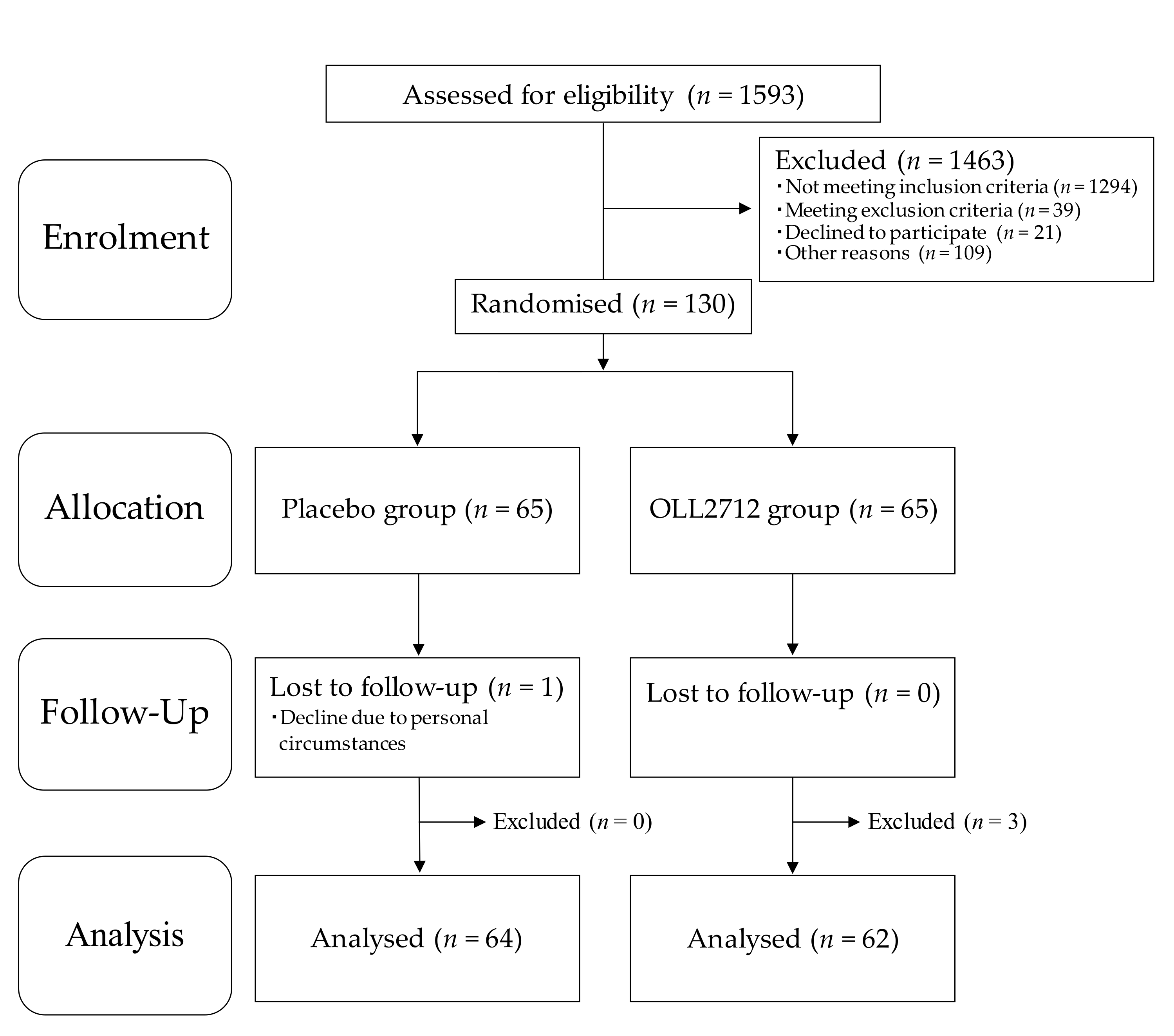
| Placebo Yogurt (n = 64) | OLL2712 Yogurt (n = 62) | |||
|---|---|---|---|---|
| Characteristics | Week 0 | Week 12 | Week 0 | Week 12 |
| Age (years) | 51.2 ± 7.6 | NA | 50.6 ± 6.9 | NA |
| Male/Female | 44/20 | NA | 42/20 | NA |
| BW (kg) | 69.4 ± 12.3 | 70.3 ± 12.5 ** | 69.1 ± 11.0 | 69.8 ± 10.8 ** |
| BMI (kg/m2) | 24.9 ± 3.2 | 25.2 ± 3.4 ** | 24.7 ± 3.3 | 25.0 ± 3.2 ** |
| SBP (mmHg) | 123.3 ± 14.3 | 128.3 ± 14.2 ** | 126.6 ± 14.4 | 133.1 ± 13.2 ** |
| DBP (mmHg) | 74.4 ± 10.7 | 79.7 ± 9.7 ** | 77.4 ± 11.0 | 82.0 ± 11.2 ** |
| HR (beats/min) | 76.4 ± 11.4 | 76.7 ± 12.1 | 76.7 ± 12.2 | 73.8 ± 12.2 * |
| Variable | Group | Week 0 | Week 4 | Week 8 | Week 12 |
|---|---|---|---|---|---|
| Total energy (kcal/day) | Placebo | 1817 ± 414 | 1831 ± 500 | 1860 ± 450 | 1854 ± 497 |
| OLL2712 | 1838 ± 355 | 1775 ± 404 | 1809 ± 407 | 1857 ± 376 | |
| Protein (g/day) | Placebo | 69.0 ± 20.7 | 69.2 ± 20.3 | 68.9 ± 18.3 | 70.2 ± 20.0 |
| OLL2712 | 67.2 ± 15.8 | 65.5 ± 14.9 | 67.3 ± 17.9 | 68.5 ± 18.9 | |
| Fat (g/day) | Placebo | 62.6 ± 20.2 | 63.3 ± 22.8 | 63.9 ± 19.9 | 64.5 ± 23.8 |
| OLL2712 | 65.8 ± 18.0 | 62.9 ± 19.0 | 63.0 ± 21.7 | 65.9 ± 18.9 | |
| Carbohydrate (g/day) | Placebo | 232 ± 58 | 233 ± 72 | 239 ± 66 | 236 ± 76 |
| OLL2712 | 230 ± 51 | 223 ± 59 | 229 ± 52 | 234 ± 52 | |
| Dietary fiber (g/day) | Placebo | 11.0 ± 3.3 | 11.1 ± 4.0 | 11.3 ± 3.8 | 11.3 ± 4.0 |
| OLL2712 | 10.8 ± 3.1 | 10.6 ± 3.3 | 11.0 ± 3.1 | 11.3 ± 2.5 |
| Variable | Group | Week 0 | Week 4 | Week 8 | Week 12 | 12-Week Change |
|---|---|---|---|---|---|---|
| FBG (mg/dL) | Placebo | 103.4 ± 7.9 | 101.0 ± 8.9 | 104.6 ± 9.6 | 101.4 ± 11.8 | −2.0 ± 10.8 |
| OLL2712 | 103.8 ± 7.8 | 102.3 ± 9.7 | 103.6 ± 10.3 | 102.1 ± 9.5 | −1.7 ± 8.1 | |
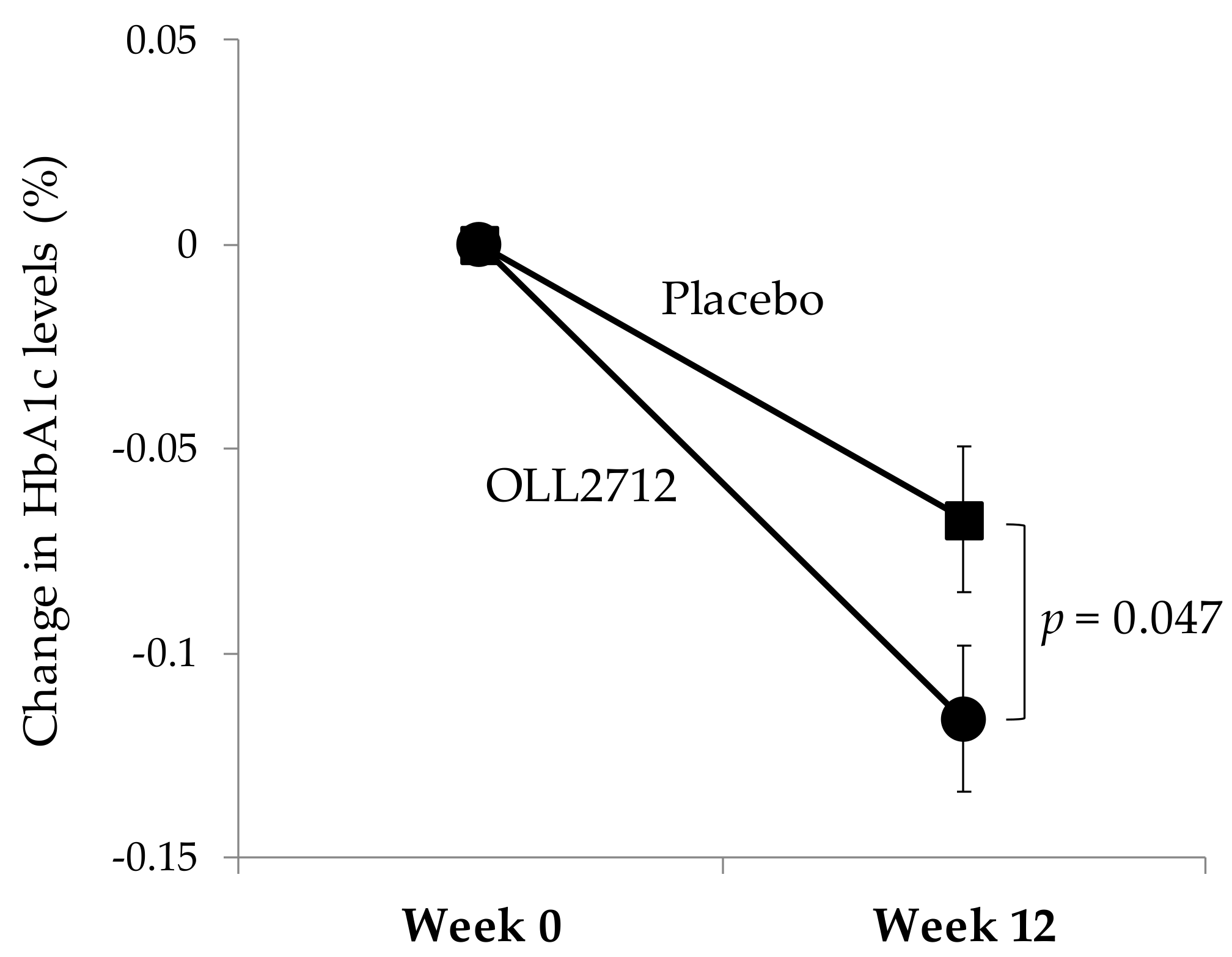
| HbA1c (%) | Placebo | 5.85 ± 0.21 | 5.79 ± 0.22** | 5.89 ± 0.22 ** | 5.78 ± 0.24 ** | −0.07 ± 0.14 |
| OLL2712 | 5.86 ± 0.22 | 5.82 ± 0.25* | 5.89 ± 0.26 | 5.74 ± 0.26 ** | −0.12 ± 0.14 # | |
| GA (%) | Placebo | 14.8 ± 1.2 | 14.7 ± 1.2 | 14.5 ± 1.2 ** | 14.5 ± 1.2 ** | −0.2 ± 0.5 |
| OLL2712 | 14.6 ± 1.1 | 14.5 ± 1.1 | 14.3 ± 1.2 ** | 14.2 ± 1.1 ** | −0.3 ± 0.5 | |
| Insulin (μU/mL) | Placebo | 5.64 ± 2.87 | 6.40 ± 3.16 * | 6.42 ± 3.28 ** | 7.31 ± 5.03 ** | 1.68 ± 4.03 |
| OLL2712 | 5.88 ± 4.78 | 5.82 ± 2.88 * | 6.16 ± 3.08 ** | 6.07 ± 3.24 * | 0.24 ± 4.41 | |
| HOMA-IR | Placebo | 1.45 ± 0.82 | 1.60 ± 0.82 | 1.68 ± 0.95 ** | 1.87 ± 1.38 * | 0.42 ± 1.12 |
| OLL2712 | 1.52 ± 1.32 | 1.48 ± 0.76 | 1.58 ± 0.79* | 1.53 ± 0.80 | 0.02 ± 1.25 |
| Variable | Group | Week 0 | Week 4 | Week 8 | Week 12 | 12-Week Change |
|---|---|---|---|---|---|---|
| IL-6 (pg/mL) | Placebo | 0.532 ± 0.268 | 0.496 ± 0.250 | 0.563 ± 0.310 | 0.601 ± 0.265 * | 0.070 ± 0.236 |
| OLL2712 | 0.801 ± 1.231 | 0.855 ± 1.456 | 0.779 ± 1.453 | 0.792 ± 1.296 | −0.001 ± 0.507 | |
| IL-8 (pg/mL) | Placebo | 4.71 ± 2.52 | 2.65 ± 1.81 ** | 6.21 ± 5.80 * | 5.72 ± 3.46 * | 1.01 ± 3.12 |
| OLL2712 | 5.24 ± 2.11 | 2.85 ± 1.76 ** | 5.41 ± 2.23 | 6.66 ± 8.41 | 1.38 ± 8.56 | |
| MCP-1 (pg/mL) | Placebo | 26.8 ± 21.7 | 16.6 ± 7.8 ** | 21.2 ± 11.1 ** | 24.9 ± 17.2 | −1.95 ± 24.53 |
| OLL2712 | 25.3 ± 11.9 | 16.6 ± 9.1 ** | 21.2 ± 10.8 ** | 23.5 ± 11.1 * | −2.10 ± 8.87 | |
| TNF-α (pg/mL) | Placebo | 9.79 ± 5.22 | 6.79 ± 8.36 | 11.04 ± 5.41 | 10.09 ± 4.69 | 0.29 ± 3.64 |
| OLL2712 | 9.93 ± 4.37 | 5.17 ± 3.34 | 10.99 ± 4.84 | 10.23 ± 5.41 | 0.20 ± 4.62 | |
| hsCRP (mg/dL) | Placebo | 0.058 ± 0.067 | NA | NA | 0.079 ± 0.098 ** | 0.030 ± 0.078 |
| OLL2712 | 0.048 ± 0.049 | NA | NA | 0.054 ± 0.049 | 0.006 ± 0.031 | |
| Adiponectin (μg/mL) | Placebo | 5038 ± 1924 | 5202 ± 2275 | 5233 ± 2090 | 5478 ± 2156 ** | 439 ± 780 |
| OLL2712 | 5472 ± 2002 | 5468 ± 2136 | 5559 ± 1975 | 6047 ± 2185 ** | 576 ± 1078 |
| Variable | Group | Week 0 | Week 4 | Week 8 | Week 12 | 12-Week Change |
|---|---|---|---|---|---|---|
| FBG (mg/dL) | Placebo | 107.0 ± 5.6 | 104.1 ± 8.2 | 108.1 ± 8.6 | 104.9 ± 10.4 | −2.2 ± 10.4 |
| OLL2712 | 107.6 ± 5.6 | 104.9 ± 9.4* | 107.1 ± 9.4 | 104.8 ± 8.7 * | −2.8 ± 8.5 | |
| HbA1c (%) | Placebo | 5.92 ± 0.21 | 5.87 ± 0.21** | 5.98 ± 0.21 ** | 5.85 ± 0.25 ** | −0.07 ± 0.16 |
| OLL2712 | 5.90 ± 0.22 | 5.87 ± 0.24 | 5.94 ± 0.24 | 5.77 ± 0.26 ** | −0.12 ± 0.14 | |
| GA (%) | Placebo | 14.9 ± 1.2 | 14.9 ± 1.2 | 14.7 ± 1.1 * | 14.7 ± 1.2 * | −0.2 ± 0.5 |
| OLL2712 | 14.7 ± 1.2 | 14.7 ± 1.2 | 14.4 ± 1.2 ** | 14.3 ± 1.1 ** | −0.3 ± 0.5 | |
| Insulin (μU/mL) | Placebo | 5.70 ± 2.47 | 6.43 ± 2.98 * | 6.51 ± 3.65 | 7.34 ± 4.77 * | 1.64 ± 3.90 |
| OLL2712 | 6.24 ± 5.28 | 5.82 ± 2.92 | 6.19 ± 2.67 | 5.87 ± 2.67 | −0.37 ± 4.89 | |
| HOMA-IR | Placebo | 1.51 ± 0.66 | 1.66 ± 0.78 | 1.77 ± 1.09 | 1.95 ± 1.39 | 0.44 ± 1.13 |
| OLL2712 | 1.67 ± 1.48 | 1.52 ± 0.80 | 1.65 ± 0.75 | 1.52 ± 0.72 | −0.15 ± 1.41 |
| Variable | Group | Week 0 | Week 4 | Week 8 | Week 12 | 12-Week Change |
|---|---|---|---|---|---|---|
| FBG (mg/dL) | Placebo | 107.1 ± 8.2 | 103.9 ± 8.1 | 109.3 ± 10.9 | 105.5 ± 10.2 | −1.6 ± 10.5 |
| OLL2712 | 104.7 ± 8.7 | 102.6 ± 8.4 | 103.7 ± 11.7 | 100.0 ± 11.4 | −4.7 ± 10.6 | |
| HbA1c (%) | Placebo | 5.94 ± 0.18 | 5.88 ± 0.20 | 6.01 ± 0.21 | 5.90 ± 0.26 | −0.03 ± 0.17 |
| OLL2712 | 5.94 ± 0.23 | 5.92 ± 0.23 | 5.99 ± 0.24 | 5.89 ± 0.29 | −0.05 ± 0.17 | |
| GA (%) | Placebo | 14.6 ± 1.5 | 14.6 ± 1.5 | 14.5 ± 1.4 | 14.5 ± 1.5 | −0.1 ± 0.5 |
| OLL2712 | 14.4 ± 1.1 | 14.3 ± 1.0 | 14.1 ± 1.0 ** | 14.2 ± 1.1 * | −0.3 ± 0.5 | |
| Insulin (μU/mL) | Placebo | 8.61 ± 2.88 | 9.13 ± 2.66 | 9.38 ± 3.30 | 10.88 ± 5.03 | 2.27 ± 5.30 |
| OLL2712 | 10.53 ± 6.93 | 8.40 ± 3.29 | 9.09 ± 3.15 | 8.68 ± 4.02 | −1.85 ± 7.81 | |
| HOMA-IR | Placebo | 2.23 ± 0.89 | 2.34 ± 0.70 | 2.57 ± 1.05 | 2.89 ± 1.51 | 0.60 ± 1.55 |
| OLL2712 | 2.74 ± 1.97 | 2.12 ± 0.84 | 2.32 ± 0.76 | 2.15 ± 0.97 | −0.59 ± 2.22 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toshimitsu, T.; Gotou, A.; Sashihara, T.; Hachimura, S.; Shioya, N.; Suzuki, S.; Asami, Y. Effects of 12-Week Ingestion of Yogurt Containing Lactobacillus plantarum OLL2712 on Glucose Metabolism and Chronic Inflammation in Prediabetic Adults: A Randomized Placebo-Controlled Trial. Nutrients 2020, 12, 374. https://doi.org/10.3390/nu12020374
Toshimitsu T, Gotou A, Sashihara T, Hachimura S, Shioya N, Suzuki S, Asami Y. Effects of 12-Week Ingestion of Yogurt Containing Lactobacillus plantarum OLL2712 on Glucose Metabolism and Chronic Inflammation in Prediabetic Adults: A Randomized Placebo-Controlled Trial. Nutrients. 2020; 12(2):374. https://doi.org/10.3390/nu12020374
Chicago/Turabian StyleToshimitsu, Takayuki, Ayako Gotou, Toshihiro Sashihara, Satoshi Hachimura, Nobuhiko Shioya, Satoru Suzuki, and Yukio Asami. 2020. "Effects of 12-Week Ingestion of Yogurt Containing Lactobacillus plantarum OLL2712 on Glucose Metabolism and Chronic Inflammation in Prediabetic Adults: A Randomized Placebo-Controlled Trial" Nutrients 12, no. 2: 374. https://doi.org/10.3390/nu12020374
APA StyleToshimitsu, T., Gotou, A., Sashihara, T., Hachimura, S., Shioya, N., Suzuki, S., & Asami, Y. (2020). Effects of 12-Week Ingestion of Yogurt Containing Lactobacillus plantarum OLL2712 on Glucose Metabolism and Chronic Inflammation in Prediabetic Adults: A Randomized Placebo-Controlled Trial. Nutrients, 12(2), 374. https://doi.org/10.3390/nu12020374





