Carrot Intake and Risk of Colorectal Cancer: A Prospective Cohort Study of 57,053 Danes
Abstract
1. Introduction
2. Materials and Methods
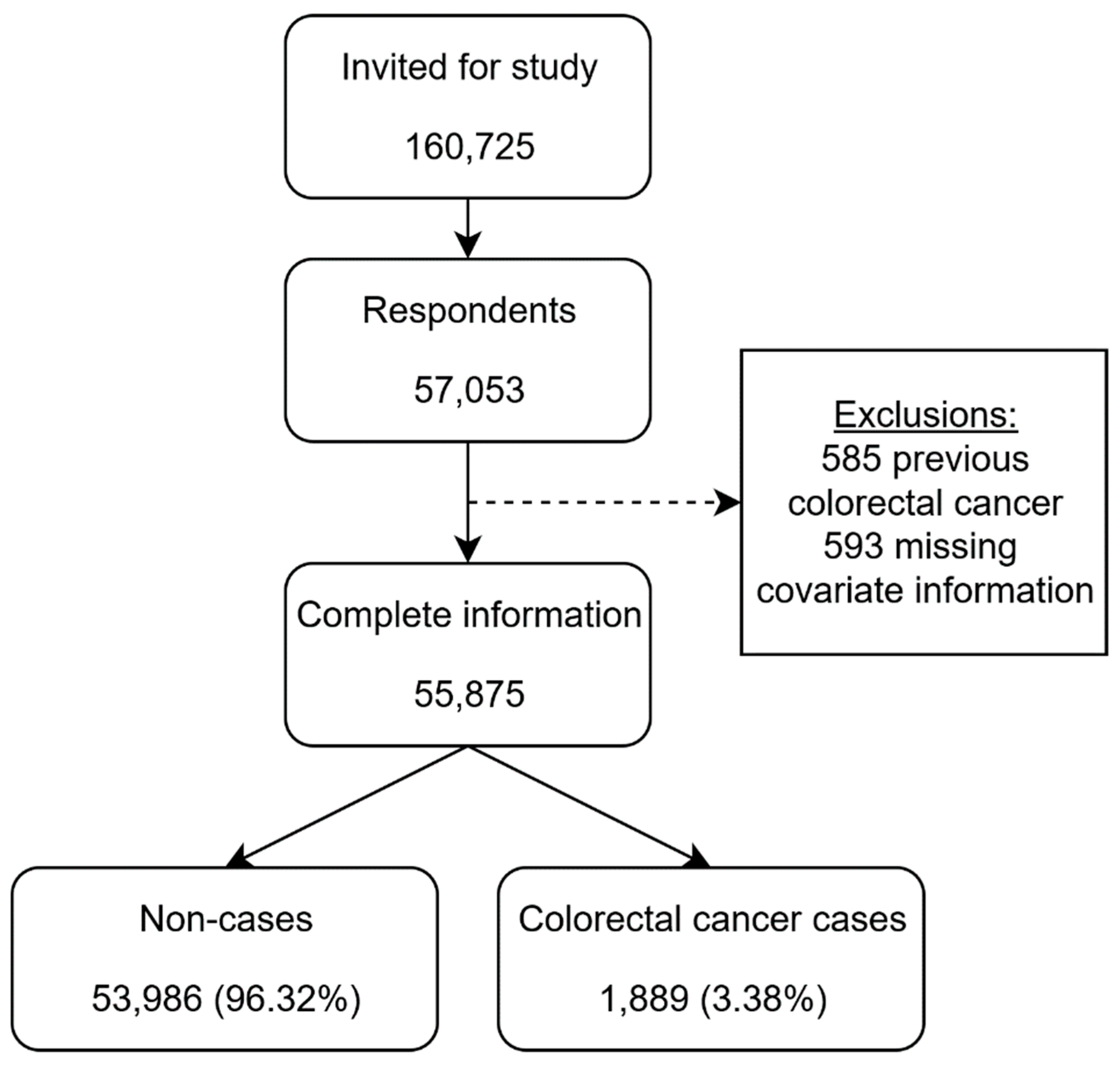
2.1. Study Population
2.2. Data from Registries
2.3. Exposure
2.4. Outcome
2.5. Covariates
2.6. Ethics
2.7. Statistical Analysis
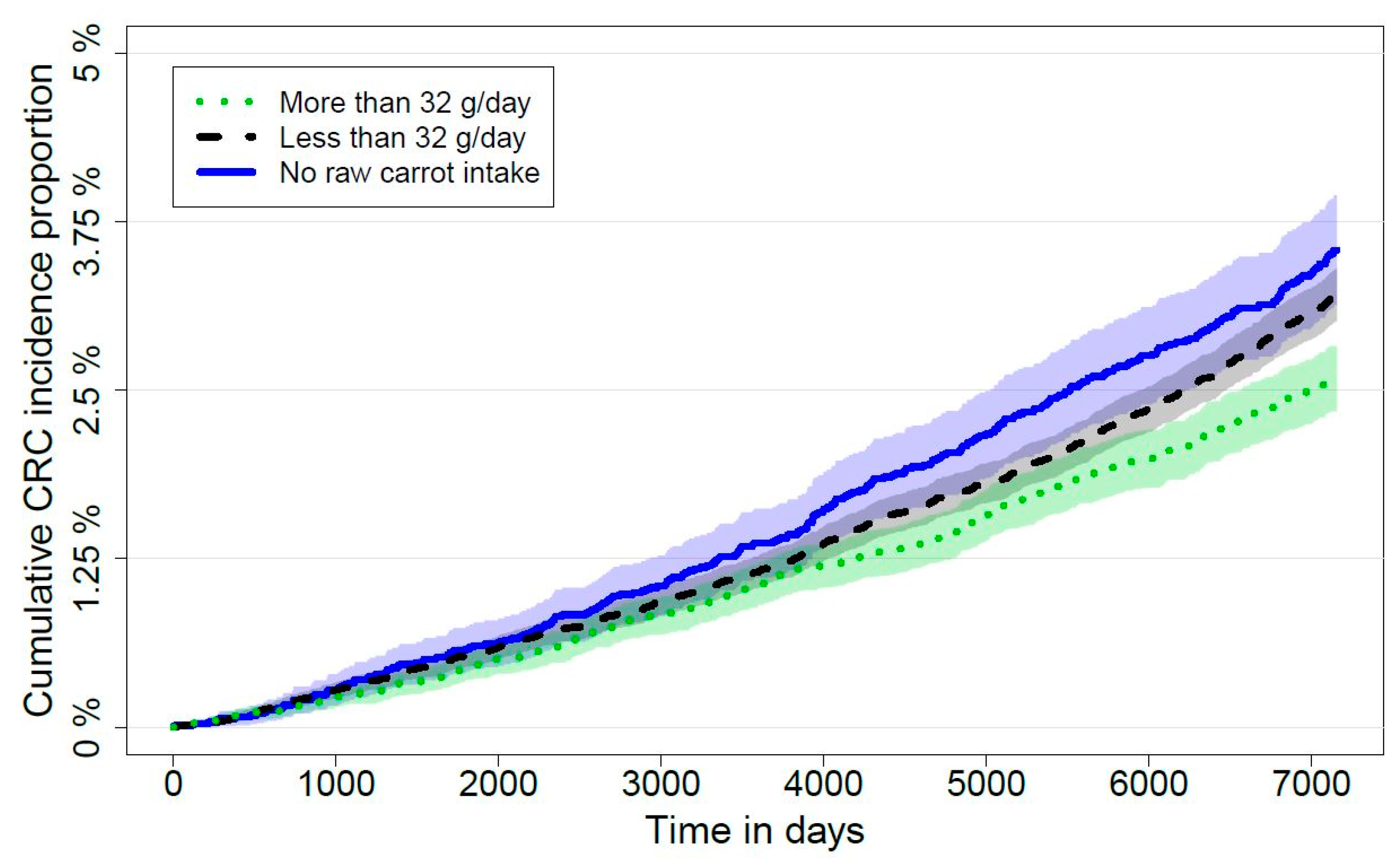
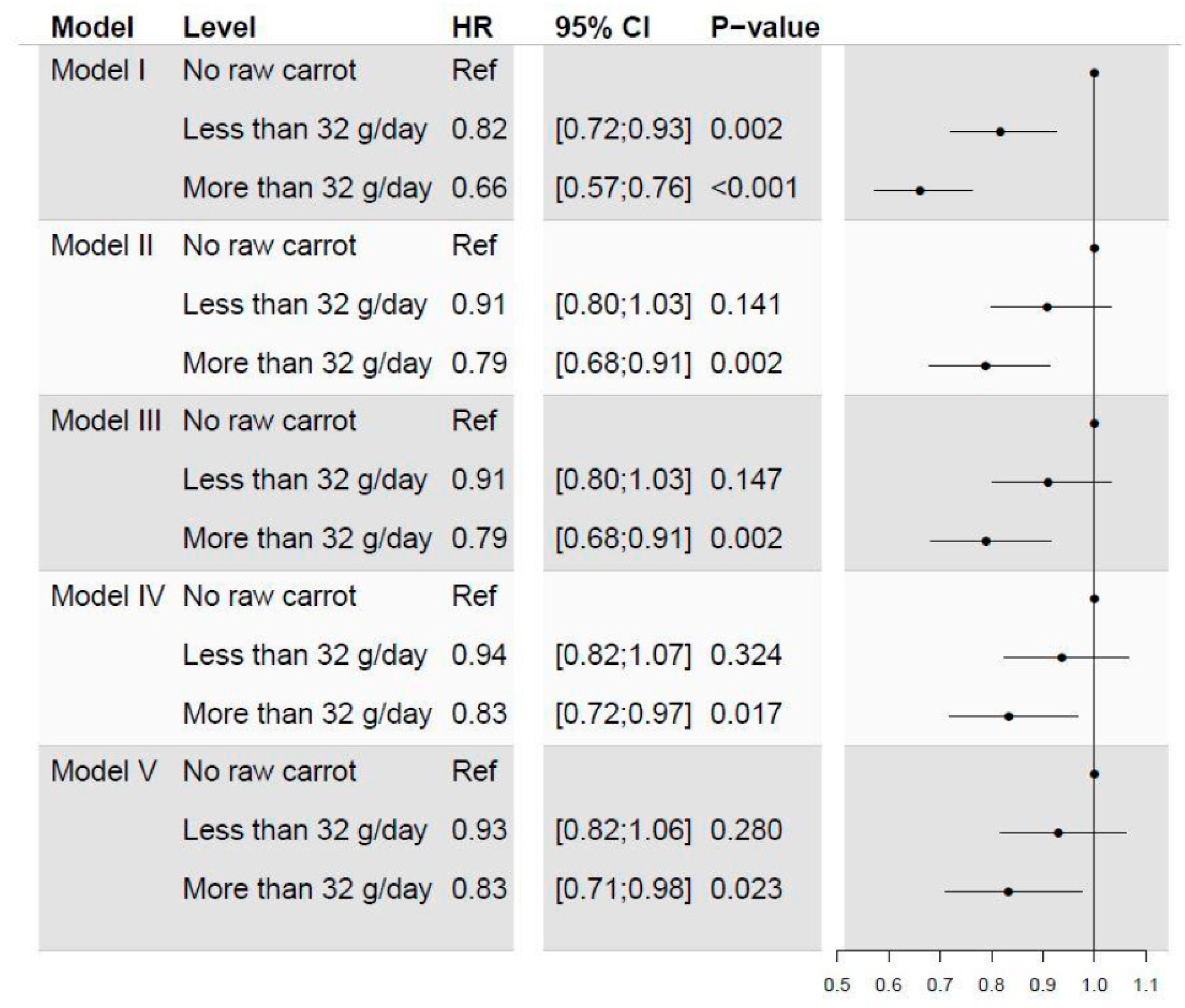
3. Results
3.1. Comparison of High, Low and No Intake of Carrots
3.2. Sensitivity Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| COX | Cyclooxygenase |
| CRC | Colorectal cancer |
| FaOH | Falcarinol |
| FaDOH | Falcarindiol |
| HR | Hazard ratio |
| MET | Metabolic equivalents |
| NSAID | Nonsteroidal anti-inflammatory drug |
References
- Block, G.; Patterson, B.; Subar, A. Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutr. Cancer 1992, 18, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, K.A.; Potter, J.D. Vegetables, fruit, and cancer prevention: A review. J. Am. Diet. Assoc. 1996, 96, 1027–1039. [Google Scholar] [CrossRef]
- Greenwald, P.; Clifford, C.K.; Milner, J.A. Diet and cancer prevention. Eur. J. Cancer 2001, 37, 948–965. [Google Scholar] [CrossRef]
- Key, T.J. Fruit and vegetables and cancer risk. Br. J. Cancer 2011, 104, 6–11. [Google Scholar] [CrossRef]
- Boffetta, P.; Couto, E.; Wichmann, J.; Ferrari, P.; Trichopoulos, D.; Bueno-de-Mesquita, H.B.; van Duijnhoven, F.J.; Büchner, F.L.; Key, T.; Boeing, H.; et al. Fruit and vegetable intake and overall cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC). J. Natl. Cancer Inst. 2010, 102, 529–537. [Google Scholar] [CrossRef]
- Higdon, J.V.; Delage, B.; Williams, D.E.; Dashwood, R.H. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol. Res. 2007, 55, 224–236. [Google Scholar] [CrossRef]
- Liu, R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef]
- Brandt, K.; Christensen, L.P.; Hansen-Møller, J.; Hansen, S.L.; Haraldsdottir, J.; Jespersen, L.; Purup, S.; Kharazmi, A.; Barkholt, V.; Frøkiær, H.; et al. Health promoting compounds in vegetables and fruits: A systematic approach for identifying plant components with impact on human health. Trends Food Sci. Technol. 2014, 15, 384–393. [Google Scholar] [CrossRef]
- Huang, J.; Weinstein, S.J.; Yu, K.; Männistö, S.; Albanes, D. Serum beta carotene and overall and cause-specific mortality. Circ. Res. 2018, 123, 1339–1349. [Google Scholar] [CrossRef]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L.; Valanis, B.; Williams, J.H.; et al. Effects of a combination of β(beta)-carotene and vitamin A on lung cancer and cardiovascular disease. N. Eng. J. Med. 1996, 334, 1150–1155. [Google Scholar] [CrossRef]
- Greenberg, E.R.; Baron, J.A.; Karagas, M.R.; Stukel, T.A.; Nierenberg, D.W.; Stevens, M.M.; Mandel, J.S.; Haile, R.W. Mortality associated with low plasma concentration of beta carotene and the effect of oral supplementation. JAMA 1996, 275, 699–703. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, M.E.; Carroll, Y.; Corridan, B.; Olmedilla, B.; Granado, F.; Blanco, I.; Van den Berg, H.; Hininger, I.; Rousell, A.M.; Chopra, M.; et al. A European carotenoid database to assess carotenoid intakes and its use in a five country comparative study. Br. J. Nutr. 2001, 85, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Feskanich, D.; Rimm, E.B.; Colditz, G.A.; Speizer, F.E.; Willett, W.C.; Giovannucci, E. Intake of specific carotenoids and risk of lung cancer in 2 prospective US cohorts. Am. J. Clin. Nutr. 2000, 72, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Knekt, P.; Järvinen, R.; Teppo, L.; Aromaa, A.; Seppänen, R. Role of various carotenoids in lung cancer prevention. J. Natl. Cancer Inst. 1999, 91, 182–184. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, H.; Yang, W.; Song, F.; Yan, S.; Wang, C.; Fu, W.; Li, H.; Lyu, C.; Gan, Y.; et al. Is carrot consumption associated with a decreased risk of lung cancer? A meta-analysis of observational studies. Br. J. Nutr. 2019, 122, 488–498. [Google Scholar] [CrossRef]
- Chen, H.; Shao, F.; Zhang, F.; Miao, Q. Association between dietary carrot intake and breast cancer: A meta-analysis. Medicine 2018, 97, e12164. [Google Scholar] [CrossRef]
- Fallahzadeh, H.; Jalali, A.; Momayyezi, M.; Bazm, S. Effect of carrot intake in the prevention of gastric cancer: A meta-analysis. J. Gastric Cancer 2015, 15, 256–261. [Google Scholar] [CrossRef][Green Version]
- Xu, X.; Cheng, Y.; Li, S.; Zhu, Y.; Xu, X.; Zheng, X.; Mao, Q.; Xie, L. Dietary carrot consumption and the risk of prostate cancer. Eur. J. Nutr. 2014, 53, 1615–1623. [Google Scholar] [CrossRef]
- The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Eng. J. Med. 1994, 330, 1029–1035. [Google Scholar] [CrossRef]
- Yang, R.L.; Yan, Z.H.; Lu, Y. Cytotoxic phenylpropanoids from carrot. J. Agric. Food Chem. 2008, 56, 3024–3027. [Google Scholar] [CrossRef]
- Kobaek-Larsen, M.; Baatrup, G.; Notabi, M.K.; El-Houri, R.B.; Pipó-Ollé, E.; Christensen, E.A.; Christensen, L.P. Dietary polyacetylenic oxylipins falcarinol and falcarindiol prevent inflammation and colorectal neoplastic transformation: A mechanistic and dose-response study in a rat model. Nutrients 2019, 11, 2223. [Google Scholar] [CrossRef] [PubMed]
- Kobaek-Larsen, M.; Nielsen, D.S.; Kot, W.; Krych, Ł.; Christensen, L.P.; Baatrup, G. Effect of the dietary polyacetylenes falcarinol and falcarindiol on the gut microbiota composition in a rat model of colorectal cancer. BMC Res. Notes 2018, 11, 411. [Google Scholar] [CrossRef] [PubMed]
- Kobaek-Larsen, M.; El-Houri, R.B.; Christensen, L.P.; Al-Najami, I.; Fretté, X.; Baatrup, G. Dietary polyacetylenes, falcarinol and falcarindiol, isolated from carrots prevents the formation of neoplastic lesions in the colon of azoxymethane-induced rats. Food Funct. 2017, 8, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Zaini, R.G.; Brandt, K.; Clench, M.R.; Le Maitre, C.L. Effects of bioactive compounds from carrots (Daucus carota L.), polyacetylenes, beta-carotene and lutein on human lymphoid leukaemia cells. Anticancer Agents Med. Chem. 2012, 12, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Purup, S.; Larsen, E.; Christensen, L.P. Differential effects of falcarinol and related aliphatic C17-polyacetylenes on intestinal cell proliferation. J. Agric. Food Chem. 2009, 57, 8290–8296. [Google Scholar] [CrossRef]
- Young, J.F.; Duthie, S.J.; Milne, L.; Christensen, L.P.; Duthie, G.G.; Bestwick, C.S. Biphasic effect of falcarinol on Caco-2 cell proliferation, DNA damage, and apoptosis. J. Agric. Food Chem. 2007, 55, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Kobæk-Larsen, M.; Christensen, L.P.; Vach, W.; Ritskes-Hoitinga, J.; Brandt, K. Inhibitory effects of feeding with carrots or (−)-falcarinol on development of azoxymethane-Induced preneoplastic lesions in the rat colon. J. Agric. Food Chem. 2005, 53, 1823–1827. [Google Scholar] [CrossRef]
- Kramer, M.; Mühleis, A.; Conrad, J.; Leitenberger, M.; Beifuss, U.; Carle, R.; Kammerer, D.R. Quantification of polyacetylenes in apiaceous plants by high-performance liquid chromatography coupled with diode array detection. Z. Naturforsch. C 2011, 66, 319–327. [Google Scholar] [CrossRef]
- Zidorn, C.; Johrer, K.; Ganzera, M.; Schubert, B.; Sigmund, E.M.; Mader, J.; Greil, R.; Ellmerer, E.P.; Stuppner, H. Polyacetylenes from the Apiaceae vegetables carrot, celery, fennel, parsley, and parsnip and their cytotoxic activities. J. Agric. Food Chem. 2005, 53, 2518–2523. [Google Scholar] [CrossRef]
- Christensen, L.P.; Brandt, K. Bioactive polyacetylenes in food plants of the Apiaceae family: Occurrence, bioactivity and analysis. J. Pharm. Biomed. Anal. 2006, 41, 683–693. [Google Scholar] [CrossRef]
- Matsunaga, H.; Katano, M.; Yamamoto, H.; Fujito, H.; Mori, M.; Takata, K. Cytotoxic activity of polyacetylene compounds in Panax ginseng C. A. Meyer. Chem. Pharm. Bull. 1990, 38, 3480–3482. [Google Scholar] [CrossRef] [PubMed]
- Um, Y.R.; Kong, C.K.; Lee, J.I.; Kim, Y.A.; Nam, T.J.; Seo, Y. Evaluation of chemical constituents from Glehnia littoralis for antiproliferative activity against HT-29 human colon cancer cells. Process Biochem. 2010, 45, 114–119. [Google Scholar] [CrossRef]
- Metzger, B.T.; Barnes, D.M.; Reed, J.D. Purple carrot (Daucus carota L.) polyacetylenes decrease lipopolysaccharide-induced expression of inflammatory proteins in macrophage and endothelial cells. J. Agric. Food Chem. 2008, 56, 3554–3560. [Google Scholar] [CrossRef] [PubMed]
- Alanko, J.; Kurahashi, Y.; Yoshimoto, T.; Yamamoto, S.; Baba, K. Panaxynol, a polyacetylene compound isolated from oriental medicines, inhibits mammalian lipoxygenases. Biochem. Pharmacol. 1994, 48, 1979–1981. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jeon, J.; Meyerhardt, J.A. Diet and lifestyle in survivors of colorectal cancer. Hematol. Oncol. Clin. N. Am. 2015, 29, 1–27. [Google Scholar] [CrossRef]
- Vogelstein, B.; Kinzler, K.W. The multistep nature of cancer. Trends Genet. 1993, 9, 138–141. [Google Scholar] [CrossRef]
- Lucas, C.; Barnich, N.; Nguyen, H.T.T. Microbiota, inflammation and colorectal cancer. Int. J. Mol. Sci. 2017, 18, 1310. [Google Scholar] [CrossRef]
- Kyrø, C.; Skeie, G.; Loft, S.; Overvad, K.; Christensen, J.; Tjønneland, A.; Olsen, A. Adherence to a healthy Nordic food index is associated with a lower incidence of colorectal cancer in women: The Diet, Cancer and Health cohort study. Br. J. Nutr. 2013, 109, 920–927. [Google Scholar] [CrossRef]
- Leenders, M.; Siersema, P.; Overvad, K.; Tjønneland, A.; Olsen, A.; Boutron-Ruault, M.; Bastide, N.; Fagherazzi, G.; Katzke, V.; Kühn, T.; et al. Subtypes of fruit and vegetables, variety in consumption and risk of colon and rectal cancer in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2015, 137, 2705–2714. [Google Scholar] [CrossRef]
- Gupta, R.A.; Dubois, R.N. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat. Rev. Cancer 2001, 1, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.A.; DuBois, R.N.; Wallace, M.C. New avenues for the prevention of colorectal cancer: Targeting cyclo-oxygenase-2 activity. Best Pract. Res. Clin. Gastroenterol. 2002, 16, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Castellone, M.D.; Teramoto, H.; Gutkind, J.S. Cyclooxygenase-2 and colorectal cancer chemoprevention: The beta-catenin connection. Cancer Res. 2006, 66, 11085–11088. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.L.; Purup, S.; Christensen, L.P. Bioactivity of falcarinol and the influence of processing and storage on its content in carrots (Daucus carota L.). J. Sci. Food Agric. 2003, 83, 1010–1017. [Google Scholar] [CrossRef]
- Christensen, L.P. Aliphatic C17-polyacetylenes of the falcarinol type as potential health promoting compounds in food plants of the Apiaceae family. Recent Pat. Food Nutr. Agric. 2011, 3, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Aguiló-Aguayo, I.; Brunton, N.; Rai, D.K.; Balagueró, E.; Hossain, M.B.; Valverde, J. Polyacetylene levels in carrot juice, effect of pH and thermal processing. Food Chem. 2014, 152, 370–377. [Google Scholar] [CrossRef]
- Link, L.B.; Potter, J.D. Raw versus cooked vegetables and cancer risk. Cancer Epidemiol. Biomarkers Prev. 2004, 13, 1422–1435. [Google Scholar]
- Tjønneland, A.; Olsen, A.; Boll, K.; Stripp, C.; Christensen, J.; Engholm, G.; Overvad, K. Study design, exposure variables, and socioeconomic determinants of participation in Diet, Cancer and Health: A population-based prospective cohort study of 57,053 men and women in Denmark. Scand. J. Public Health 2007, 35, 432–441. [Google Scholar] [CrossRef]
- Overvad, K.; Tjønneland, A.; Haraldsdóttir, J.; Ewertz, M.; Jensen, O.M. Development of a semiquantitative food frequency questionnaire to assess food, energy and nutrient intake in Denmark. Int. J. Epidemiol. 1991, 20, 900–905. [Google Scholar] [CrossRef]
- Tjønneland, A.; Overvad, K.; Haraldsdóttir, J.; Bang, S.; Ewertz, M.; Jensen, O.M. Validation of a semiquantitative food frequency questionnaire developed in Denmark. Int. J. Epidemiol. 1991, 20, 906–912. [Google Scholar] [CrossRef]
- Pedersen, C.B. The Danish Civil Registration System. Scand. J. Public Health 2011, 39 (Suppl. 7), 22–25. [Google Scholar] [CrossRef] [PubMed]
- Gjerstorff, M.L. The Danish Cancer Registry. Scand. J. Public Health 2011, 39 (Suppl. 7), 42–45. [Google Scholar] [CrossRef] [PubMed]
- Lauritsen, J. Foodcalc v.1.3. 2019. Available online: https://github.com/jesperldk/FoodCalc (accessed on 1 December 2019).
- Bach, V.; Kidmose, U.; Kristensen, H.L.; Edelenbos, M. Eating quality of carrots (Daucus carota L.) grown in one conventional and three organic cropping systems over three years. J. Agric. Food Chem. 2015, 63, 9803–9811. [Google Scholar] [CrossRef] [PubMed]
- Dawid, C.; Dunemann, F.; Schwab, W.; Nothnagel, T.; Hofmann, T. Bioactive C17-Polyacetylenes in carrots (Daucus carota L.): Current knowledge and future perspectives. J. Agric. Food Chem. 2015, 63, 9211–9222. [Google Scholar] [CrossRef] [PubMed]
- Sundhedsstyrelsen (Danish Health Authorities). Forebyggelsespakke Alkohol (Prevention package Alcohol). Available online: https://www.sst.dk/da/Udgivelser/2018/Forebyggelsespakke-Alkohol (accessed on 1 December 2019).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.r-project.org (accessed on 1 December 2019).
- Gerds, T.A.; Ozenne, B. Publish: Format Output of Various Routines in a Suitable Way for Reports and Publication. R Package Version. Available online: https://cran.r-project.org/package=Publish (accessed on 2 November 2019).
- Therneau, T. _A Package for Survival Analysis in S_. Version 2.38. Available online: https://cran.r-project.org/package=survival (accessed on 2 November 2019).
- Franceschi, S.; Parpinel, M.; La Vecchia, C.; Favero, A.; Talamini, R.; Negri, E. Role of different types of vegetables and fruit in the prevention of cancer of the colon, rectum, and breast. Epidemiology 1998, 9, 338–341. [Google Scholar] [CrossRef]
- Heydenreuter, W.; Kunold, E.; Sieber, S.A. Alkynol natural products target ALDH2 in cancer cells by irreversible binding to the active site. Chem. Commun. 2015, 51, 15784–15787. [Google Scholar] [CrossRef]
- Kreutzmann, S.; Christensen, L.P.; Edelenbos, M. Investigation of bitterness in carrots (Daucus carota L.) based on quantitative chemical and sensory analyses. LWT - Food Sci. Technol. 2008, 41, 193–205. [Google Scholar] [CrossRef]
- Miller, T.M.; Abdel-Maksoud, M.F.; Crane, L.A.; Marcus, A.C.; Byers, T.E. Effects of social approval bias on self-reported fruit and vegetable consumption: A randomized controlled trial. Nutr. J. 2008, 7, 18. [Google Scholar] [CrossRef]
- Althubaiti, A. Information bias in health research: Definition, pitfalls, and adjustment methods. J. Multidiscip. Healthc. 2016, 9, 211–217. [Google Scholar] [CrossRef]
- Hansen-Møller, J.; Hansen, S.L.; Christensen, L.P.; Jespersen, L.; Brandt, K.; Haraldsdóttir, J. Quantification of polyacetylenes by LC–MS in human plasma after intake of fresh carrot juice (Daucus carota L.). In Proceedings of the Dietary Phytochemicals and Human Health, Salamanca, Spain, 18–20 April 2002; pp. 203–204. [Google Scholar]
- Haraldsdóttir, J.; Jespersen, L.; Hansen-Møller, J.; Hansen, S.L.; Christensen, L.P.; Brandt, K. Recent developments in bioavailability of falcarinol. In Proceedings of the Health Promoting Compounds in Vegetables and Fruits, Karrebæksminde, Denmark, 6–8 November 2002. [Google Scholar]
| Variable | Level | CRC a n = 1889 | No CRC n= 53,986 | Total n = 55,875 | p-Value |
|---|---|---|---|---|---|
| Raw carrot intake | None | 306 (3.9) a | 7610 (96.1) b | 7916 | |
| 0–32 g/day | 1105 (3.5) | 30,440 (96.5) | 31,545 | ||
| Over 32 g/day | 478 (2.9) | 15,936 (97.1) | 16,414 | <0.001 | |
| Gender | Female | 857 (2.9) | 28,373 (97.1) | 29,230 | |
| Male | 1032 (3.9) | 25,613 (96.1) | 26,645 | <0.001 | |
| Smoking | Non-smoker | 586 (3.0) | 18,993 (97.0) | 19,579 | |
| Former smoker | 603 (3.7) | 15,513 (96.3) | 16,116 | ||
| Current smoker | 700 (3.5) | 19,480 (96.5) | 20,180 | <0.001 | |
| NSAID intake | No | 1347 (3.6) | 36,311 (96.4) | 37,658 | |
| Yes | 542 (3.0) | 17,675 (97.0) | 18,217 | <0.001 | |
| Body Mass Index | Normal | 717 (3.0) | 23,386 (97.0) | 24,103 | |
| Low | 16 (3.4) | 459 (96.6) | 475 | ||
| High | 1156 (3.7) | 30,141 (96.3) | 31,297 | <0.001 | |
| Previous cerebral or | No | 1830 (3.4) | 52,278 (96.6) | 54,108 | |
| coronary artery thrombosis | Yes | 59 (3.3) | 1708 (96.7) | 1767 | 0.974 |
| Alcohol intake | Within recommendation | 1006 (3.2) | 30,918 (96.8) | 31,924 | |
| No alcohol | 41 (3.2) | 1254 (96.8) | 1295 | ||
| Over recommendation | 842 (3.7) | 21,814 (96.3) | 22,656 | 0.001 | |
| Age group | 50–54 years | 634 (2.7) | 22,990 (97.3) | 23,624 | |
| 55–59 years | 591 (3.4) | 16,701 (96.6) | 17,292 | ||
| 60–65 years | 664 (4.4) | 14,295 (95.6) | 14,959 | <0.001 | |
| Other root | 1st quartile | 478 (3.4) | 13,457 (96.6) | 13,935 | |
| vegetables | 2nd quartile | 486 (3.5) | 13,481 (96.5) | 13,967 | |
| 3rd quartile | 496 (3.5) | 13,489 (96.5) | 13,985 | ||
| 4th quartile | 429 (3.1) | 13,559 (96.9) | 13,988 | 0.116 | |
| All other vegetables | 1st quartile | 485 (3.5) | 13,444 (96.5) | 13,929 | |
| 2nd quartile | 481 (3.4) | 13,500 (96.6) | 13,981 | ||
| 3rd quartile | 466 (3.3) | 13,491 (96.7) | 13,957 | ||
| 4th quartile | 457 (3.3) | 13,551 (96.7) | 14,008 | 0.738 | |
| METs—h/week | 1st quartile | 498 (3.5) | 13,721 (96.5) | 14,219 | |
| 2nd quartile | 467 (3.3) | 13,783 (96.7) | 14,250 | ||
| 3rd quartile | 466 (3.4) | 13,073 (96.6) | 13,539 | ||
| 4th quartile | 458 (3.3) | 13,409 (96.7) | 13,867 | 0.677 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deding, U.; Baatrup, G.; Christensen, L.P.; Kobaek-Larsen, M. Carrot Intake and Risk of Colorectal Cancer: A Prospective Cohort Study of 57,053 Danes. Nutrients 2020, 12, 332. https://doi.org/10.3390/nu12020332
Deding U, Baatrup G, Christensen LP, Kobaek-Larsen M. Carrot Intake and Risk of Colorectal Cancer: A Prospective Cohort Study of 57,053 Danes. Nutrients. 2020; 12(2):332. https://doi.org/10.3390/nu12020332
Chicago/Turabian StyleDeding, Ulrik, Gunnar Baatrup, Lars Porskjær Christensen, and Morten Kobaek-Larsen. 2020. "Carrot Intake and Risk of Colorectal Cancer: A Prospective Cohort Study of 57,053 Danes" Nutrients 12, no. 2: 332. https://doi.org/10.3390/nu12020332
APA StyleDeding, U., Baatrup, G., Christensen, L. P., & Kobaek-Larsen, M. (2020). Carrot Intake and Risk of Colorectal Cancer: A Prospective Cohort Study of 57,053 Danes. Nutrients, 12(2), 332. https://doi.org/10.3390/nu12020332





