Long-Term Blackcurrant Supplementation Modified Gut Microbiome Profiles in Mice in an Age-Dependent Manner: An Exploratory Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. DNA Extraction PCR Amplification and Sequencing of Taxonomic Marker
2.3. Sequence Data Processing
2.4. Statistical Analysis
3. Results
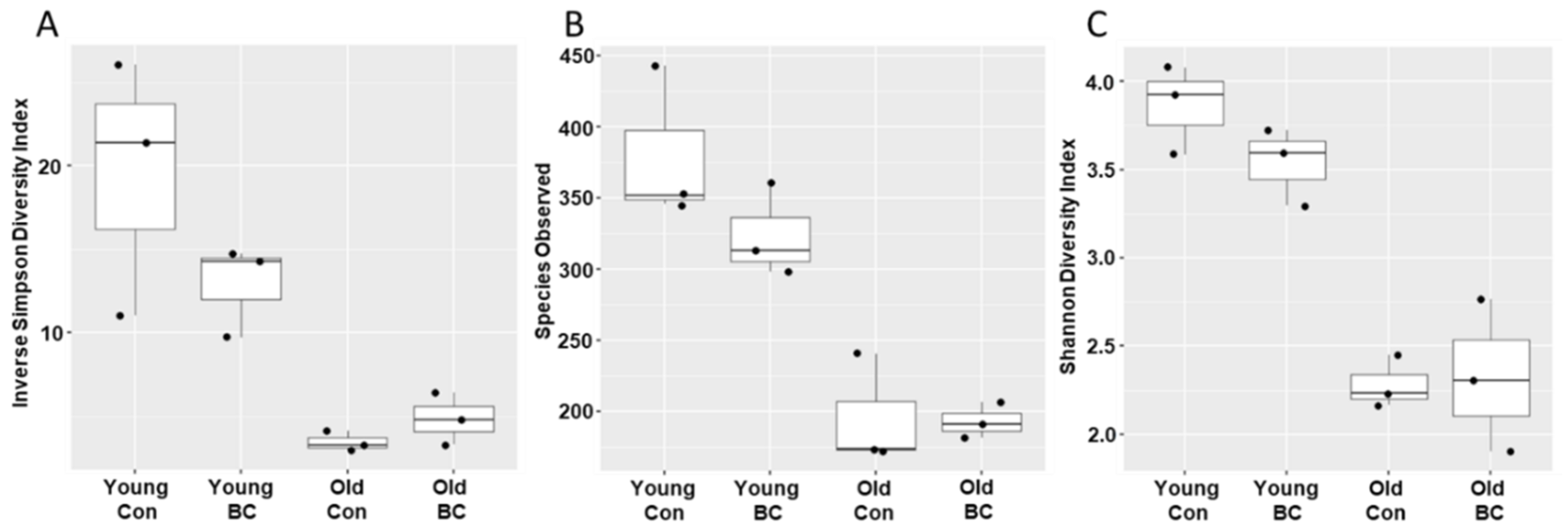
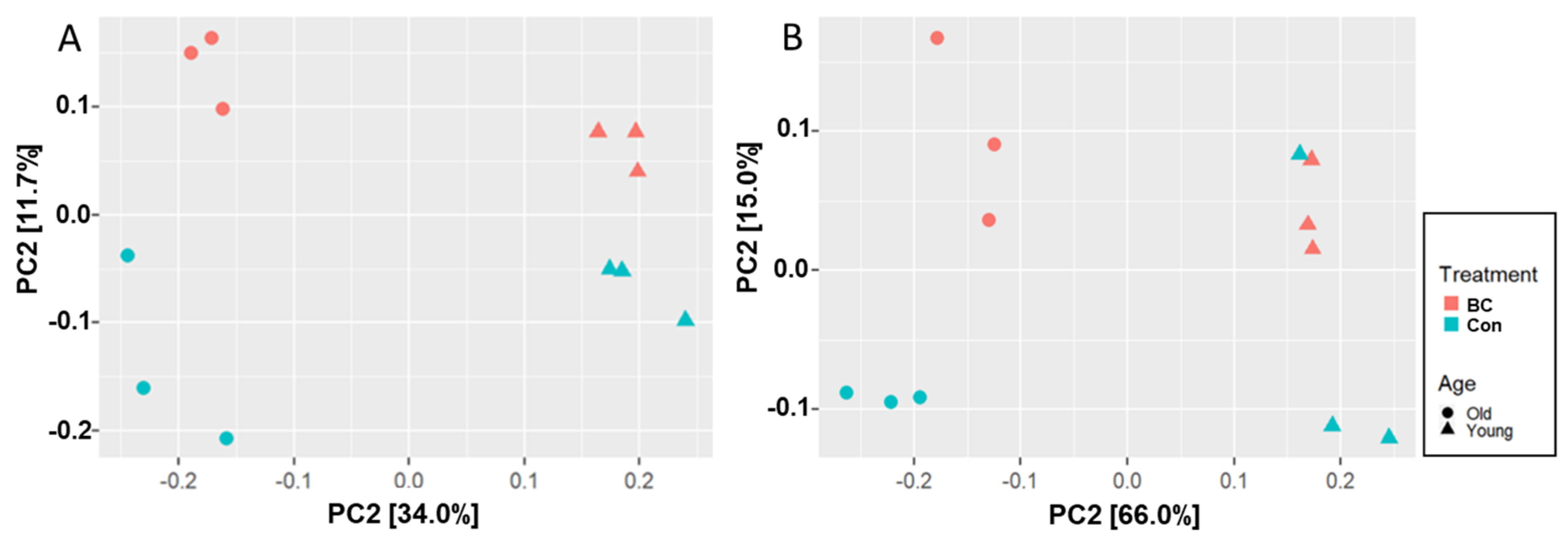
3.1. Age and BC Modified the Gut Microbial Community Composition
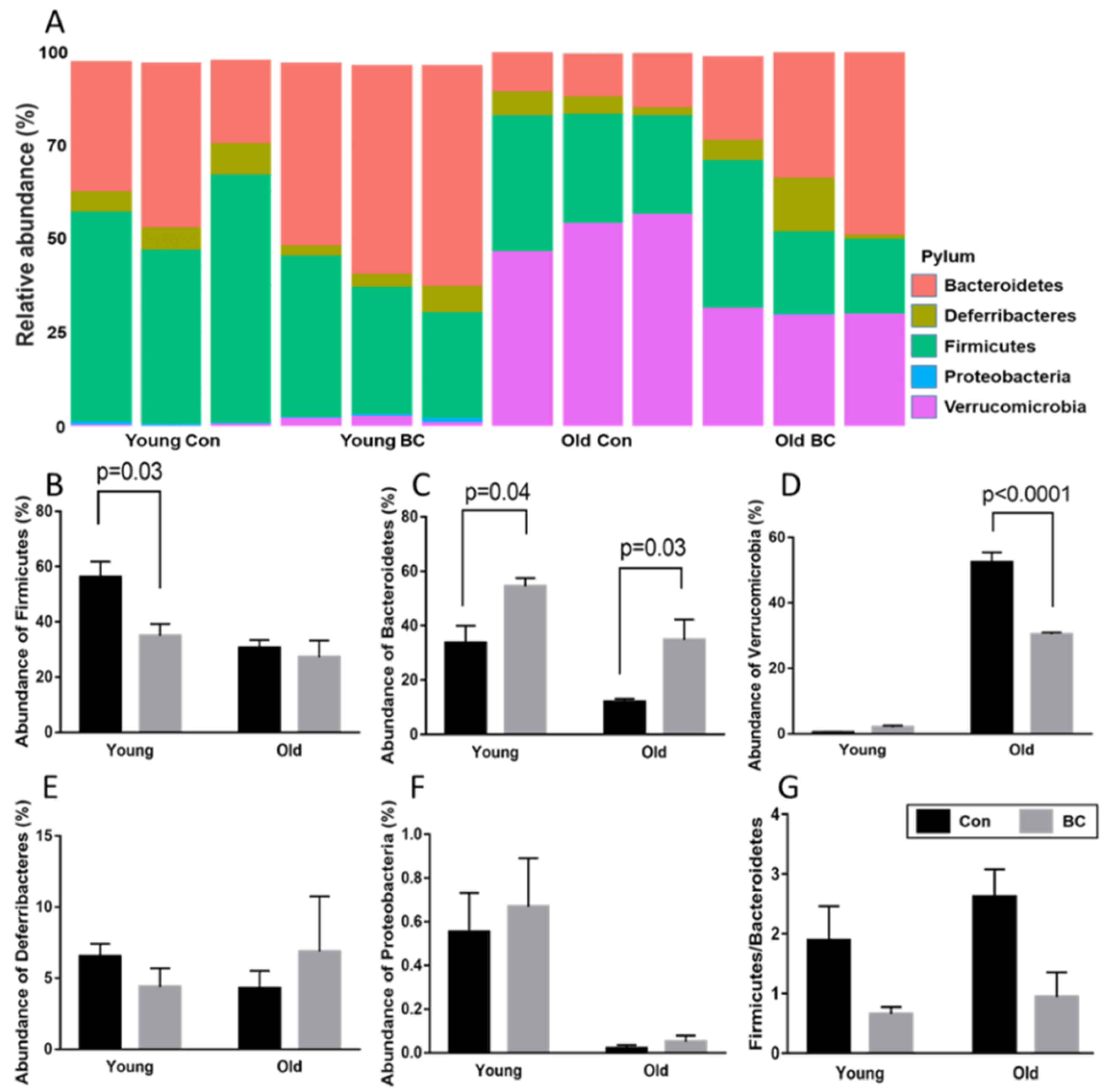
3.2. Age and BC Modified the Phylum Composition in Mice
3.3. Age and BC Modified the Abundance of OTUs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gopalan, A.; Reuben, S.C.; Ahmed, S.; Darvesh, A.S.; Hohmann, J.; Bishayee, A. The health benefits of blackcurrants. Food Funct. 2012, 3, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, S.G.; Park, Y.K.; Ku, C.S.; Pham, T.X.; Wegner, C.J.; Yang, Y.; Koo, S.I.; Chun, O.K.; Lee, J.Y. Blueberry, blackberry, and blackcurrant differentially affect plasma lipids and pro-inflammatory markers in diet-induced obesity mice. Nutr. Res. Pract. 2016, 10, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Puupponen-Pimia, R.; Nohynek, L.; Meier, C.; Kahkonen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Molan, A.-L.; Liu, Z.; Kruger, M. The ability of blackcurrant extracts to positively modulate key markers of gastrointestinal function in rats. World J. Microbiol. Biotechnol. 2010, 26, 1735–1743. [Google Scholar] [CrossRef]
- Lee, S.G.; Vance, T.M.; Nam, T.G.; Kim, D.O.; Koo, S.I.; Chun, O.K. Contribution of Anthocyanin Composition to Total Antioxidant Capacity of Berries. Plant Foods Hum. Nutr. 2015, 70, 427–432. [Google Scholar] [CrossRef]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Faria, A.; Fernandes, I.; Norberto, S.; Mateus, N.; Calhau, C. Interplay between anthocyanins and gut microbiota. J. Agric. Food Chem. 2014, 62, 6898–6902. [Google Scholar] [CrossRef]
- Aura, A.M.; Martin-Lopez, P.; O’Leary, K.A.; Williamson, G.; Oksman-Caldentey, K.M.; Poutanen, K.; Santos-Buelga, C. In vitro metabolism of anthocyanins by human gut microflora. Eur. J. Nutr. 2005, 44, 133–142. [Google Scholar] [CrossRef]
- Eker, M.E.; Aaby, K.; Budic-Leto, I.; Brncic, S.R.; El, S.N.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; Pascual-Teresa, S. A Review of Factors Affecting Anthocyanin Bioavailability: Possible Implications for the Inter-Individual Variability. Foods 2019, 9, 2. [Google Scholar] [CrossRef] [Green Version]
- Rechner, A.R.; Kuhnle, G.; Bremner, P.; Hubbard, G.P.; Moore, K.P.; Rice-Evans, C.A. The metabolic fate of dietary polyphenols in humans. Free Radic. Biol. Med. 2002, 33, 220–235. [Google Scholar] [CrossRef]
- Esposito, D.; Damsud, T.; Wilson, M.; Grace, M.H.; Strauch, R.; Li, X.; Lila, M.A.; Komarnytsky, S. Black Currant Anthocyanins Attenuate Weight Gain and Improve Glucose Metabolism in Diet-Induced Obese Mice with Intact, but Not Disrupted, Gut Microbiome. J. Agric. Food Chem. 2015, 63, 6172–6180. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.; Fang, S.; He, M.; Huang, X.; Yang, H.; Yang, B.; Chen, C.; Huang, L. Age-based dynamic changes of phylogenetic composition and interaction networks of health pig gut microbiome feeding in a uniformed condition. BMC Vet. Res. 2019, 15, 172. [Google Scholar] [CrossRef] [PubMed]
- Bartley, J.M.; Zhou, X.; Kuchel, G.A.; Weinstock, G.M.; Haynes, L. Impact of Age, Caloric Restriction, and Influenza Infection on Mouse Gut Microbiome: An Exploratory Study of the Role of Age-Related Microbiome Changes on Influenza Responses. Front. Immunol. 2017, 8, 1164. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.; Meehan, C.J.; Koenig, J.E.; Dhanani, A.S.; Rose, R.A.; Howlett, S.E.; Beiko, R.G. Microbial shifts in the aging mouse gut. Microbiome 2014, 2, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flurkey, K.; Currer, J.M.; Harrison, D. Mouse models in aging research. In The Mouse in Biomedical Research; Elsevier: Amsterdam, The Netherlands, 2007; pp. 637–672. [Google Scholar]
- Sakaki, J.; Melough, M.; Lee, S.G.; Kalinowski, J.; Koo, S.I.; Lee, S.K.; Chun, O.K. Blackcurrant Supplementation Improves Trabecular Bone Mass in Young but Not Aged Mice. Nutrients 2018, 10, 1671. [Google Scholar] [CrossRef] [Green Version]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [Green Version]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Werlein, H.-D.; Kütemeyer, C.; Schatton, G.; Hubbermann, E.; Schwarz, K. Influence of elderberry and blackcurrant concentrates on the growth of microorganisms. Food Control 2005, 16, 729–733. [Google Scholar] [CrossRef]
- Molan, A.L.; Liu, Z.; Plimmer, G. Evaluation of the effect of blackcurrant products on gut microbiota and on markers of risk for colon cancer in humans. Phytother. Res. 2014, 28, 416–422. [Google Scholar] [CrossRef]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Bjorksten, B.; Engstrand, L.; Jenmalm, M.C. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin. Exp. Allergy 2014, 44, 842–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, J.D.; Parikh, I.; Green, S.J.; Chlipala, G.; Mohney, R.P.; Keaton, M.; Bauer, B.; Hartz, A.M.S.; Lin, A.L. Age Drives Distortion of Brain Metabolic, Vascular and Cognitive Functions, and the Gut Microbiome. Front. Aging Neurosci. 2017, 9, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihajlovski, A.; Dore, J.; Levenez, F.; Alric, M.; Brugere, J.F. Molecular evaluation of the human gut methanogenic archaeal microbiota reveals an age-associated increase of the diversity. Environ. Microbiol. Rep. 2010, 2, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Sylvia, K.E.; Jewell, C.P.; Rendon, N.M.; St John, E.A.; Demas, G.E. Sex-specific modulation of the gut microbiome and behavior in Siberian hamsters. Brain Behav. Immun. 2017, 60, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Org, E.; Mehrabian, M.; Parks, B.W.; Shipkova, P.; Liu, X.; Drake, T.A.; Lusis, A.J. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 2016, 7, 313–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.C.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.P.; et al. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe 2017, 21, 455–466. [Google Scholar] [CrossRef]
- Scott, K.P.; Gratz, S.W.; Sheridan, P.O.; Flint, H.J.; Duncan, S.H. The influence of diet on the gut microbiota. Pharmacol. Res. 2013, 69, 52–60. [Google Scholar] [CrossRef]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef] [Green Version]
- Ley, R.E.; Backhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, Y.; Gao, W.; Wang, B.; Zhao, H.; Zeng, Y.; Ji, Y.; Hao, D. Diversity analysis of gut microbiota in osteoporosis and osteopenia patients. PeerJ 2017, 5, e3450. [Google Scholar] [CrossRef] [Green Version]
- Remely, M.; Aumueller, E.; Jahn, D.; Hippe, B.; Brath, H.; Haslberger, A.G. Microbiota and epigenetic regulation of inflammatory mediators in type 2 diabetes and obesity. Benef. Microbes 2014, 5, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Yang, J.; Hsu, M.; Lee, R.P.; Grojean, E.M.; Ly, A.; Tseng, C.H.; Heber, D.; Li, Z. Decaffeinated green and black tea polyphenols decrease weight gain and alter microbiome populations and function in diet-induced obese mice. Eur. J. Nutr. 2018, 57, 2759–2769. [Google Scholar] [CrossRef] [PubMed]
- George, N.S.; Cheung, L.; Luthria, D.L.; Santin, M.; Dawson, H.D.; Bhagwat, A.A.; Smith, A.D. Pomegranate peel extract alters the microbiome in mice and dysbiosis caused by Citrobacter rodentium infection. Food Sci. Nutr. 2019, 7, 2565–2576. [Google Scholar]
- Kong, F.; Hua, Y.; Zeng, B.; Ning, R.; Li, Y.; Zhao, J. Gut microbiota signatures of longevity. Curr. Biol. 2016, 26, R832–R833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Sparks, J.B.; Karyala, S.V.; Settlage, R.; Luo, X.M. Host adaptive immunity alters gut microbiota. ISME J. 2015, 9, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, B.P.; Klopfleisch, R.; Loh, G.; Blaut, M. Commensal Akkermansia muciniphila exacerbates gut inflammation in Salmonella Typhimurium-infected gnotobiotic mice. PLoS ONE 2013, 8, e74963. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, J.; Ji, K.; Zhang, P. Bamboo shoot fiber prevents obesity in mice by modulating the gut microbiota. Sci. Rep. 2016, 6, 32953. [Google Scholar] [CrossRef]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Shang, Q.; Shan, X.; Cai, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2016, 7, 3224–3232. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, H.; He, S.; Lou, Q.; Yu, M.; Tang, M.; Tu, L. Metabolism and prebiotics activity of anthocyanins from black rice (Oryza sativa L.) in vitro. PLoS ONE 2018, 13, e0195754. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Li, H.; Xu, X.; Li, C.; Zhou, G. The gut microbiota in young and middle-aged rats showed different responses to chicken protein in their diet. BMC Microbiol. 2016, 16, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Nutrition | % (1720 Kj/100g) |
| Carbohydrate | 89.2 |
| Protein | 3.9 |
| Fat | 3.8 |
| Trans Fat | 0.5 |
| Saturated Fat | 0.8 |
| Ash | 1.7 |
| Total anthocyanin composition | % |
| Delphinidin-3-rutinoside | 35–50 |
| Delphinidin-3-glucoside | 5–20 |
| Cyanidin-3-rutinoside | 30–45 |
| Cyanidin-3-glucoside | 3–10 |
| Regulation | Phylum | Class | Order | Family | Genus | OTU | p-Value |
|---|---|---|---|---|---|---|---|
| Decreased | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Lachnospiraceae_unclassified | Otu0045 | 0.0013 |
| Otu0047 | 0.0008 | ||||||
| Otu0059 | 0.0045 | ||||||
| Otu0061 | 0.0046 | ||||||
| Otu0074 | 0.0100 | ||||||
| Otu0089 | 0.0012 | ||||||
| Otu0124 | 0.0012 | ||||||
| Otu0125 | 0.0031 | ||||||
| Otu0201 | 0.0039 | ||||||
| Otu0314 | 0.0072 | ||||||
| Otu0351 | 0.0028 | ||||||
| Otu0353 | 0.0028 | ||||||
| Blautia | Otu0029 | 0.0049 | |||||
| Otu0204 | 0.0036 | ||||||
| Otu0288 | 0.0046 | ||||||
| Acetatifactor | Otu0121 | 0.0016 | |||||
| Coprococcus | Otu0141 | 0.0002 | |||||
| Roseburia | Otu0069 | 0.0080 | |||||
| Ruminococcaceae | Ruminococcaceae_unclassified | Otu0099 | 0.0013 | ||||
| Otu0159 | 0.0007 | ||||||
| Otu0227 | 0.0005 | ||||||
| Oscillibacter | Otu0039 | 0.0003 | |||||
| Otu0211 | 0.0034 | ||||||
| Bacteroidetes | Bacteroidia | Bacteroidales | Bacteroidaceae | Bacteroides | Otu0010 | 0.0000 | |
| Otu0012 | 0.0053 | ||||||
| Otu0014 | 0.0014 | ||||||
| Otu0017 | 0.0024 | ||||||
| Otu0018 | 0.0079 | ||||||
| Otu0073 | 0.0000 | ||||||
| Otu0220 | 0.0085 | ||||||
| Otu0241 | 0.0063 | ||||||
| Porphyromonadaceae | Butyricimonas | Otu0006 | 0.0005 | ||||
| Otu0134 | 0.0008 | ||||||
| Otu0333 | 0.0040 | ||||||
| Rikenellaceae | Alistipes | Otu0130 | 0.0003 | ||||
| Bacteroidetes_unclassified | Bacteroidetes_unclassified | Bacteroidetes_unclassified | Bacteroidetes_unclassified | Otu0070 | 0.0074 | ||
| Cyanobacteria | Melainabacteria | Gastranaerophilales | Gastranaerophilales_unclassified | Gastranaerophilales_unclassified | Otu0020 | 0.0001 | |
| Clostridiales_unclassified | Clostridiales_unclassified | Otu0064 | 0.0036 | ||||
| Proteobacteria | Deltaproteobacteria | Desulfovibrionales | Desulfovibrionaceae | Desulfovibrio | Otu0184 | 0.0028 | |
| Tenericutes | Mollicutes | RF9 | RF9_unclassified | RF9_unclassified | Otu0181 | 0.0043 | |
| Increased | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Lachnospiraceae_unclassified | Otu0036 | 0.0092 |
| Otu0156 | 0.0024 | ||||||
| Ruminococcaceae | Anaerotruncus | Otu0114 | 0.0001 | ||||
| Erysipelotrichia | Erysipelotrichales | Erysipelotrichaceae | Incertae_Sedis | Otu0057 | 0.0007 | ||
| Bacteroidetes | Bacteroidia | Bacteroidales | Rikenellaceae | Alistipes | Otu0013 | 0.0027 | |
| Verrucomicrobia | Verrucomicrobiae | Verrucomicrobiales | Verrucomicrobiaceae | Akkermansia | Otu0127 | 0.0029 |
| Regulation | Phylum | Class | Order | Family | Genus | OTU | p-Value |
|---|---|---|---|---|---|---|---|
| Decreased | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Lachnospiraceae_unclassified | Otu0053 | 0.0027 |
| Acetatifactor | Otu0058 | 0.0072 | |||||
| Blautia | Otu0269 | 0.0012 | |||||
| Ruminococcaceae | Incertae_Sedis | Otu0340 | 0.0028 | ||||
| Increased | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Lachnospiraceae_unclassified | Otu0265 | 0.0068 |
| Lachnospiraceae_unclassified | Otu0156 | 0.0016 | |||||
| Roseburia | Otu0142 | 0.0073 | |||||
| Marvinbryantia | Otu0254 | 0.002 |
| Phylum | Class | Order | Family | Genus | OTU | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| Age | BC | Age * BC | ||||||
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Lachnospiraceae_unclassified | Otu0025 | 0.0004 | 0.0006 | 0.0004 |
| Otu0119 | 0.0032 | 0.0037 | 0.0037 | |||||
| Otu0123 | 0.0031 | 0.0018 | 0.0031 | |||||
| Otu0143 | 0.2415 | 0.0578 | 0.0007 | |||||
| Otu0217 | 0.6811 | 0.4186 | 0.0092 | |||||
| Otu0290 | 0.0085 | 0.0085 | 0.0085 | |||||
| Otu0332 | 0.0011 | 0.0011 | 0.0011 | |||||
| Otu0433 | 0 | 0 | 0 | |||||
| Incertae_Sedis | Otu0081 | 0.0446 | 0.0446 | 0.0036 | ||||
| Otu0219 | 1E-05 | 0.0002 | 0.0002 | |||||
| Otu0320 | 0.0085 | 0.0085 | 0.0085 | |||||
| Blautia | Otu0050 | 0.0046 | 0.0017 | 0.0019 | ||||
| Otu0190 | 0.0001 | 2E-05 | 0.0001 | |||||
| Roseburia | Otu0118 | 0.0065 | 0.0026 | 0.0082 | ||||
| Ruminococcaceae | Anaerotruncus | Otu0126 | 0.0001 | 0.0011 | 0.003 | |||
| Intestinimonas | Otu0152 | 0.0006 | 0.009 | 0.009 | ||||
| Oscillibacter | Otu0221 | 0.0667 | 0.0222 | 0.0028 | ||||
| Pseudoflavonifractor | Otu0092 | 0.1241 | 0.1149 | 0.0066 | ||||
| vadinBB60 | vadinBB60_unclassified | Otu0323 | 0.0805 | 0.0805 | 0.004 | |||
| Verrucomicrobia | Verrucomicrobiae | Verrucomicrobiales | Verrucomicrobiaceae | Akkermansia | Otu0001 | 0 | 0.0002 | 7E-05 |
| Bacteroidetes | Bacteroidia | Bacteroidales | Bacteroidaceae | Bacteroides | Otu0376 | 0.004 | 0.004 | 0.004 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, L.; Lee, S.G.; Melough, M.M.; Sakaki, J.R.; Maas, K.R.; Koo, S.I.; Chun, O.K. Long-Term Blackcurrant Supplementation Modified Gut Microbiome Profiles in Mice in an Age-Dependent Manner: An Exploratory Study. Nutrients 2020, 12, 290. https://doi.org/10.3390/nu12020290
Cao L, Lee SG, Melough MM, Sakaki JR, Maas KR, Koo SI, Chun OK. Long-Term Blackcurrant Supplementation Modified Gut Microbiome Profiles in Mice in an Age-Dependent Manner: An Exploratory Study. Nutrients. 2020; 12(2):290. https://doi.org/10.3390/nu12020290
Chicago/Turabian StyleCao, Lei, Sang Gil Lee, Melissa M. Melough, Junichi R. Sakaki, Kendra R. Maas, Sung I. Koo, and Ock K. Chun. 2020. "Long-Term Blackcurrant Supplementation Modified Gut Microbiome Profiles in Mice in an Age-Dependent Manner: An Exploratory Study" Nutrients 12, no. 2: 290. https://doi.org/10.3390/nu12020290
APA StyleCao, L., Lee, S. G., Melough, M. M., Sakaki, J. R., Maas, K. R., Koo, S. I., & Chun, O. K. (2020). Long-Term Blackcurrant Supplementation Modified Gut Microbiome Profiles in Mice in an Age-Dependent Manner: An Exploratory Study. Nutrients, 12(2), 290. https://doi.org/10.3390/nu12020290







