The Association between Purine-Rich Food Intake and Hyperuricemia: A Cross-Sectional Study in Chinese Adult Residents
Abstract
1. Introduction
2. Materials and Methods
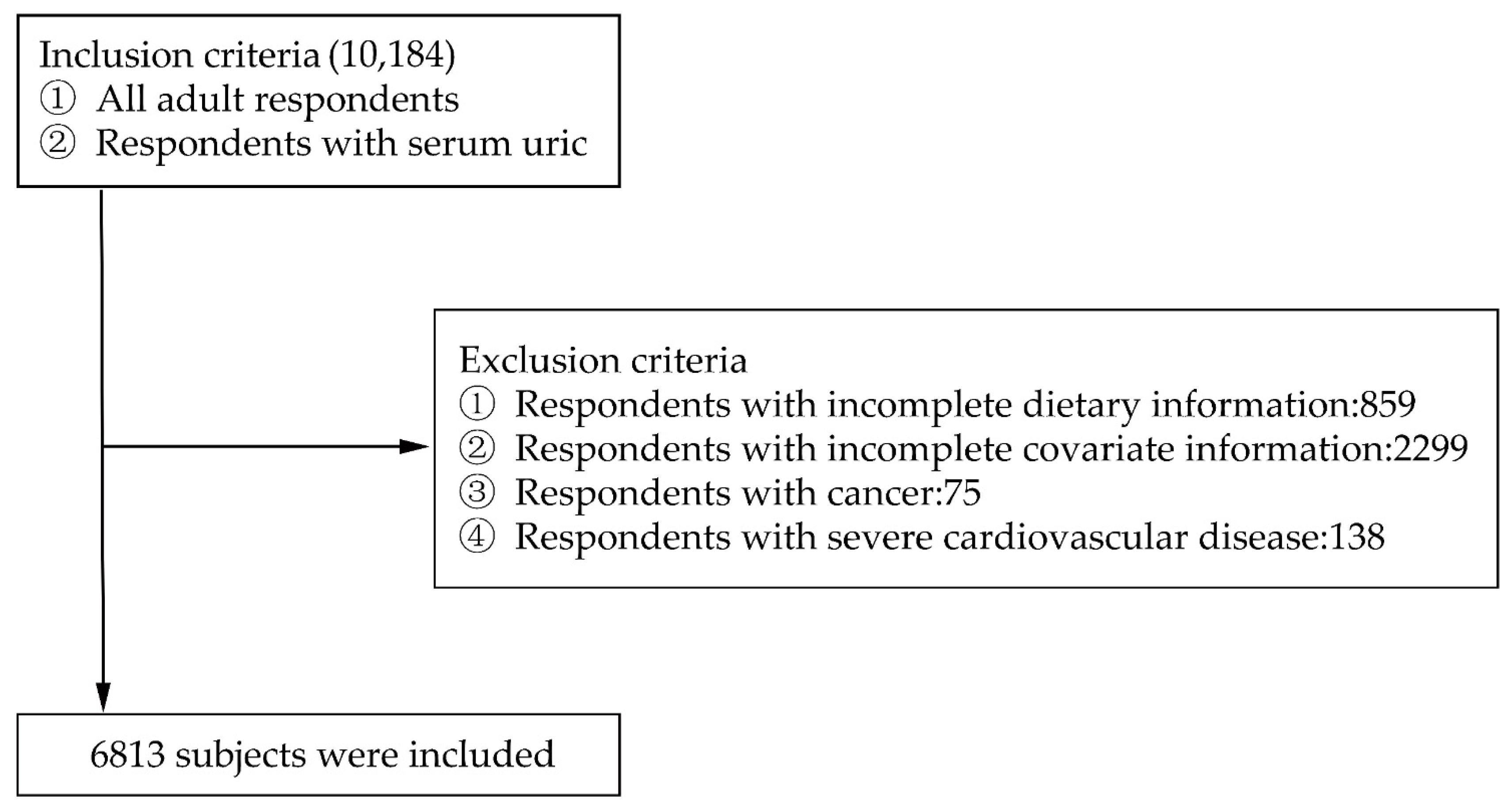
2.1. Data Collection and Samples
2.2. Definition of Hyperuricemia
2.3. Definition and Evaluation of Purine-Rich Food
2.4. Assessment of Covariates
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. The Association of Dietary Purine-Rich Food Intake and Hyperuricemia
3.3. Interaction of the Effect of Animal-Derived Food Intake on Hyperuricemia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, Y.; Sun, J.; Zhang, P.; Zhong, F.; Cai, J.; Ma, A. Association of dietary fiber intake with hyperuricemia in U.S. adults. Food Funct. 2019, 10, 4932–4940. [Google Scholar] [CrossRef]
- Cea, S.L.; Rothenbacher, D.; Choi, H.K.; Garcia, R.L. Contemporary epidemiology of gout in the UK general population. Arthritis Res. Ther. 2011, 13, R39. [Google Scholar]
- Choi, H.K.; Mount, D.B.; Reginato, A.M. Pathogenesis of gout. Ann. Intern. Med. 2005, 143, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Ford, E.S.; Li, C.; Curhan, G. Prevalence of the metabolic syndrome in patients with gout: The Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007, 57, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Annemans, L.; Spaepen, E.; Gaskin, M.; Bonnemaire, M.; Malier, V.; Gilbert, T.; Nuki, G. Gout in the UK and Germany: Prevalence, comorbidities and management in general practice 2000-2005. Ann. Rheum. Dis. 2008, 67, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Kang, D.H.; Feig, D.; Kivlighn, S.; Kanellis, J.; Watanabe, S.; Tuttle, K.R.; Rodriguez-Iturbe, B.; Herrera-Acosta, J.; Mazzali, M. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension 2003, 41, 1183–1190. [Google Scholar] [CrossRef]
- Wang, J.; Qin, T.; Chen, J.; Li, Y.; Wang, L.; Huang, H.; Li, J. Hyperuricemia and risk of incident hypertension: A systematic review and meta-analysis of observational studies. PLoS ONE 2014, 9, e114259. [Google Scholar] [CrossRef]
- Chang, H.Y.; Tung, C.W.; Lee, P.H.; Lei, C.C.; Hsu, Y.C.; Chang, H.H.; Yang, H.F.; Lu, L.C.; Jong, M.C.; Chen, C.Y.; et al. Hyperuricemia as an independent risk factor of chronic kidney disease in middle-aged and elderly population. Am. J. Med. Sci. 2010, 339, 509–515. [Google Scholar] [CrossRef]
- Borghi, C.; Rosei, E.A.; Bardin, T.; Dawson, J.; Dominiczak, A.; Kielstein, J.T.; Manolis, A.J.; Perez-Ruiz, F.; Mancia, G. Serum uric acid and the risk of cardiovascular and renal disease. J. Hypertens. 2015, 33, 1729–1741. [Google Scholar] [CrossRef]
- Nakanishi, N.; Okamoto, M.; Yoshida, H.; Matsuo, Y.; Suzuki, K.; Tatara, K. Serum uric acid and risk for development of hypertension and impaired fasting glucose or Type II diabetes in Japanese male office workers. Eur. J. Epidemiol. 2003, 18, 523–530. [Google Scholar] [CrossRef]
- Lu, J.; Hou, X.; Yuan, X.; Cui, L.; Liu, Z.; Li, X.; Ma, L.; Cheng, X.; Xin, Y.; Wang, C.; et al. Knockout of the urate oxidase gene provides a stable mouse model of hyperuricemia associated with metabolic disorders. Kidney Int. 2018, 93, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hou, W.; Zhang, X.; Hu, L.; Tang, Z. Hyperuricemia and risk of stroke: A systematic review and meta-analysis of prospective studies. Atherosclerosis 2014, 232, 265–270. [Google Scholar] [CrossRef]
- De Oliveira, E.P.; Burini, R.C. High plasma uric acid concentration: Causes and consequences. Diabetol. Metab. Syndr. 2012, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, D.; Unwin, R. The pathophysiology of hyperuricaemia and its possible relationship to cardiovascular disease, morbidity and mortality. BMC Nephrol 14 (2013) 164Gustafsson, D.; Unwin, R. The pathophysiology of hyperuricaemia and its possible relationship to cardiovascular disease, morbidity and mortality. BMC Nephrol. 2013, 14, 164. [Google Scholar]
- Tsumuraya, Y.; Hirayama, T.; Tozuka, E.; Furuta, W.; Utsugi, S.; Tsuchiya, A.; Hishida, A.; Kumagai, H. Impact of hyperuricaemia on the chronic kidney disease-associated risk factors in a community-based population. Nephrology (Carlton) 2015, 20, 399–404. [Google Scholar] [CrossRef]
- Yan, D.; Tu, Y.; Jiang, F.; Wang, J.; Zhang, R.; Sun, X.; Wang, T.; Wang, S.; Bao, Y.; Hu, C.; et al. Uric Acid is independently associated with diabetic kidney disease: A cross-sectional study in a Chinese population. PLoS ONE 2015, 10, e0129797. [Google Scholar] [CrossRef]
- Silva, M.; Diniz, M.; Coelho, C.G.; Vidigal, P.G.; Telles, R.W.; Barreto, S.M. Intake of selected foods and beverages and serum uric acid levels in adults: ELSA-Brasil (2008-2010). Public Health Nutr. 2020, 23, 506–514. [Google Scholar] [CrossRef]
- Trifiro, G.; Morabito, P.; Cavagna, L.; Ferrajolo, C.; Pecchioli, S.; Simonetti, M.; Bianchini, E.; Medea, G.; Cricelli, C.; Caputi, A.P.; et al. Epidemiology of gout and hyperuricaemia in Italy during the years 2005–2009: A nationwide population-based study. Ann. Rheum. Dis. 2013, 72, 694–700. [Google Scholar] [CrossRef]
- Zhu, Y.; Pandya, B.J.; Choi, H.K. Prevalence of gout and hyperuricemia in the US general population: The National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011, 63, 3136–3141. [Google Scholar] [CrossRef]
- Guan, S.; Tang, Z.; Fang, X.; Wu, X.; Liu, H.; Wang, C.; Hou, C. Prevalence of hyperuricemia among Beijing post-menopausal women in 10 years. Arch. Gerontol. Geriatr. 2016, 64, 162–166. [Google Scholar] [CrossRef]
- Kramer, H.M.; Curhan, G. The association between gout and nephrolithiasis: The National Health and Nutrition Examination Survey III, 1988–1994. Am. J. Kidney Dis. 2002, 40, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Hak, A.E.; Curhan, G.C.; Grodstein, F.; Choi, H.K. Menopause, postmenopausal hormone use and risk of incident gout. Ann. Rheum. Dis. 2010, 69, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Puig, J.G.; Martinez, M.A.; Mora, M.; Fraile, J.M.; Montoya, F.; Torres, R.J. Serum urate, metabolic syndrome, and cardiovascular risk factors. A population-based study. Nucleosides Nucleotides Nucleic Acids 2008, 27, 620–623. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.M.; Wang, Y.L.; Liu, B.C. Prevalence of hyperuricemia among Chinese adults: A national cross-sectional survey using multistage, stratified sampling. J. Nephrol. 2014, 27, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Han, C.; Wu, D.; Xia, X.; Gu, J.; Guan, H.; Shan, Z.; Teng, W. Prevalence of Hyperuricemia and Gout in Mainland China from 2000 to 2014: A Systematic Review and Meta-Analysis. Biomed. Res. Int. 2015, 2015, 762820. [Google Scholar]
- Wang, W.; Zhang, D.; Xu, C.; Wu, Y.; Duan, H.; Li, S.; Tan, Q. Heritability and Genome-Wide Association Analyses of Serum Uric Acid in Middle and Old-Aged Chinese Twins. Front. Endocrinol. (Lausanne) 2018, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.; Ye, H.; Guan, H.; Guo, Y.; Li, X.; Li, M.; Yang, F.; Pan, H.; Yang, Y. Analysis on Purine in Different Kinds of Fresh Fungi and Dried Fungi. Food Nutr. China 2014, 20, 62–64. [Google Scholar]
- Zhang, Y.; Wang, D.; Guan, H.; Guo, Y.; Li, M.; Pan, H. Study on the Purine Content in Common Dried Legumes and Legume Products. Food Nutr. China 2014, 20, 61–63. [Google Scholar]
- Pan, H.; Rong, S.; Zou, L.; Wang, Z.; Yang, Y. The contents of purine in common animal foods in China. Acta Nutr. Sin. 2012, 34, 74–78. [Google Scholar]
- Rong, S.; Zou, L.; Wang, Z.; Pan, H.; Yang, Y. Purine in common plant food in China. J. Hyg. Res. 2012, 41, 92–95. [Google Scholar]
- Fam, A.G. Gout, diet, and the insulin resistance syndrome. J. Rheumatol. 2002, 29, 1350–1355. [Google Scholar] [PubMed]
- Choi, H.K.; Atkinson, K.; Karlson, E.W.; Willett, W.; Curhan, G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N. Engl. J. Med. 2004, 350, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Liu, S.; Curhan, G. Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: The Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2005, 52, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Villegas, R.; Xiang, Y.B.; Elasy, T.; Xu, W.H.; Cai, H.; Cai, Q.; Linton, M.F.; Fazio, S.; Zheng, W.; Shu, X.O. Purine-rich foods, protein intake, and the prevalence of hyperuricemia: The Shanghai Men’s Health Study. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Bardin, T.; Richette, P. Definition of hyperuricemia and gouty conditions. Curr. Opin. Rheumatol. 2014, 26, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J. Interpretation of “consensus of multidisciplinary experts on diagnosis and treatment of hyperuricemia related diseases in China”. China Med. News 2017, 32, 22. [Google Scholar]
- Yu, K.H.; See, L.C.; Huang, Y.C.; Yang, C.H.; Sun, J.H. Dietary factors associated with hyperuricemia in adults. Semin. Arthritis Rheum. 2008, 37, 243–250. [Google Scholar] [CrossRef]
- Qiu, L.; Cheng, X.Q.; Wu, J.; Liu, J.T.; Xu, T.; Ding, H.T.; Liu, Y.H.; Ge, Z.M.; Wang, Y.J.; Han, H.J.; et al. Prevalence of hyperuricemia and its related risk factors in healthy adults from Northern and Northeastern Chinese provinces. BMC Public Health 2013, 13, 664. [Google Scholar] [CrossRef]
- Miao, Z.; Li, C.; Chen, Y.; Zhao, S.; Wang, Y.; Wang, Z.; Chen, X.; Xu, F.; Wang, F.; Sun, R.; et al. Dietary and lifestyle changes associated with high prevalence of hyperuricemia and gout in the Shandong coastal cities of Eastern China. J. Rheumatol. 2008, 35, 1859–1864. [Google Scholar]
- Lyu, L.C.; Hsu, C.Y.; Yeh, C.Y.; Lee, M.S.; Huang, S.H.; Chen, C.L. A case-control study of the association of diet and obesity with gout in Taiwan. Am. J. Clin. Nutr. 2003, 78, 690–701. [Google Scholar] [CrossRef]
- Zgaga, L.; Theodoratou, E.; Kyle, J.; Farrington, S.M.; Agakov, F.; Tenesa, A.; Walker, M.; McNeill, G.; Wright, A.F.; Rudan, I.; et al. The association of dietary intake of purine-rich vegetables, sugar-sweetened beverages and dairy with plasma urate, in a cross-sectional study. PLoS ONE 2012, 7, e38123. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Tomas, N.; Mena-Sanchez, G.; Diaz-Lopez, A.; Martinez-Gonzalez, M.A.; Babio, N.; Corella, D.; Freixer, G.; Romaguera, D.; Vioque, J.; Alonso-Gomez, A.M.; et al. Cross-sectional association between non-soy legume consumption, serum uric acid and hyperuricemia: The PREDIMED-Plus study. Eur. J. Nutr. 2020, 59, 2195–2206. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.K.; Fung, T.T.; Lu, N.; Keller, S.F.; Curhan, G.C.; Choi, H.K. The Dietary Approaches to Stop Hypertension (DASH) diet, Western diet, and risk of gout in men: Prospective cohort study. BMJ 2017, 357, j1794. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, T.; Zhao, H.N.; Yue, W.W.; Yu, H.P.; Liu, C.X.; Yin, J.; Jia, R.Y.; Nie, H.W. The prevalence of hyperuricemia in China: A meta-analysis. BMC Public Health 2011, 11, 832. [Google Scholar]
- Kuo, C.F.; Grainge, M.J.; Zhang, W.; Doherty, M. Global epidemiology of gout: Prevalence, incidence and risk factors. Nat. Rev. Rheumatol. 2015, 11, 649–662. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, X.; Wu, W.; Zhang, D. A meta-analysis of alcohol consumption and the risk of gout. Clin. Rheumatol. 2013, 32, 1641–1648. [Google Scholar] [CrossRef]
- Li, Y.; Stamler, J.; Xiao, Z.; Folsom, A.; Tao, S.; Zhang, H. Serum uric acid and its correlates in Chinese adult populations, urban and rural, of Beijing. The PRC-USA Collaborative Study in Cardiovascular and Cardiopulmonary Epidemiology. Int. J. Epidemiol. 1997, 26, 288–296. [Google Scholar] [CrossRef][Green Version]
- Woo, J.; Swaminathan, R.; Cockram, C.; Lau, E.; Chan, A. Association between serum uric acid and some cardiovascular risk factors in a Chinese population. Postgrad. Med. J. 1994, 70, 486–491. [Google Scholar] [CrossRef][Green Version]
| Variable | Total (Number, %) | Hyperuricemia (Number, %) | Nonhyperuricemia (Number, %) | χ2 | p |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 3218 (47.23) | 676 (60.85) | 2542 (44.58) | 98.700 | <0.0001 |
| Female | 3595 (52.77) | 435 (39.15) | 3160 (55.42) | ||
| Age (years) | |||||
| <45 | 2370 (34.79) | 311 (27.99) | 2059 (36.11) | 39.581 | <0.0001 |
| 45–59 | 2479 (36.39) | 403 (36.28) | 2076 (36.41) | ||
| ≥60 | 1964 (28.83) | 397 (35.73) | 1567 (27.48) | ||
| Region | |||||
| Urban areas | 2227 (32.69) | 442 (39.78) | 1785 (31.30) | 30.383 | <0.0001 |
| Rural areas | 4586 (67.31) | 669 (60.22) | 3917 (68.70) | ||
| Education level | |||||
| None | 1588 (23.31) | 260 (23.40) | 1328 (23.29) | 11.762 | 0.0382 |
| Grad from primary | 1315 (19.30) | 203 (18.27) | 1112 (19.50) | ||
| Lower middle school degree | 2278 (33.43) | 345 (31.05) | 1933 (33.90) | ||
| Upper middle school degree | 818 (12.01) | 140 (12.60) | 678 (11.89) | ||
| Technical or vocational degree | 494 (7.25) | 97 (8.73) | 397 (6.96) | ||
| University or college degree | 320 (4.70) | 66 (5.94) | 254 (4.46) | ||
| BMI (kg/m2) | |||||
| <18.5 | 439 (6.44) | 34 (3.06) | 405 (7.10) | 158.564 | <0.0001 |
| ≥18.5 & <24 | 3643 (53.47) | 447 (40.23) | 3196 (56.05) | ||
| ≥24 | 2731 (40.09) | 630 (56.71) | 2101 (36.85) | ||
| Alcohol consumption | |||||
| No -drinker (1 year or above) | 4539 (66.62) | 631 (56.80) | 3908 (68.54) | 57.649 | <0.0001 |
| Current drinker | 2274 (33.38) | 480 (43.20) | 1794 (31.46) | ||
| Hypertension | |||||
| Patient | 2095 (30.75) | 509 (45.81) | 1586 (27.81) | 141.470 | <0.0001 |
| Nonpatient | 4718 (69.25) | 602 (54.19) | 4116 (72.19) | ||
| Diabetes | |||||
| Patient | 210 (3.08) | 56 (5.04) | 154 (2.70) | 17.039 | <0.0001 |
| Nonpatient | 6603 (96.92) | 1055 (94.96) | 5548 (97.30) | ||
| Smoking status | |||||
| Nonsmoker | 4685 (68.77) | 675 (60.76) | 4010 (70.33) | 39.649 | <0.0001 |
| Smoker | 2128 (31.23) | 436 (39.24) | 1692 (29.67) |
| Dietary Factors | Intake (Averages, g/Day) | p | ||
|---|---|---|---|---|
| All | Hyperuricemia | Nonhyperuricemia | ||
| Purine-rich food | ||||
| Red meat | 83.92 | 98.03 | 81.17 | <0.0001 |
| Legumes | 62.37 | 70.79 | 60.72 | 0.0002 |
| Seafood | 38.31 | 47.80 | 36.46 | <0.0001 |
| Poultry | 18.34 | 21.70 | 17.69 | 0.0017 |
| Purine-rich vegetables | 11.12 | 9.97 | 11.34 | 0.3695 |
| Purine-rich fungi | 2.32 | 3.14 | 2.16 | 0.0083 |
| Other food | ||||
| Dark vegetables | 118.88 | 122.77 | 118.13 | 0.0091 |
| Other vegetables | 212.18 | 212.87 | 212.04 | 0.5814 |
| Fruits | 52.54 | 53.84 | 52.29 | 0.2435 |
| Refined grains | 369.95 | 359.74 | 371.93 | 0.0272 |
| Wholegrains | 15.71 | 14.27 | 15.99 | 0.0670 |
| Tubers | 28.89 | 26.73 | 29.31 | 0.1233 |
| Variable | Intake (g/Day) | OR, 95% CI (10−1g/Day) | p | |
|---|---|---|---|---|
| Hyperuricemia | Nonhyperuricemia | |||
| Seafood | 47.80 | 36.46 | 1.022 (1.013, 1.032) | <0.0001 |
| Purine-rich fungi | 3.14 | 2.16 | 1.039 (0.990, 1.090) | 0.1202 |
| Legumes | 70.79 | 60.72 | 1.014 (1.006, 1.022) | 0.0003 |
| Red meat | 98.03 | 81.17 | 1.028 (1.019, 1.036) | <0.0001 |
| Poultry | 21.70 | 17.69 | 1.019 (1.004, 1.035) | 0.0156 |
| Variables | OR, 95% CI (10−1g/Day) | p |
|---|---|---|
| Animal-derived food | ||
| Model1 | 1.025 (1.019, 1.030) | <0.0001 |
| Model2 | 1.025 (1.019, 1.031) | <0.0001 |
| Model3 | 1.024 (1.018, 1.030) | <0.0001 |
| Legumes | ||
| Model1 | 1.014 (1.007, 1.022) | 0.0003 |
| Model2 | 1.015 (1.008, 1.023) | <0.0001 |
| Model3 | 1.011 (1.003, 1.019) | 0.0080 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aihemaitijiang, S.; Zhang, Y.; Zhang, L.; Yang, J.; Ye, C.; Halimulati, M.; Zhang, W.; Zhang, Z. The Association between Purine-Rich Food Intake and Hyperuricemia: A Cross-Sectional Study in Chinese Adult Residents. Nutrients 2020, 12, 3835. https://doi.org/10.3390/nu12123835
Aihemaitijiang S, Zhang Y, Zhang L, Yang J, Ye C, Halimulati M, Zhang W, Zhang Z. The Association between Purine-Rich Food Intake and Hyperuricemia: A Cross-Sectional Study in Chinese Adult Residents. Nutrients. 2020; 12(12):3835. https://doi.org/10.3390/nu12123835
Chicago/Turabian StyleAihemaitijiang, Sumiya, Yaqin Zhang, Li Zhang, Jiao Yang, Chen Ye, Mairepaiti Halimulati, Wei Zhang, and Zhaofeng Zhang. 2020. "The Association between Purine-Rich Food Intake and Hyperuricemia: A Cross-Sectional Study in Chinese Adult Residents" Nutrients 12, no. 12: 3835. https://doi.org/10.3390/nu12123835
APA StyleAihemaitijiang, S., Zhang, Y., Zhang, L., Yang, J., Ye, C., Halimulati, M., Zhang, W., & Zhang, Z. (2020). The Association between Purine-Rich Food Intake and Hyperuricemia: A Cross-Sectional Study in Chinese Adult Residents. Nutrients, 12(12), 3835. https://doi.org/10.3390/nu12123835







