Role of Gut Microbiota on Onset and Progression of Microvascular Complications of Type 2 Diabetes (T2DM)
Abstract
1. Introduction
2. Materials and Methods
3. Gut Microbiota and T2DM
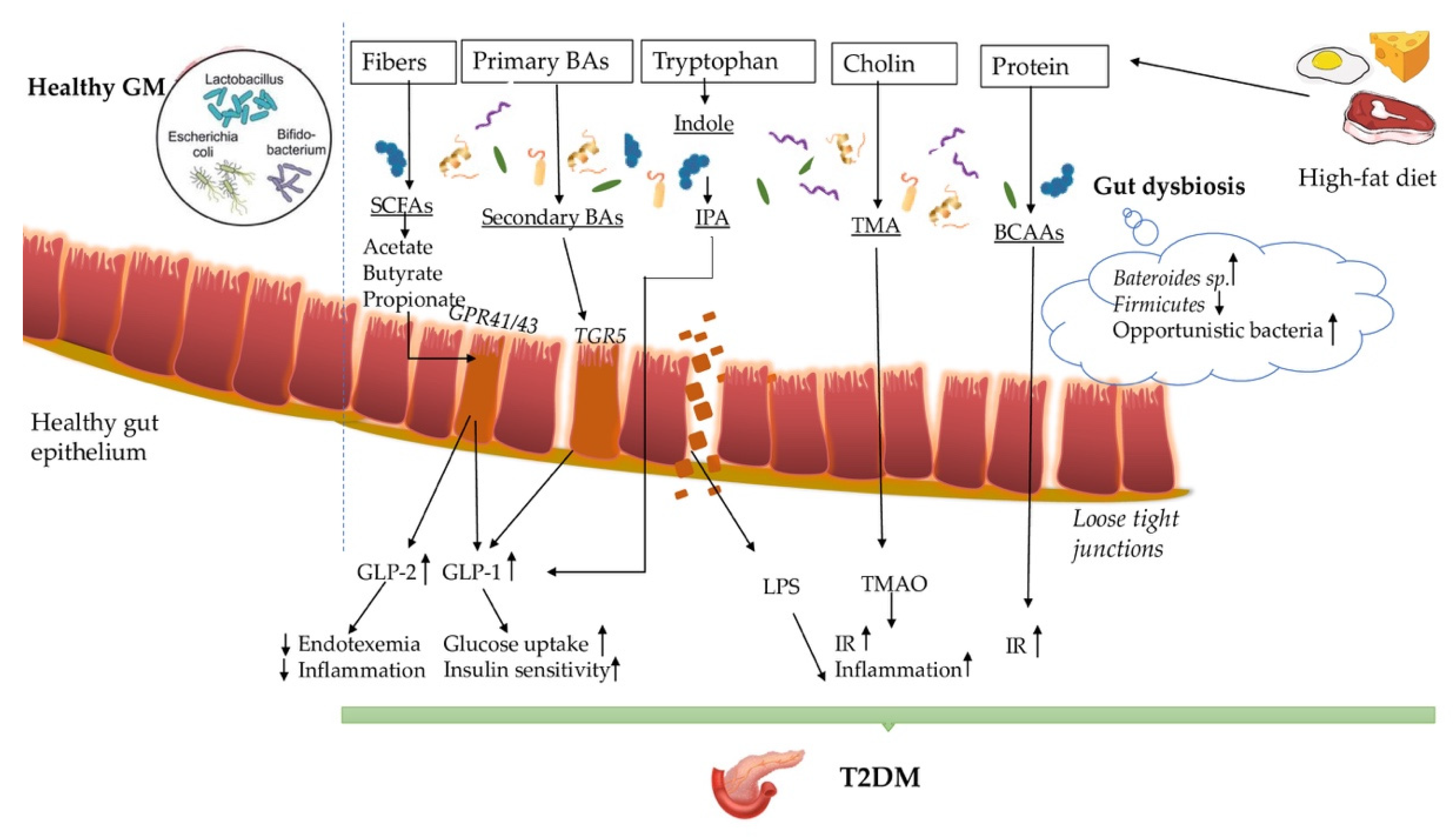
4. The Imunopathogenesis behind Gut Dysbiosis-T2DM
4.1. Short-Chain Fatty Acids (SCFAs)
4.2. Bile Acids (BAs)
4.3. Trimethylamine Oxide (TMAO)
4.4. Indole Propionic Acids and Branched Chain Amino Acids (BCAAs)
4.5. Hydrogen Sulfide (H2S)
4.6. Immune System in GM-T2DM
5. Gut Microbiota and Diabetic Retinopathy
6. Gut Microbiota and Diabetic Neuropathy
| Main Focus | Species | Salient Findings | Country | Year and Reference |
|---|---|---|---|---|
| therapy | mice | GLP-1based therapy for functional insulin secretion requires an eubiotic intestinal microbiota environment | France | 2017 |
| [110] | ||||
| neuropathy | mice | The ENS structural and functional integrity depends on TLR4 and nuclear factor–κB activation using microbiota components such as LPS | USA | 2012 |
| [105] | ||||
| neuropathy | mice | TLR2 activation by different microbial products regulates the intestinal neuromuscular function | Italy | 2015 |
| [106] | ||||
| neuropathy | mice | TLR9 in the neuromuscular junction development is considered a key element in neuronal activity | USA | 2016 |
| [107] | ||||
| neuropathy | mice | Normal functioning of intestinal neurons requires commensal intestinal microbiota | Canada | 2013 |
| [108] | ||||
| neuropathy | mice | Functional gut-brain signaling requires an intact microbiome | Canada | 2015 |
| [109] | ||||
| diet/therapy | mice | Diet supplementation with SCFAs in GF mice induced chromatin changes affecting the host epigenome similar to those associated with colonization | USA | 2016 |
| [111] | ||||
| neuropathy | humans + murine | Mutation of the MeCP2 gene leads to a disruption in the neuronal communication that explains the intestinal dysmotility in Rett syndrome | Canada | 2015 |
| [112] | ||||
| neuropathy | mice | An important imbalance of the nitric oxide synthesized at neuronal level was observed in the MeCP2 mutation that can be a cause for the dysregulation of the GI transit | Canada | 2016 |
| [113] | ||||
| diet/diabetic neuropathy | mice | High palmitic acid exposure is a contributing factor in diabetic neuropathy causing gastrointestinal dysregulation | Sweden | 2013 |
| [116] | ||||
| diet/neuropathy | mice | HFD-fed mice had a decrease in the inhibitory neuromuscular transmission and lost myenteric inhibitory motor neurons correlated with intestinal dysbiosis | USA | 2020 |
| [125] | ||||
| neuropathy | murine | The enteric neural network can be activated directly by bacterial RNA and DNA fragments that bind to TLR | Italy | 2009 |
| [104] | ||||
| diet/neuropathy | mice | HFD-induced dysmotility is due to apoptosis or necrosis of the myenteric inhibitory motor neurons | USA | 2013 |
| [115] | ||||
| diet/diabetic neuropathy | mice | Type 2 diabetes and HFD influence the ENS; diabetic dysmotility is caused by nerve damage | USA | 2015 |
| [117] | ||||
| diabetic neuropathy | humans | Oxidative stress causes loss of enteric neurons that may be inducing diabetic dysmotility; antioxidants are a possible therapeutic option in diabetic motility disorders | USA | 2011 |
| [118] | ||||
| diet/neuropathy | mice | Western diet induces neurodegeneration and dysmotility through TLR4 activation, even without overt endotoxemia or hyperglycemia | USA | 2017 |
| [122] | ||||
| neuropathy | mice | Early exposure to intestinal microbiota is necessary for physiological development of the ENS | Canada | 2014 |
| [126] |
7. Gut Microbiota and Diabetic Nephropathy
8. Therapy and Future Perspectives
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| T2DM | type 2 diabetes mellitus |
| IR | insulin resistance |
| GM | gut microbiota |
| SCFAs | short chain fatty acids |
| TMAO | trimethylamine N-oxide |
| GLP-1 | glucagonlike peptide-1 |
| LPS | lipopolyscaccharide |
| MR | Mendelian randomization |
| PG | peptidoglycan |
| TLR-4 | toll-like receptor-4 |
| GPR | G-protein-coupled receptors |
| BAs | bile acids |
| CDCA | chenodeoxycholic acid |
| CA | cholic acid |
| DCA | deoxycholic acid |
| LCA | lithocholic acid |
| FXR | farnesoid X receptor |
| TMA | trimethylamine |
| FMO3 | flavin monooxygenase 3 |
| TMAO | trimethylamine oxide |
| VLDL | very-low-density lipoproteins |
| HOMA-IR | homeostasis model assessment of insulin resistance |
| BCAAs | Branched chain amino acids |
| IPA | 3-Indolepropionic acid |
| H2S | Hydrogen Sulfide |
| Th17/Treg | T helper 17/regulatory T cell |
| GALT | gut-associated lymphoid tissues |
| NK | natural killer |
| ILCs | The Innate Lymphoid Cells |
| STAT4 | signal transducer and activator of transcription 4 |
| DR | Diabetic retinopathy |
| TUDCA | taurochenodeoxycholate |
| DPN | distal polyneuropathy |
| DM | diabetes mellitus |
| GI | gastrointestinal |
| ENS | enteric nervous system |
| VN | vagus nerve |
| RNA | ribonucleic acid |
| DANN | deoxyribonucleic acid |
| GF | germ-free |
| MeCP2 | methyl CpG binding protein |
| RTT | Rett syndrome |
| HFD | high fat diet |
| NO | nitric oxide |
| nNOS | neuronal nitric oxide synthase |
| DKD | diabetic kidney disease |
| CKD | chronic kidney disease |
| RAS | renin-angiotensin system |
| DN | diabetic nephropathy |
| Olfr78 | olfactory receptor Olfr78 |
| PS | phenyl sulfate |
| SP | severe proteinuria |
| MP | mild proteinuria |
| CCP | cordyceps cicadae polysaccharides |
| TGF-β1 | transforming growth factor-beta 1 |
| FMT | fecal microbiota transplantation |
| BHID | Bekhogainsam decoction |
| JSD | Jowiseungki decoction |
| MAPK | mitogen-activated protein kinase |
References
- World Health Organization. Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 8 June 2020).
- IDF. IDF Atlas, 8th ed.; International Diabetes Federation: Watermael-Boitsfort, Belgium, 2018. [Google Scholar]
- American Diabetes Association. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S135–S151. [Google Scholar] [CrossRef] [PubMed]
- Sircana, A.; Framarin, L.; Leone, N.; Berrutti, M.; Castellino, F.; Parente, R.; De Michieli, F.; Paschetta, E.; Musso, G. Altered Gut Microbiota in Type 2 Diabetes: Just a Coincidence? Curr. Diabetes Rep. 2018, 18, 98. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Sun, L.; Zhang, S.; Zhao, X.; Gang, X.; Wang, G. Evaluating the Causal Role of Gut Microbiota in Type 1 Diabetes and Its Possible Pathogenic Mechanisms. Front. Endocrinol. 2020, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Hasain, Z.; Mokhtar, N.M.; Kamaruddin, N.A.; Mohamed Ismail, N.A.; Razalli, N.H.; Gnanou, J.V.; Raja Ali, R.A. Gut Microbiota and Gestational Diabetes Mellitus: A Review of Host-Gut Microbiota Interactions and Their Therapeutic Potential. Front. Cell. Infect. Microbiol. 2020, 10, 188. [Google Scholar] [CrossRef]
- Sharma, S.; Tripathi, P. Gut Microbiome and Type 2 Diabetes: Where We Are and Where to Go? J. Nutr. Biochem. 2019, 63, 101–108. [Google Scholar] [CrossRef]
- Sharma, M.; Li, Y.; Stoll, M.L.; Tollefsbol, T.O. The Epigenetic Connection between the Gut Microbiome in Obesity and Diabetes. Front. Genet. 2020, 10, 1329. [Google Scholar] [CrossRef]
- Altveş, S.; Yildiz, H.K.; Vural, H.C. Interaction of the Microbiota with the Human Body in Health and Diseases. Biosci. Microbiota Food Health 2020, 39, 23–32. [Google Scholar] [CrossRef]
- Manchanayake, L.N. The Impact of Gut Microbiota on Host Obesity. J. Gastrointest. Dig. Syst. 2019, 9, 591. [Google Scholar] [CrossRef]
- Takagi, T.; Naito, Y.; Inoue, R.; Kashiwagi, S.; Uchiyama, K.; Mizushima, K.; Tsuchiya, S.; Dohi, O.; Yoshida, N.; Kamada, K.; et al. Differences in Gut Microbiota Associated with Age, Sex, and Stool Consistency in Healthy Japanese Subjects. J. Gastroenterol. 2018, 54, 53–63, Erratum in 2019, 54, 96-98. [Google Scholar] [CrossRef]
- Pasolli, E.; Asnicar, F.; Manara, S.; Zolfo, M.; Karcher, N.; Armanini, F.; Beghini, F.; Manghi, P.; Tett, A.; Ghensi, P.; et al. Extensive Unexplored Human Microbiome Diversity Revealed by Over 150,000 Genomes from Metagenomes Spanning Age, Geography, and Lifestyle. Cell 2019, 176, 649–662.e20. [Google Scholar] [CrossRef]
- Mishra, S.P.; Karunakar, P.; Taraphder, S.; Yadav, H. Free Fatty Acid Receptors 2 and 3 as Microbial Metabolite Sensors to Shape Host Health: Pharmacophysiological View. Biomedicines 2020, 8, 154. [Google Scholar] [CrossRef]
- Clarke, G.; Sandhu, K.V.; Griffin, B.T.; Dinan, T.G.; Cryan, J.F.; Hyland, N.P. Gut Reactions: Breaking Down Xenobiotic-Microbiome Interactions. Pharmacol. Rev. 2019, 71, 198–224. [Google Scholar] [CrossRef]
- Komaroff, A.L. The Microbiome and Risk for Obesity and Diabetes. JAMA 2017, 317, 355–356. [Google Scholar] [CrossRef]
- Takahashi, D.; Hoshina, N.; Kabumoto, Y.; Maeda, Y.; Suzuki, A.; Tanabe, H.; Isobe, J.; Yamada, T.; Muroi, K.; Yanagisawa, Y.; et al. Microbiota-Derived Butyrate Limits the Autoimmune Response by Promoting the Differentiation of Follicular Regulatory T Cells. EBioMedicine 2020, 58, 102913. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, E.; Egea-Zorrilla, A.; Plaza-Díaz, J.; Aragón-Vela, J.; Muñoz-Quezada, S.; Tercedor-Sánchez, L.; Abadia-Molina, F. The Gut Microbiota and Its Implication in the Development of Atherosclerosis and Related Cardiovascular Diseases. Nutrients 2020, 12, 605. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.; Sandhu, K.V.; Bastiaanssen, T.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Meroni, M.; Longo, M.; Dongiovanni, P. Alcohol or Gut Microbiota: Who Is the Guilty? Int. J. Mol. Sci. 2019, 20, 4568. [Google Scholar] [CrossRef]
- Rukavina Mikusic, N.L.; Kouyoumdzian, N.M.; Choi, M.R. Gut Microbiota and Chronic Kidney Disease: Evidences and Mechanisms That Mediate a New Communication in the Gastrointestinal-Renal Axis. Pflugers Arch. 2020, 472, 303–320. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Li, Q.; Chang, Y.; Zhang, K.; Chen, H.; Tao, S.; Zhang, Z. Implication of the Gut Microbiome Composition of Type 2 Diabetic Patients from Northern China. Sci. Rep. 2020, 10, 5450. [Google Scholar] [CrossRef]
- Yang, Q.; Lin, S.L.; Kwok, M.K.; Leung, G.M.; Schooling, C.M. The Roles of 27 Genera of Human Gut Microbiota in Ischemic Heart Disease, Type 2 Diabetes Mellitus, and Their Risk Factors: A Mendelian Randomization Study. Am. J. Epidemiol. 2018, 187, 1916–1922. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of Gut Microbiota in Type 2 Diabetes Pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Yassour, M.; Lim, M.Y.; Yun, H.S.; Tickle, T.L.; Sung, J.; Song, Y.-M.; Lee, K.; Franzosa, E.A.; Morgan, X.C.; Gevers, D.; et al. Sub-Clinical Detection of Gut Microbial Biomarkers of Obesity and Type 2 Diabetes. Genome Med. 2016, 8, 17. [Google Scholar] [CrossRef]
- Sedighi, M.; Razavi, S.; Navab-Moghadam, F.; Khamseh, M.E.; Alaei-Shahmiri, F.; Mehrtash, A.; Amirmozafari, N. Comparison of Gut Microbiota in Adult Patients with Type 2 Diabetes and Healthy Individuals. Microb. Pathog. 2017, 111, 362–369. [Google Scholar] [CrossRef]
- Ahmad, A.; Yang, W.; Chen, G.; Shafiq, M.; Javed, S.; Ali Zaidi, S.S.; Shahid, R.; Liu, C.; Bokhari, H. Analysis of Gut Microbiota of Obese Individuals with Type 2 Diabetes and Healthy Individuals. PLoS ONE 2019, 14, e0226372. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Díaz-Rizzolo, D.A.; Kostov, B.; López-Siles, M.; Serra, A.; Colungo, C.; González-de-Paz, L.; Martinez-Medina, M.; Sisó-Almirall, A.; Gomis, R. Healthy Dietary Pattern and Their Corresponding Gut Microbiota Profile Are Linked to a Lower Risk of Type 2 Diabetes, Independent of the Presence of Obesity. Clin. Nutr. 2020, 39, 524–532. [Google Scholar] [CrossRef]
- Peng, W.; Huang, J.; Yang, J.; Zhang, Z.; Yu, R.; Fayyaz, S.; Zhang, S.; Qin, Y.-H. Integrated 16S rRNA Sequencing, Metagenomics, and Metabolomics to Characterize Gut Microbial Composition, Function, and Fecal Metabolic Phenotype in Non-obese Type 2 Diabetic Goto-Kakizaki Rats. Front. Microbiol. 2020, 10, 3141. [Google Scholar] [CrossRef]
- Fujio-Vejar, S.; Vasquez, Y.; Morales, P.; Magne, F.; Vera-Wolf, P.; Ugalde, J.A.; Navarrete, P.; Gotteland, M. The Gut Microbiota of Healthy Chilean Subjects Reveals a High Abundance of the Phylum Verrucomicrobia. Front. Microbiol. 2017, 8, 1221. [Google Scholar] [CrossRef]
- Adachi, K.; Sugiyama, T.; Yamaguchi, Y.; Tamura, Y.; Izawa, S.; Hijikata, Y.; Ebi, M.; Funaki, Y.; Ogasawara, N.; Goto, C.; et al. Gut Microbiota Disorders Cause Type 2 Diabetes Mellitus and Homeostatic Disturbances in Gut-Related Metabolism in Japanese Subjects. J. Clin. Biochem. Nutr. 2019, 64, 231–238. [Google Scholar] [CrossRef]
- Suceveanu, A.I.; Pantea Stoian, A.; Parepa, R.I.; Voinea, C.; Hainarosie, R.; Manuc, D.; Nitipir, C.; Mazilu, L. Gut Microbiota Patterns in Obese and Type 2 Diabetes. (T2D) Patients from Romanian Black Sea Coast Region. Rev. Chim. 2018, 69, 2260–2267. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing a Complex Relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef]
- Zhu, T.; Goodarzi, M.O. Metabolites Linking the Gut Microbiome with Risk for Type 2 Diabetes. Curr. Nutr. Rep. 2020, 9, 83–93. [Google Scholar] [CrossRef]
- Mandaliya, D.K.; Seshadri, S. Short Chain Fatty Acids, Pancreatic Dysfunction and Type 2 Diabetes. Pancreatology 2019, 19, 280–284. [Google Scholar] [CrossRef]
- Ohira, H.; Tsutsui, W.; Fujioka, Y. Are Short Chain Fatty Acids in Gut Microbiota Defensive Players for Inflammation and Atherosclerosis? J. Atheroscler. Thromb. 2017, 24, 660–672. [Google Scholar] [CrossRef]
- Aoki, R.; Kamikado, K.; Suda, W.; Takii, H.; Mikami, Y.; Suganuma, N.; Hattori, M.; Koga, Y. A Proliferative Probiotic Bifidobacterium Strain in the Gut Ameliorates Progression of Metabolic Disorders via Microbiota Modulation and Acetate Elevation. Sci. Rep. 2017, 7, 43522. [Google Scholar] [CrossRef]
- Oliveira, R.B.; Canuto, L.P.; Collares-Buzato, C.B. Intestinal Luminal Content from High-Fat-Fed Prediabetic Mice Changes Epithelial Barrier Function In Vitro. Life Sci. 2019, 216, 10–21. [Google Scholar] [CrossRef]
- Gomes, J.; Costa, J.A.; Alfenas, R. Metabolic Endotoxemia and Diabetes Mellitus: A Systematic Review. Metabolism 2017, 68, 133–144. [Google Scholar] [CrossRef]
- Veprik, A.; Laufer, D.; Weiss, S.; Rubins, N.; Walker, M.D. GPR41 Modulates Insulin Secretion and Gene Expression in Pancreatic β-Cells and Modifies Metabolic Homeostasis in Fed and Fasting States. FASEB J. 2016, 30, 3860–3869. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut Bacteria Selectively Promoted by Dietary Fibers Alleviate Type 2 Diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef]
- Rey, F.E.; Faith, J.J.; Bain, J.; Muehlbauer, M.J.; Stevens, R.D.; Newgard, C.B.; Gordon, J.I. Dissecting the In Vivo Metabolic Potential of Two Human Gut Acetogens. J. Biol. Chem. 2010, 285, 22082–22090. [Google Scholar] [CrossRef]
- Sanna, S.; van Zuydam, N.R.; Mahajan, A.; Kurilshikov, A.; Vich Vila, A.; Võsa, U.; Mujagic, Z.; Masclee, A.; Jonkers, D.; Oosting, M.; et al. Causal Relationships Among the Gut Microbiome, Short-Chain Fatty Acids and Metabolic Diseases. Nat. Genet. 2019, 51, 600–605. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of Propionate and Butyrate by the Human Colonic Microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Pingitore, A.; Chambers, E.S.; Hill, T.; Maldonado, I.R.; Liu, B.; Bewick, G.; Morrison, D.J.; Preston, T.; Wallis, G.A.; Tedford, C.; et al. The Diet-Derived Short Chain Fatty Acid Propionate Improves Beta-Cell Function in Humans and Stimulates Insulin Secretion from Human Islets In Vitro. Diabetes Obes. Metab. 2017, 19, 257–265. [Google Scholar] [CrossRef]
- Jergens, A.E.; Guard, B.C.; Redfern, A.; Rossi, G.; Mochel, J.P.; Pilla, R.; Chandra, L.; Seo, Y.J.; Steiner, J.M.; Lidbury, J.; et al. Microbiota-Related Changes in Unconjugated Fecal Bile Acids Are Associated with Naturally Occurring, Insulin-Dependent Diabetes Mellitus in Dogs. Front. Vet. Sci. 2019, 6, 199. [Google Scholar] [CrossRef]
- Molinaro, A.; Wahlström, A.; Marschall, H.U. Role of Bile Acids in Metabolic Control. Trends Endocrinol. Metab. 2018, 29, 31–41. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- Liu, H.; Hu, C.; Zhang, X.; Jia, W. Role of Gut Microbiota, Bile Acids and Their Cross-Talk in the Effects of Bariatric Surgery on Obesity and Type 2 Diabetes. J. Diabetes Investig. 2018, 9, 13–20. [Google Scholar] [CrossRef]
- Rajani, C.; Jia, W. Bile Acids and Their Effects on Diabetes. Front. Med. 2018, 12, 608–623. [Google Scholar] [CrossRef]
- Nerild, H.H.; Christensen, M.B.; Knop, F.K.; Brønden, A. Preclinical Discovery and Development of Colesevelam for the Treatment of Type 2 Diabetes. Expert Opin. Drug Discov. 2018, 13, 1161–1167. [Google Scholar] [CrossRef]
- Mudaliar, S.; Henry, R.R.; Sanyal, A.J.; Morrow, L.; Marschall, H.U.; Kipnes, M.; Adorini, L.; Sciacca, C.I.; Clopton, P.; Castelloe, E.; et al. Efficacy and Safety of the Farnesoid X Receptor Agonist Obeticholic Acid in Patients with Type 2 Diabetes and Nonalcoholic Fatty Liver Disease. Gastroenterology 2013, 145, 574–582.e1. [Google Scholar] [CrossRef]
- Schoeler, M.; Caesar, R. Dietary Lipids, Gut Microbiota and Lipid Metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, R.; Ge, X.; Han, L.; Yu, P.; Gong, X.; Meng, Q.; Zhang, Y.; Fan, H.; Zheng, L.; Liu, Z.; et al. Gut Microbe-Generated Metabolite Trimethylamine N-Oxide and the Risk of Diabetes: A Systematic Review and Dose-Response Meta-Analysis. Obes. Rev. 2019, 20, 883–894. [Google Scholar] [CrossRef]
- Andraos, S.; Lange, K.; Clifford, S.A.; Jones, B.; Thorstensen, E.B.; Kerr, J.A.; Wake, M.; Saffery, R.; Burgner, D.P.; O’Sullivan, J.M. Plasma Trimethylamine N-Oxide and Its Precursors: Population Epidemiology, Parent-Child Concordance, and Associations with Reported Dietary Intake in 11- to 12-Year-Old Children and Their Parents. Curr. Dev. Nutr. 2020, 4, nzaa103. [Google Scholar] [CrossRef]
- Qi, J.; You, T.; Li, J.; Pan, T.; Xiang, L.; Han, Y.; Zhu, L. Circulating Trimethylamine N-Oxide and the Risk of Cardiovascular Diseases: A Systematic Review and Meta-Analysis of 11 Prospective Cohort Studies. J. Cell. Mol. Med. 2018, 22, 185–194. [Google Scholar] [CrossRef]
- Chhibber-Goel, J.; Singhal, V.; Parakh, N.; Bhargava, B.; Sharma, A. The Metabolite Trimethylamine-N-Oxide Is an Emergent Biomarker of Human Health. Curr. Med. Chem. 2017, 24, 3942–3953. [Google Scholar] [CrossRef]
- Heianza, Y.; Sun, D.; Li, X.; DiDonato, J.A.; Bray, G.A.; Sacks, F.M.; Qi, L. Gut Microbiota Metabolites, Amino Acid Metabolites and Improvements in Insulin Sensitivity and Glucose Metabolism: The POUNDS Lost Trial. Gut 2019, 68, 263–270. [Google Scholar] [CrossRef]
- Roberts, A.B.; Gu, X.; Buffa, J.A.; Hurd, A.G.; Wang, Z.; Zhu, W.; Gupta, N.; Skye, S.M.; Cody, D.B.; Levison, B.S.; et al. Development of a Gut Microbe-Targeted Nonlethal Therapeutic to Inhibit Thrombosis Potential. Nat. Med. 2018, 24, 1407–1417. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial Tryptophan Catabolites in Health and Disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef]
- De Mello, V.D.; Paananen, J.; Lindström, J.; Lankinen, M.A.; Shi, L.; Kuusisto, J.; Pihlajamäki, J.; Auriola, S.; Lehtonen, M.; Rolandsson, O.; et al. Indolepropionic Acid and Novel Lipid Metabolites Are Associated with a Lower Risk of Type 2 Diabetes in the Finnish Diabetes Prevention Study. Sci. Rep. 2017, 7, 46337. [Google Scholar] [CrossRef]
- Mercer, K.E.; Yeruva, L.; Pack, L.; Graham, J.L.; Stanhope, K.L.; Chintapalli, S.V.; Wankhade, U.D.; Shankar, K.; Havel, P.J.; Adams, S.H.; et al. Xenometabolite Signatures in the UC Davis Type 2 Diabetes Mellitus Rat Model Revealed Using a Metabolomics Platform Enriched with Microbe-Derived Metabolites. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G157–G169. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-Z.; Stirling, K.; Yang, J.-J.; Zhang, L. Gut Microbiota and Diabetes: From Correlation to Causality and Mechanism. World J. Diabetes. 2020, 11, 293–308. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite Profiles and the Risk of Developing Diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef]
- Bloomgarden, Z. Diabetes and Branched-Chain Amino Acids: What Is the Link? J. Diabetes 2018, 10, 350–352. [Google Scholar] [CrossRef]
- Barton, L.L.; Ritz, N.L.; Fauque, G.D.; Lin, H.C. Sulfur Cycling and the Intestinal Microbiome. Dig. Dis. Sci. 2017, 62, 2241–2257. [Google Scholar] [CrossRef]
- Gheibi, S.; Samsonov, A.P.; Gheibi, S.; Vazquez, A.B.; Kashfi, K. Regulation of Carbohydrate Metabolism by Nitric Oxide and Hydrogen Sulfide: Implications in Diabetes. Biochem. Pharmacol. 2020, 176, 113819. [Google Scholar] [CrossRef]
- Bełtowski, J.; Wójcicka, G.; Jamroz-Wiśniewska, A. Hydrogen Sulfide in the Regulation of Insulin Secretion and Insulin Sensitivity: Implications for the Pathogenesis and Treatment of Diabetes Mellitus. Biochem. Pharmacol. 2018, 149, 60–76. [Google Scholar] [CrossRef]
- Tomasova, L.; Konopelski, P.; Ufnal, M. Gut Bacteria and Hydrogen Sulfide: The New Old Players in Circulatory System Homeostasis. Molecules 2016, 21, 1558. [Google Scholar] [CrossRef]
- Wu, L.; Yang, W.; Jia, X.; Yang, G.; Duridanova, D.; Cao, K.; Wang, R. Pancreatic Islet Overproduction of H2S and Suppressed Insulin Release in Zucker Diabetic Rats. Lab. Investig. 2009, 89, 59–67. [Google Scholar] [CrossRef]
- Taniguchi, S.; Kang, L.; Kimura, T.; Niki, I. Hydrogen Sulphide Protects Mouse Pancreatic β-Cells from Cell Death Induced by Oxidative Stress, but Not by Endoplasmic Reticulum Stress. Br. J. Pharmacol. 2011, 162, 1171–1178. [Google Scholar] [CrossRef]
- Moffa, S.; Mezza, T.; Cefalo, C.; Cinti, F.; Impronta, F.; Sorice, G.P.; Santoro, A.; Di Giuseppe, G.; Pontecorvi, A.; Giaccari, A. The Interplay between Immune System and Microbiota in Diabetes. Mediators Inflamm. 2019, 2019, 9367404. [Google Scholar] [CrossRef]
- Belizário, J.E.; Faintuch, J.; Garay-Malpartida, M. Gut Microbiome Dysbiosis and Immunometabolism: New Frontiers for Treatment of Metabolic Diseases. Mediators Inflamm. 2018, 2018, 2037838. [Google Scholar] [CrossRef]
- Daryabor, G.; Atashzar, M.R.; Kabelitz, D.; Meri, S.; Kalantar, K. The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front. Immunol. 2020, 11, 1582. [Google Scholar] [CrossRef]
- Liu, F.; Wang, H.; Feng, W.; Ye, X.; Sun, X.; Jiang, C.; Chu, X.; Zhang, P.; Jiang, C.; Wang, Y.; et al. Type 1 Innate Lymphoid Cells Are Associated with Type 2 Diabetes. Diabetes Metab. 2019, 45, 341–346. [Google Scholar] [CrossRef]
- Galle-Treger, L.; Sankaranarayanan, I.; Hurrell, B.P.; Howard, E.; Lo, R.; Maazi, H.; Lewis, G.; Banie, H.; Epstein, A.L.; Hu, P.; et al. Costimulation of Type-2 Innate Lymphoid Cells by GITR Promotes Effector Function and Ameliorates Type 2 Diabetes. Nat. Commun. 2019, 10, 713. [Google Scholar] [CrossRef]
- Ali, M.; Mali, V.; Haddox, S.; AbdelGhany, S.M.; El-Deek, S.; Abulfadl, A.; Matrougui, K.; Belmadani, S. Essential Role of IL-12 in Angiogenesis in Type 2 Diabetes. Am. J. Pathol. 2017, 187, 2590–2601. [Google Scholar] [CrossRef]
- Wu, W.; Liu, H.-P.; Chen, F.; Liu, H.; Cao, A.T.; Yao, S.; Sun, M.; Evans-Marin, H.L.; Zhao, Y.; Zhao, Q.; et al. Commensal A4 Bacteria Inhibit Intestinal Th2-Cell Responses through Induction of Dendritic Cell TGF-β Production. Eur. J. Immunol. 2016, 46, 1162–1167. [Google Scholar] [CrossRef]
- Wang, Q.; McLoughlin, R.M.; Cobb, B.A.; Charrel-Dennis, M.; Zaleski, K.J.; Golenbock, D.; Tzianabos, A.O.; Kasper, D.L. A Bacterial Carbohydrate Links Innate and Adaptive Responses through Toll-Like Receptor 2. J. Exp. Med. 2006, 203, 2853–2863. [Google Scholar] [CrossRef]
- Weaver, J.R.; Nadler, J.L.; Taylor-Fishwick, D.A. Interleukin-12 (IL-12)/STAT4 Axis Is an Important Element for β-Cell Dysfunction Induced by Inflammatory Cytokines. PLoS ONE 2015, 10, e0142735. [Google Scholar] [CrossRef]
- Flaxman, S.R.; Bourne, R.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Vision Loss Expert Group of the Global Burden of Disease Study. Global Causes of Blindness and Distance Vision Impairment 1990–2020: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef]
- Romero-Aroca, P.; Navarro-Gil, R.; Valls-Mateu, A.; Sagarra-Alamo, R.; Moreno-Ribas, A.; Soler, N. Differences in Incidence of Diabetic Retinopathy between Type 1 and 2 Diabetes Mellitus: A Nine-Year Follow-Up Study. Br. J. Ophthalmol. 2017, 101, 1346–1351. [Google Scholar] [CrossRef]
- Grzybowski, A.; Brona, P.; Kim, S.J. Microbial Flora and Resistance in Ophthalmology: A Review. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 851–862. [Google Scholar] [CrossRef]
- Wen, X.; Miao, L.; Deng, Y.; Bible, P.W.; Hu, X.; Zou, Y.; Liu, Y.; Guo, S.; Liang, J.; Chen, T.; et al. The Influence of Age and Sex on Ocular Surface Microbiota in Healthy Adults. Invest. Ophthalmol. Vis. Sci. 2017, 58, 6030–6037. [Google Scholar] [CrossRef]
- Kugadas, A.; Gadjeva, M. Impact of Microbiome on Ocular Health. Ocul. Surf. 2016, 14, 342–349. [Google Scholar] [CrossRef]
- Ham, B.; Hwang, H.B.; Jung, S.H.; Chang, S.; Kang, K.D.; Kwon, M.J. Distribution and Diversity of Ocular Microbial Communities in Diabetic Patients Compared with Healthy Subjects. Curr. Eye Res. 2018, 43, 314–324. [Google Scholar] [CrossRef]
- St Leger, A.J.; Caspi, R.R. Visions of Eye Commensals: The Known and the Unknown About How the Microbiome Affects Eye Disease. BioEssays 2018, 40, e1800046. [Google Scholar] [CrossRef]
- Beli, E.; Yan, Y.; Moldovan, L.; Vieira, C.P.; Gao, R.; Duan, Y.; Prasad, R.; Bhatwadekar, A.; White, F.A.; Townsend, S.D.; et al. Restructuring of the Gut Microbiome by Intermittent Fasting Prevents Retinopathy and Prolongs Survival in db/db Mice. Diabetes 2018, 67, 1867–1879. [Google Scholar] [CrossRef]
- Haluzík, M.; Mráz, M. Intermittent Fasting and Prevention of Diabetic Retinopathy: Where Do We Go from Here? Diabetes 2018, 67, 1745–1747. [Google Scholar] [CrossRef]
- Rowan, S.; Taylor, A. The Role of Microbiota in Retinal Disease. Adv. Exp. Med. Biol. 2018, 1074, 429–435. [Google Scholar] [CrossRef]
- Floyd, J.L.; Grant, M.B. The Gut-Eye Axis: Lessons Learned from Murine Models. Ophthalmol. Ther. 2020, 9, 499–513. [Google Scholar] [CrossRef]
- Baim, A.D.; Movahedan, A.; Farooq, A.V.; Skondra, D. The Microbiome and Ophthalmic Disease. Exp. Biol. Med. (Maywood) 2019, 244, 419–429. [Google Scholar] [CrossRef]
- Paul, S.; Ali, A.; Katare, R. Molecular Complexities Underlying the Vascular Complications of Diabetes Miellitus—A Comprehensive Review. J. Diabetes Complicat. 2020, 8, 107613. [Google Scholar] [CrossRef]
- Hicks, C.W.; Selvin, E. Epidemiology of Peripheral Neuropathy and Lower Extremity Disease in Diabetes. Curr. Diabetes Rep. 2019, 19, 86. [Google Scholar] [CrossRef]
- Preguiça, I.; Alves, A.; Nunes, S.; Gomes, P.; Fernandes, R.; Viana, S.D.; Reis, F. Diet-Induced Rodent Models of Diabetic Peripheral Neuropathy, Retinopathy and Nephropathy. Nutrients 2020, 12, 250. [Google Scholar] [CrossRef]
- Sellin, J.H.; Chang, E.B. Therapy Insight: Gastrointestinal Complications of Diabetes—Pathophysiology and Management. Nat. Clin. Pract. Gastroenterol. Hepatol. 2008, 5, 162–171. [Google Scholar] [CrossRef]
- Grasset, E.; Burcelin, R. The Gut Microbiota to the Brain Axis in the Metabolic Control. Rev. Endocr. Metab. Disord. 2019, 20, 427–438. [Google Scholar] [CrossRef]
- Yarandi, S.S.; Srinivasan, S. Diabetic Gastrointestinal Motility Disorders and the Role of Enteric Nervous System: Current Status and Future Directions. Neurogastroenterol. Motil. 2014, 26, 611–624. [Google Scholar] [CrossRef]
- Sanders, K.M.; Koh, S.D.; Ward, S.M. Interstitial Cells of Cajal as Pacemakers in the Gastrointestinal Tract. Annu. Rev. Physiol. 2006, 68, 307–343. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, W.; Li, Y.; Cong, Y. Enteroendocrine Cells: Sensing Gut Microbiota and Regulating Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2020, 26, 11–20. [Google Scholar] [CrossRef]
- Strandwitz, P.; Kim, K.H.; Terekhova, D.; Liu, J.K.; Sharma, A.; Levering, J.; McDonald, D.; Dietrich, D.; Ramadhar, T.R.; Lekbua, A.; et al. GABA-Modulating Bacteria of the Human Gut Microbiota. Nat. Microbiol. 2019, 4, 396–403. [Google Scholar] [CrossRef]
- Denou, E.; Lolmède, K.; Garidou, L.; Pomie, C.; Chabo, C.; Lau, T.C.; Fullerton, M.D.; Nigro, G.; Zakaroff-Girard, A.; Luche, E.; et al. Defective NOD2 Peptidoglycan Sensing Promotes Diet-Induced Inflammation, Dysbiosis, and Insulin Resistance. EMBO Mol. Med. 2015, 7, 259–274. [Google Scholar] [CrossRef]
- Barajon, I.; Serrao, G.; Arnaboldi, F.; Opizzi, E.; Ripamonti, G.; Balsari, A.; Rumio, C. Toll-Like Receptors 3,4, and 7 Are Expressed in the Enteric Nervous System and Dorsal Root Ganglia. J. Histochem. Cytochem. 2009, 57, 1013–1023. [Google Scholar] [CrossRef]
- Anitha, M.; Vijay-Kumar, M.; Sitaraman, S.V.; Gewirtz, A.T.; Srinivasan, S. Gut Microbial Products Regulate Murine Gastrointestinal Motility via Toll-Like Receptor 4 Signaling. Gastroenterology 2012, 143, 1006–1016. [Google Scholar] [CrossRef]
- Brun, P.; Gobbo, S.; Caputi, V.; Spagnol, L.; Schirato, G.; Pasqualin, M.; Levorato, E.; Palù, G.; Giron, M.C.; Castagliuolo, I. Toll Like Receptor-2 Regulates Production of Glial-Derived Neurotrophic Factors in Murine Intestinal Smooth Muscle Cells. Mol. Cell. Neurosci. 2015, 68, 24–35. [Google Scholar] [CrossRef]
- Patel, V.; Patel, A.M.; McArdle, J.J. Synaptic Abnormalities of Mice Lacking Toll-Like Receptor (TLR)-9. Neuroscience 2016, 324, 1–10. [Google Scholar] [CrossRef]
- McVey Neufeld, K.A.; Mao, Y.K.; Bienenstock, J.; Foster, J.A.; Kunze, W.A. The Microbiome is Essential for Normal Gut Intrinsic Primary Afferent Neuron Excitability in the Mouse. Neurogastroenterol. Motil. 2013, 25, 183–188. [Google Scholar] [CrossRef]
- McVey Neufeld, K.A.; Perez-Burgos, A.; Mao, Y.K.; Bienenstock, J.; Kunze, W.A. The Gut Microbiome Restores Intrinsic and Extrinsic Nerve Function in Germ-Free Mice Accompanied by Changes in Calbindin. Neurogastroenterol. Motil. 2015, 27, 627–636. [Google Scholar] [CrossRef]
- Grasset, E.; Puel, A.; Charpentier, J.; Collet, X.; Christensen, J.E.; Tercé, F.; Burcelin, R. A Specific Gut Microbiota Dysbiosis of Type 2 Diabetic Mice Induces GLP-1 Resistance through an Enteric NO-Dependent and Gut-Brain Axis Mechanism. Cell. Metab. 2017, 25, 1075–1090. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Kreznar, J.H.; Romano, K.A.; Vivas, E.I.; Barrett-Wilt, G.A.; Rabaglia, M.E.; Keller, M.P.; Attie, A.D.; Rey, F.E.; Denu, J.M. Diet-Microbiota Interactions Mediate Global Epigenetic Programming in Multiple Host Tissues. Mol. Cell. 2016, 64, 982–992. [Google Scholar] [CrossRef]
- Wahba, G.; Schock, S.C.; Claridge, E.; Bettolli, M.; Grynspan, D.; Humphreys, P.; Staines, W.A. MeCP2 in the Enteric Nervous System. Neurogastroenterol. Motil. 2015, 27, 1156–1161. [Google Scholar] [CrossRef]
- Wahba, G.; Schock, S.C.; Cudd, S.; Grynspan, D.; Humphreys, P.; Staines, W.A. Activity and MeCP2-Dependent Regulation of nNOS Levels in Enteric Neurons. Neurogastroenterol. Motil. 2016, 28, 1723–1730. [Google Scholar] [CrossRef]
- Furness, J.B. The Enteric Nervous System and Neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef]
- Stenkamp-Strahm, C.M.; Kappmeyer, A.J.; Schmalz, J.T.; Gericke, M.; Balemba, O. High-Fat Diet Ingestion Correlates with Neuropathy in the Duodenum Myenteric Plexus of Obese Mice with Symptoms of Type 2 Diabetes. Cell. Tissue Res. 2013, 354, 381–394. [Google Scholar] [CrossRef]
- Voss, U.; Sand, E.; Olde, B.; Ekblad, E.; Donath, M. Enteric Neuropathy Can Be Induced by High Fat Diet In Vivo and Palmitic Acid Exposure In Vitro. PLoS ONE 2013, 8, e81413. [Google Scholar] [CrossRef]
- Stenkamp-Strahm, C.M.; Nyavor, Y.E.A.; Kappmeyer, A.J.; Horton, S.; Gericke, M.; Balemba, O.B. Prolonged High Fat Diet Ingestion, Obesity, and Type 2 Diabetes Symptoms Correlate with Phenotypic Plasticity in Myenteric Neurons and Nerve Damage in the Mouse Duodenum. Cell. Tissue Res. 2015, 361, 411–426. [Google Scholar] [CrossRef]
- Chandrasekharan, B.; Anitha, M.; Blatt, R.; Shahnavaz, N.; Kooby, D.; Staley, C.; Mwangi, S.; Jones, D.P.; Sitaraman, S.V.; Srinivasan, S. Colonic Motor Dysfunction in Human Diabetes Is Associated with Enteric Neuronal Loss and Increased Oxidative Stress. Neurogastroenterol. Motil. 2011, 23, 131–138. [Google Scholar] [CrossRef]
- Grover, M.; Farrugia, G.; Lurken, M.S.; Bernard, C.E.; Faussone-Pellegrini, M.S.; Smyrk, T.C.; Parkman, H.P.; Abell, T.L.; Snape, W.J.; Hasler, W.L.; et al. Cellular Changes in Diabetic and Idiopathic Gastroparesis. Gastroenterology 2011, 140, 1575–1585. [Google Scholar] [CrossRef]
- Everard, A.; Lazarevic, V.; Gaïa, N.; Johansson, M.; Ståhlman, M.; Backhed, F.; Delzenne, N.M.; Schrenzel, J.; François, P.; Cani, P.D. Microbiome of Prebiotic-Treated Mice Reveals Novel Targets Involved in Host Response during Obesity. ISME J. 2014, 8, 2116–2130. [Google Scholar] [CrossRef]
- Reichardt, F.; Chassaing, B.; Nezami, B.G.; Li, G.; Tabatabavakili, S.; Mwangi, S.; Uppal, K.; Liang, B.; Vijay-Kumar, M.; Jones, D.; et al. Western Diet Induces Colonic Nitrergic Myenteric Neuropathy and Dysmotility in Mice via Saturated Fatty Acid- and Lipopolysaccharide- Induced TLR4 Signalling. J. Physiol. 2017, 595, 1831–1846. [Google Scholar] [CrossRef]
- Van Hul, M.; Geurts, L.; Plovier, H.; Druart, C.; Everard, A.; Ståhlman, M.; Rhimi, M.; Chira, K.; Teissedre, P.L.; Delzenne, N.M.; et al. Reduced Obesity, Diabetes and Steatosis upon Cinnamon and Grape Pomace Are Associated with Changes in Gut Microbiota and Markers of Gut Barrier. Am. J. Physiol. Endocrinol. Metab. 2017, 314, E334–E352. [Google Scholar] [CrossRef]
- Grenham, S.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-Gut-Microbe Communication in Health and Disease. Front. Physiol. 2011, 2, 94. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.; Borojevic, R.; Verdu, E.F.; Huizinga, J.D.; Ratcliffe, E.M. Intestinal Microbiota Influence the Early Postnatal Development of the Enteric Nervous System. Neurogastroenterol. Motil. 2014, 26, 98–107. [Google Scholar] [CrossRef]
- Nyavor, Y.; Brands, C.R.; May, G.; Kuther, S.; Nicholson, J.; Tiger, K.; Tesnohlidek, A.; Yasuda, A.; Starks, K.; Litvinenko, D.; et al. High-Fat Diet-Induced Alterations to Gut Microbiota and Gut-Derived Lipoteichoic Acid Contributes to the Development of Enteric. Neurogastroenterol. Motil. 2020, 32, e13838. [Google Scholar] [CrossRef]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef]
- Miranda-Diaz, A.G.; Pazarin-Villasenor, L.; Yanowsky-Escatell, F.G.; Andrade-Sierra, J. Oxidative Stress in Diabetic Nephropathy with Early Chronic Kidney Disease. J. Diabetes Res. 2016, 2016, 7047238. [Google Scholar] [CrossRef]
- Urushihara, M.; Kagami, S. Role of the Intrarenal Renin–Angiotensin System in the Progression of Renal Disease. Pediatr. Nephrol. 2017, 32, 1471–1479. [Google Scholar] [CrossRef]
- Ramezani, A.; Massy, Z.A.; Meijers, B.; Evenepoel, P.; Vanholder, R.; Raj, D.S. Role of the Gut Microbiome in Uremia: A Potential Therapeutic Target. Am. J. Kidney Dis. 2016, 67, 483–498. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.H.; Andersen, G.L. Chronic Kidney Disease Alters Intestinal Microbial Flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef]
- Vallon, V.; Docherty, N.G. Intestinal Regulation of Urinary Sodium Excretion and the Pathophysiology of Diabetic Kidney Disease: A Focus on Glucagon-Like Peptide 1 and Dipeptidyl Peptidase 4 in Diabetic Kidney Disease. Exp. Physiol. 2014, 99, 1140–1145. [Google Scholar] [CrossRef]
- Pluznick, J.L.; Protzko, R.J.; Gevorgyan, H.; Peterlin, Z.; Sipos, A.; Han, J.; Brunet, I.; Wan, L.X.; Rey, F.; Wang, T.; et al. Olfactory Receptor Responding to Gut Microbiota-Derived Signals Plays a Role in Renin Secretion and Blood Pressure Regulation. Proc. Natl. Acad. Sci. USA 2013, 110, 4410–4415. [Google Scholar] [CrossRef]
- Fiaccadori, E.; Cosola, C.; Sabatino, A. Targeting the Gut for Early Diagnosis, Prevention, and Cure of Diabetic Kidney Disease: Is the Phenyl Sulfate Story Another Step Forward? Am. J. Kidney Dis. 2020, 75, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.C.; Hu, Z.B.; Wang, R.; Hong, Z.H.; Lu, J.; Chen, P.P.; Zhang, J.X.; Li, X.Q.; Yuan, B.Y.; Huang, S.J.; et al. Gut Microbiota Dysbiosis-Induced Activation of the Intrarenal Renin-Angiotensin System is Involved in Kidney Injuries in Rat Diabetic Nephropathy. Acta Pharmacol. Sin. 2020, 41, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.B.; Lu, J.; Chen, P.P.; Lu, C.C.; Zhang, J.X.; Li, X.Q.; Yuan, B.Y.; Huang, S.J.; Ruan, X.Z.; Liu, B.C.; et al. Dysbiosis of Intestinal Microbiota Mediates Tubulointerstitial Injury in Diabetic Nephropathy via the Disruption of Cholesterol Homeostasis. Theranostics 2020, 10, 2803–2816. [Google Scholar] [CrossRef]
- Li, Y.; Su, X.; Gao, Y.; Lv, C.; Gao, Z.; Liu, Y.; Wang, Y.; Li, S.; Wang, Z. The Potential Role of the Gut Microbiota in Modulating Renal Function in Experimental Diabetic Nephropathy Murine Models Established in Same Environment. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165764. [Google Scholar] [CrossRef]
- Wang, S.; Xia, G.-H.; He, Y.; Liao, S.-X.; Yin, J.; Sheng, H.-F.; Zhou, H.-W. Distribution Characteristics of Trimethylamine N-Oxide and Its Association with Gut Microbiota. Nan Fang Yi Ke Da Xue Xue Bao 2016, 36, 455–460. [Google Scholar]
- Chen, Y.Y.; Chen, D.Q.; Chen, L.; Liu, J.R.; Vaziri, N.D.; Guo, Y.; Zhao, Y.Y. Microbiome-Metabolome Reveals the Contribution of Gut-Kidney Axis on Kidney Disease. J. Transl. Med. 2019, 17, 5. [Google Scholar] [CrossRef]
- Sabatino, A.; Regolisti, G.; Cosola, C.; Gesualdo, L.; Fiaccadori, E. Intestinal Microbiota in Type 2 Diabetes and Chronic Kidney Disease. Curr. Diab. Rep. 2017, 17, 16. [Google Scholar] [CrossRef]
- Mafra, D.; Barros, A.F.; Fouque, D. Dietary Protein Metabolism by Gut Microbiota and Its Consequences for Chronic Kidney Disease Patients. Future Microbiol. 2013, 8, 1317–1323. [Google Scholar] [CrossRef]
- Nyangale, E.P.; Mottram, D.S.; Gibson, G.R. Gut Microbial Activity, Implications for Health and Disease: The Potential Role of Metabolite Analysis. J. Proteome Res. 2012, 11, 5573–5585. [Google Scholar] [CrossRef]
- Barrios, C.; Beaumont, M.; Pallister, T.; Villar, J.; Goodrich, J.K.; Clark, A.; Pascual, J.; Ley, R.E.; Spector, T.D.; Bell, J.T.; et al. Gut–Microbiota–Metabolite Axis in Early Renal Function Decline. PLoS ONE 2015, 10, e0134311. [Google Scholar] [CrossRef]
- Wong, J.; Piceno, Y.M.; DeSantis, T.Z.; Pahl, M.; Andersen, G.L.; Vaziri, N.D. Expansion of Urease- and Uricase-Containing, Indole- and p- Cresol-Forming and Contraction of Short-Chain Fatty Acid-Producing Intestinal Microbiota in ESRD. Am. J. Nephrol. 2014, 39, 230–237. [Google Scholar] [CrossRef]
- Adeshirlarijaney, A.; Gewirtz, A.T. Considering Gut Microbiota in Treatment of Type 2 Diabetes Mellitus. Gut Microbes 2020, 11, 253–264. [Google Scholar] [CrossRef]
- Andreasen, A.S.; Larsen, N.; Pedersen-Skovsgaard, T.; Berg, R.M.; Møller, K.; Svendsen, K.D.; Jakobsen, M.; Pedersen, B.K. Effects of Lactobacillus acidophilus NCFM on Insulin Sensitivity and the Systemic Inflammatory Response in Human Subjects. Br. J. Nutr. 2010, 104, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Tiderencel, K.A.; Hutcheon, D.A.; Ziegler, J. Probiotics for the Treatment of Type 2 Diabetes: A Review of Randomized Controlled Trials. Diabetes Metab. Res. Rev. 2020, 36, e3213. [Google Scholar] [CrossRef]
- Stenman, L.K.; Waget, A.; Garret, C.; Klopp, P.; Burcelin, R.; Lahtinen, S. Potential Probiotic Bifidobacterium animalis ssp. Lactis 420 Prevents Weight Gain and Glucose Intolerance in Diet-Induced Obese Mice. Benef. Microbes 2014, 5, 437–445. [Google Scholar] [CrossRef]
- Remely, M.; Hippe, B.; Zanner, J.; Aumueller, E.; Brath, H.; Haslberger, A.G. Gut Microbiota of Obese, Type 2 Diabetic Individuals is Enriched in Faecalibacterium prausnitzii, Akkermansia muciniphila and Peptostreptococcus anaerobius after Weight Loss. Endocr. Metab. Immune Disord. Drug Targets 2016, 16, 99–106. [Google Scholar] [CrossRef]
- Verma, A.; Xu, K.; Du, T.; Zhu, P.; Liang, Z.; Liao, S.; Zhang, J.; Raizada, M.K.; Grant, M.B.; Li, Q. Expression of Human ACE2 in Lactobacillus and Beneficial Effects in Diabetic Retinopathy in Mice. Mol. Ther. Methods Clin. Dev. 2019, 14, 161–170. [Google Scholar] [CrossRef]
- Moreira, G.V.; Azevedo, F.F.; Ribeiro, L.M.; Santos, A.; Guadagnini, D.; Gama, P.; Liberti, E.A.; Saad, M.J.A.; Carvalho, C.R.O. Liraglutide Modulates Gut Microbiota and Reduces NAFLD in Obese Mice. J. Nutr. Biochem. 2018, 62, 143–154. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Y.; Yan, Y.; Tian, S.; Zheng, D.; Leng, D.; Wang, C.; Jiao, J.; Wang, Z.; Bai, Y. Promising Treatment for Type 2 Diabetes: Fecal Microbiota Transplantation Reverses Insulin Resistance and Impaired Islets. Front. Cell. Infect. Microbiol. 2020, 9, 455. [Google Scholar] [CrossRef]
- Ganesan, K.; Chung, S.K.; Vanamala, J.; Xu, B. Causal Relationship between Diet-Induced Gut Microbiota Changes and Diabetes: A Novel Strategy to Transplant Faecalibacterium prausnitzii in Preventing Diabetes. Int. J. Mol. Sci. 2018, 19, 3720. [Google Scholar] [CrossRef]
- Xu, J.; Liang, R.; Zhang, W.; Tian, K.; Li, J.; Chen, X.; Yu, T.; Chen, Q. Faecalibacterium prausnitzii-Derived Microbial Anti-Inflammatory Molecule Regulates Intestinal Integrity in Diabetes mellitus Mice via Modulating Tight Junction Protein Expression. J. Diabetes 2020, 12, 224–236. [Google Scholar] [CrossRef]
- Bonora, E.; Cigolini, M.; Bosello, O.; Zancanaro, C.; Capretti, L.; Zavaroni, I.; Coscelli, C.; Butturini, U. Lack of Effect of Intravenous Metformin on Plasma Concentrations of Glucose, Insulin, C-Peptide, Glucagon and Growth Hormone in Non-Diabetic Subjects. Curr. Med. Res. Opin. 1984, 9, 47–51. [Google Scholar] [CrossRef]
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Mannerås-Holm, L.; Ståhlman, M.; Olsson, L.M.; Serino, M.; Planas-Fèlix, M.; et al. Metformin Alters the Gut Microbiome of Individuals with Treatment-Naive Type 2 Diabetes, Contributing to the Therapeutic Effects of the Drug. Nat. Med. 2017, 23, 850–858. [Google Scholar] [CrossRef]
- Rosario, D.; Benfeitas, R.; Bidkhori, G.; Zhang, C.; Uhlen, M.; Shoaie, S.; Mardinoglu, A. Understanding the Representative Gut Microbiota Dysbiosis in Metformin-Treated Type 2 Diabetes Patients Using Genome-Scale Metabolic Modeling. Front. Physiol. 2018, 9, 775. [Google Scholar] [CrossRef]
- Han, K.; Bose, S.; Wang, J.-H.; Lim, S.-K.; Chin, Y.-W.; Kim, Y.-M.; Choi, H.-S.; Kim, H. In Vivo Therapeutic Effect of Combination Treatment with Metformin and Scutellaria baicalensis on Maintaining Bile Acid Homeostasis. PLoS ONE 2017, 12, e0182467. [Google Scholar] [CrossRef]
- Li, Q.; He, R.; Zhang, F.; Zhang, J.; Lian, S.; Liu, H. Combination of Oligofructose and Metformin Alters the Gut Microbiota and Improves Metabolic Profiles, Contributing to the Potentiated Therapeutic Effects on Diet-Induced Obese Animals. Front. Endocrinol. (Lausanne) 2020, 10, 939. [Google Scholar] [CrossRef]
- Chen, M.; Liao, Z.; Lu, B.; Wang, M.; Lin, L.; Zhang, S.; Li, Y.; Liu, D.; Liao, Q.; Xie, Z. Huang-Lian-Jie-Du-Decoction Ameliorates Hyperglycemia and Insulin Resistant in Association with Gut Microbiota Modulation. Front. Microbiol. 2018, 9, 2380. [Google Scholar] [CrossRef]
- Cui, H.-X.; Zhang, L.-S.; Luo, Y.; Yuan, K.; Huang, Z.-Y.; Guo, Y. A Purified Anthraquinone-Glycoside Preparation from Rhubarb Ameliorates Type 2 Diabetes Mellitus by Modulating the Gut Microbiota and Reducing Inflammation. Front. Microbiol. 2019, 10, 1423. [Google Scholar] [CrossRef]
- Yang, J.; Dong, H.; Wang, Y.; Jiang, Y.; Zhang, W.; Lu, Y.; Chen, Y.; Chen, L. Cordyceps cicadae Polysaccharides Ameliorated Renal Interstitial Fibrosis in Diabetic Nephropathy Rats by Repressing Inflammation and Modulating Gut Microbiota Dysbiosis. Int. J. Biol. Macromol. 2020, 163, 442–456. [Google Scholar] [CrossRef]
- Meng, X.; Ma, J.; Kang, A.N.; Kang, S.Y.; Jung, H.W.; Park, Y.K. A Novel Approach Based on Metabolomics Coupled with Intestinal Flora Analysis and Network Pharmacology to Explain the Mechanisms of Action of Bekhogainsam Decoction in the Improvement of Symptoms of Streptozotocin-Induced Diabetic Nephropathy in Mice. Front. Pharmacol. 2020, 11, 633. [Google Scholar] [CrossRef]
- Meng, X.; Ma, J.; Kang, S.Y.; Jung, H.W.; Park, Y.K. Jowiseungki Decoction Affects Diabetic Nephropathy in Mice through Renal Injury Inhibition as Evidenced by Network Pharmacology and Gut Microbiota Analyses. Chin. Med. 2020, 15, 24. [Google Scholar] [CrossRef]
- Zhao, T.T.; Zhang, H.J.; Yin, X.; Zhao, H.L.; Ma, L.; Yan, M.H.; Peng, L.; Wang, Q.; Dong, X.; Li, P. Tangshen Formula Modulates Gut Microbiota and Reduces Gut-Derived Toxins in Diabetic Nephropathy Rats. Biomed. Pharmacother. 2020, 129, 110325. [Google Scholar] [CrossRef]
- Xie, J.; Song, W.; Liang, X.; Zhang, Q.; Shi, Y.; Liu, W.; Shi, X. Protective Effect of Quercetin on Streptozotocin-Induced Diabetic Peripheral Neuropathy Rats through Modulating Gut Microbiota and Reactive Oxygen Species Level. Biomed. Pharmacother. 2020, 127, 110147. [Google Scholar] [CrossRef]
- Pang, B.; Zhao, L.-H.; Zhou, Q.; Zhao, T.-Y.; Wang, H.; Gu, C.-J.; Tong, X.-L. Application of Berberine on Treating Type 2 Diabetes mellitus. Int. J. Endocrinol. 2015, 2015, 905749. [Google Scholar] [CrossRef]
- Wang, Y.; Shou, J.-W.; Li, X.-Y.; Zhao, Z.-X.; Fu, J.; He, C.-Y.; Feng, R.; Ma, C.; Wen, B.-Y.; Guo, F.; et al. Berberine-Induced Bioactive Metabolites of the Gut Microbiota Improve Energy Metabolism. Metabolism 2017, 70, 72–84. [Google Scholar] [CrossRef]
- Li, Y.J.; Chen, X.; Kwan, T.K.; loh, Y.W.; Singer, J.; Liu, Y.; Ma, J.; Tan, J.; Macia, L.; Mackay, C.R.; et al. Dietary Fiber Protects against Diabetic Nephropathy through Short-Chain Fatty Acid-Mediated Activation of G Protein-Coupled Receptors GPR43 and GPR109A. J. Am. Soc. Nephrol. 2020, 31, 1267–1281. [Google Scholar] [CrossRef]
- Xie, X.; Dai, S.; Dai, Y.; Ning, J. Influence of Dietary Fiber on Chronic Kidney Disease: Base on the Gut-Kidney Axis Theory. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2020, 45, 193–197. [Google Scholar] [CrossRef]
| Main Focus | Salient Findings | Country | Year and Reference |
|---|---|---|---|
| physiopathology | The disturbed microbiota increased the production of acetate in DM rats, causing early kidney injuries of DN by activating renal RAS | China | 2020 |
| [134] | |||
| physiopathology | Tubulointerstitial injury present in DN is induced by high acetate levels produced by the GM | China | 2020 |
| [135] | |||
| physiopathology | Specific bacteria from the intestinal microbiota influences the renal function in mice with diabetic kidney disease | China | 2020 |
| [136] | |||
| physiopathology | Activation of the GLP-1/GLP-1 receptor complex attenuates proximal tubular reabsorption and growth, ameliorating early manifestation of DN | USA and Ireland | 2014 |
| [131] | |||
| physiopathology/therapy | Phenyl is correlated with early kidney damage in diabetic patients, making it a valuable potential marker to identify diabetic individuals that are at risk of developing DN; its involvement in different molecular mechanisms that lead to podocyte injury makes targeting its intestinal production a possible pharmaceutical option | Italy | 2020 |
| [133] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanase, D.M.; Gosav, E.M.; Neculae, E.; Costea, C.F.; Ciocoiu, M.; Hurjui, L.L.; Tarniceriu, C.C.; Maranduca, M.A.; Lacatusu, C.M.; Floria, M.; et al. Role of Gut Microbiota on Onset and Progression of Microvascular Complications of Type 2 Diabetes (T2DM). Nutrients 2020, 12, 3719. https://doi.org/10.3390/nu12123719
Tanase DM, Gosav EM, Neculae E, Costea CF, Ciocoiu M, Hurjui LL, Tarniceriu CC, Maranduca MA, Lacatusu CM, Floria M, et al. Role of Gut Microbiota on Onset and Progression of Microvascular Complications of Type 2 Diabetes (T2DM). Nutrients. 2020; 12(12):3719. https://doi.org/10.3390/nu12123719
Chicago/Turabian StyleTanase, Daniela Maria, Evelina Maria Gosav, Ecaterina Neculae, Claudia Florida Costea, Manuela Ciocoiu, Loredana Liliana Hurjui, Claudia Cristina Tarniceriu, Minela Aida Maranduca, Cristina Mihaela Lacatusu, Mariana Floria, and et al. 2020. "Role of Gut Microbiota on Onset and Progression of Microvascular Complications of Type 2 Diabetes (T2DM)" Nutrients 12, no. 12: 3719. https://doi.org/10.3390/nu12123719
APA StyleTanase, D. M., Gosav, E. M., Neculae, E., Costea, C. F., Ciocoiu, M., Hurjui, L. L., Tarniceriu, C. C., Maranduca, M. A., Lacatusu, C. M., Floria, M., & Serban, I. L. (2020). Role of Gut Microbiota on Onset and Progression of Microvascular Complications of Type 2 Diabetes (T2DM). Nutrients, 12(12), 3719. https://doi.org/10.3390/nu12123719










