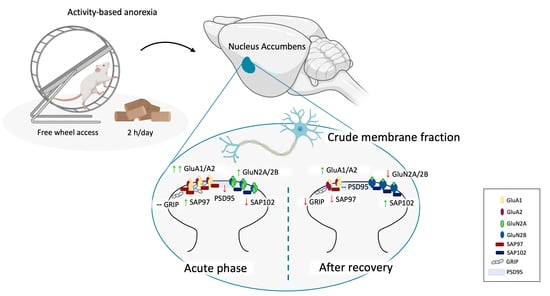
Activity-Based Anorexia Dynamically Dysregulates the Glutamatergic Synapse in the Nucleus Accumbens of Female Adolescent Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Housing
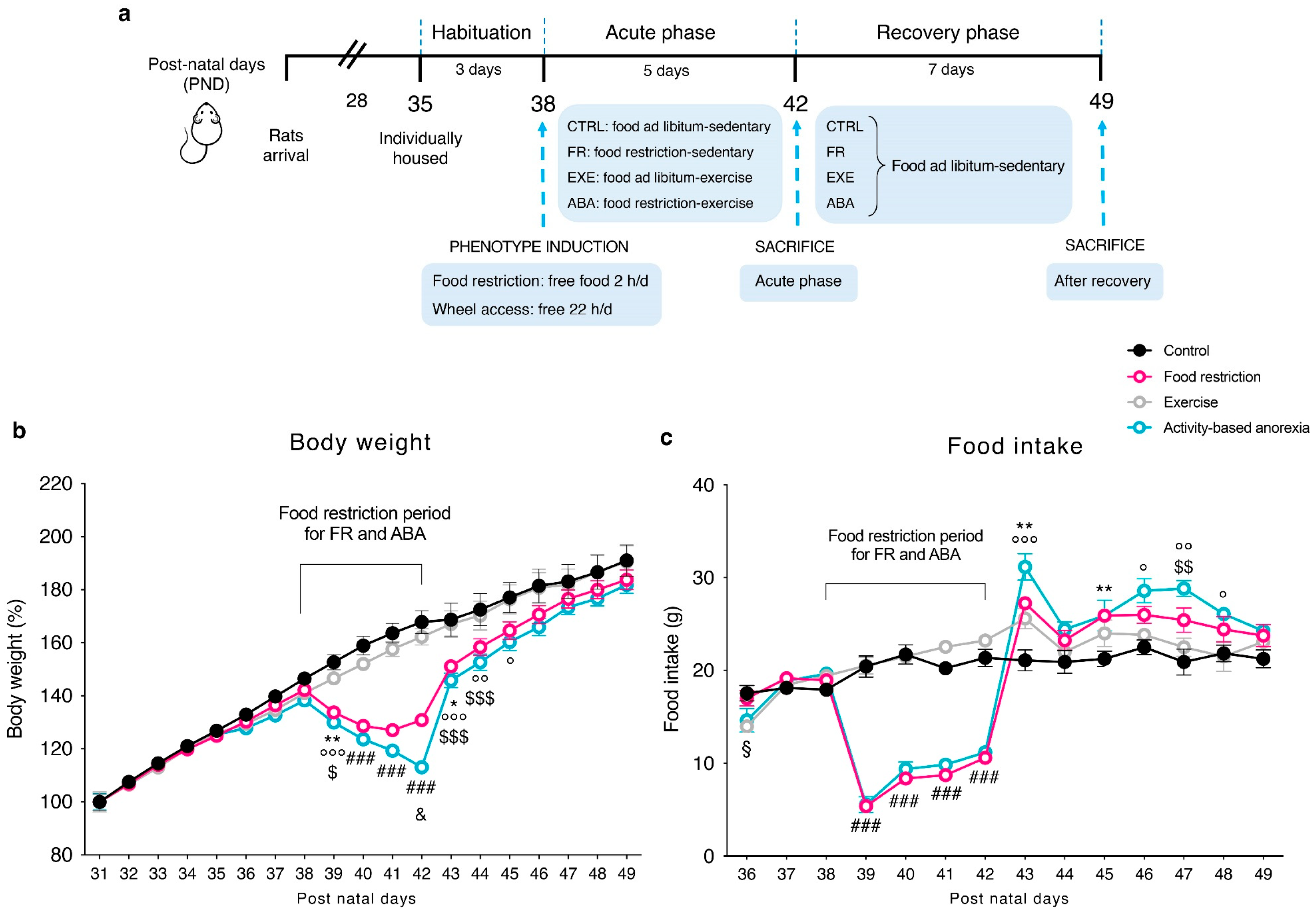
2.2. Experimental Design and Procedures
2.3. Measurements
2.4. Preparation of Protein Extracts and Western Blot Analyses
2.5. Data Analysis and Statistics
3. Results
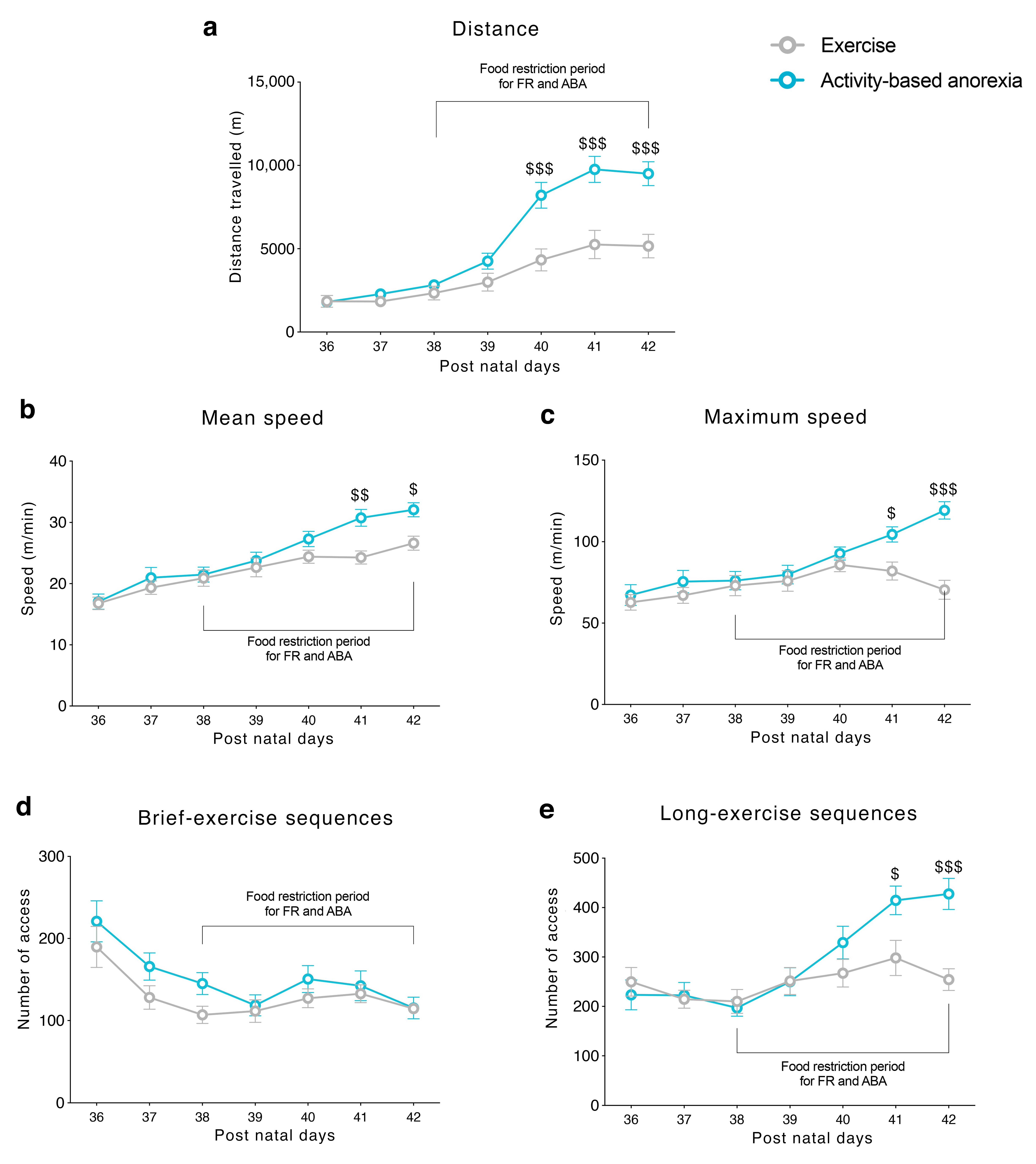
3.1. Food Restriction Elicited Hyperactive Running Behavior
3.2. Food Restriction-Evoked Hyperactivity Remodeled the Molecular Composition of the Glutamate Synapse in the NAc
3.3. Food Restriction-Evoked Hyperactivity Correlated with GluA1/A2 Ratio
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sullivan, P.F. Mortality in anorexia nervosa. Am. J. Psychiatry 1995, 152, 1073–1074. [Google Scholar]
- Arcelus, J.; Mitchell, A.J.; Wales, J.; Nielsen, S. Mortality Rates in Patients with Anorexia Nervosa and Other Eating Disorders. Arch. Gen. Psychiatry 2011, 68, 724–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klump, K.L. Puberty as a critical risk period for eating disorders: A review of human and animal studies. Horm. Behav. 2013, 64, 399–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagl, M.; Jacobi, C.; Paul, M.; Beesdo-Baum, K.; Höfler, M.; Lieb, R.; Wittchen, H.-U. Prevalence, incidence, and natural course of anorexia and bulimia nervosa among adolescents and young adults. Eur. Child Adolesc. Psychiatry 2016, 25, 903–918. [Google Scholar] [CrossRef]
- Schmidt, N.M.; Glymour, M.M.; Osypuk, T.L. Adolescence Is a Sensitive Period for Housing Mobility to Influence Risky Behaviors: An Experimental Design. J. Adolesc. Heal. 2016, 60, 431–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eddy, K.T.; Le Grange, D.; Crosby, R.D.; Hoste, R.R.; Doyle, A.C.; Smyth, A.; Herzog, D.B. Diagnostic classification of eating disorders in children and adolescents: How does DSM-IV-TR compare to empirically-derived categories? J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 277–293. [Google Scholar] [CrossRef]
- American Psychiatric Association. DSM-5, Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Philadelphia, PA, USA, 2013. [Google Scholar]
- Casper, R.C. Behavioral activation and lack of concern, core symptoms of anorexia nervosa? Int. J. Eat. Disord. 1998, 24, 381–393. [Google Scholar] [CrossRef]
- Skowron, K.; Kurnik-Łucka, M.; Dadański, E.; Bętkowska-Korpała, B.; Gil, K. Backstage of Eating Disorder—About the Biological Mechanisms behind the Symptoms of Anorexia Nervosa. Nutrients 2020, 12, 2604. [Google Scholar] [CrossRef]
- Rizk, M.; Mattar, L.; Kern, L.; Berthoz, S.; Jeanne, D.; Viltart, O.; Godart, N. Physical Activity in Eating Disorders: A Systematic Review. Nutrients 2020, 12, 183. [Google Scholar]
- Lloyd, E.; Frampton, I.; Verplanken, B.B.; Haase, A. How extreme dieting becomes compulsive: A novel hypothesis for the role of anxiety in the development and maintenance of anorexia nervosa. Med. Hypotheses 2017, 108, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Steinglass, J.E.; Walsh, B.T. Neurobiological model of the persistence of anorexia nervosa. J. Eat. Disord. 2016, 4, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivo, G.; Gaudio, S.; Schiöth, H.B. Brain and Cognitive Development in Adolescents with Anorexia Nervosa: A Systematic Review of fMRI Studies. Nutrients 2019, 11, 1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lüscher, C.; Robbins, T.W.; Everitt, B.J. The transition to compulsion in addiction. Nat. Rev. Neurosci. 2020, 21, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Zink, C.F.; Weinberger, D.R. Cracking the moody brain: The rewards of self starvation. Nat. Med. 2010, 16, 1382–1383. [Google Scholar] [CrossRef]
- Wierenga, C.E.; Ely, A.; Bischoff-Grethe, A.; Bailer, U.F.; Simmons, A.N.; Kaye, W.H. Are Extremes of Consumption in Eating Disorders Related to an Altered Balance between Reward and Inhibition? Front. Behav. Neurosci. 2014, 8, 410. [Google Scholar] [CrossRef] [Green Version]
- Kelley, A.E. Ventral striatal control of appetitive motivation: Role in ingestive behavior and reward-related learning. Neurosci. Biobehav. Rev. 2004, 27, 765–776. [Google Scholar] [CrossRef]
- Fladung, A.-K.; Schulze, U.M.E.; Scholl, F.G.; Bauer, K.A.; Gron, G. Role of the ventral striatum in developing anorexia nervosa. Transl. Psychiatry 2013, 3, e315. [Google Scholar] [CrossRef] [Green Version]
- Foldi, C.J.; Milton, L.K.; Oldfield, B.J. The Role of Mesolimbic Reward Neurocircuitry in Prevention and Rescue of the Activity-Based Anorexia (ABA) Phenotype in Rats. Neuropsychopharmacology 2017, 42, 2292–2300. [Google Scholar] [CrossRef]
- Milton, L.K.; Mirabella, P.N.; Greaves, E.; Spanswick, D.C.; Buuse, M.V.D.; Oldfield, B.J.; Foldi, C.J. Suppression of cortico-striatal circuit activity improves cognitive flexibility and prevents body weight loss in activity-based anorexia in rats. Biol. Psychiatry 2020. [Google Scholar] [CrossRef]
- O’Hara, C.B.; Campbell, I.C.; Schmidt, U. A reward-centred model of anorexia nervosa: A focussed narrative review of the neurological and psychophysiological literature. Neurosci. Biobehav. Rev. 2015, 52, 131–152. [Google Scholar] [CrossRef]
- Wang, S.; Tan, Y.; Zhang, J.-E.; Luo, M. Pharmacogenetic activation of midbrain dopaminergic neurons induces hyperactivity. Neurosci. Bull. 2013, 29, 517–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beeler, J.A.; Mourra, D.; Zanca, R.M.; Kalmbach, A.; Gellman, C.; Klein, B.Y.; Ravenelle, R.; Serrano, P.; Moore, H.; Rayport, S.; et al. Vulnerable and Resilient Phenotypes in a Mouse Model of Anorexia Nervosa. Biol. Psychiatry 2020. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.D.; Kashima, D.T.; Manz, K.M.; Grueter, C.A.; Grueter, B.A. Synaptic Plasticity in the Nucleus Accumbens: Lessons Learned from Experience. ACS Chem. Neurosci. 2017, 9, 2114–2126. [Google Scholar] [CrossRef] [PubMed]
- Scofield, M.D.; Heinsbroek, J.A.; Gipson, C.D.; Kupchik, Y.M.; Spencer, S.; Smith, A.C.W.; Roberts-Wolfe, D.; Kalivas, P.W. The Nucleus Accumbens: Mechanisms of Addiction across Drug Classes Reflect the Importance of Glutamate Homeostasis. Pharm. Rev. 2016, 68, 816–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, M.E. Synaptic mechanisms underlying persistent cocaine craving. Nat. Rev. Neurosci. 2016, 17, 351–365. [Google Scholar] [CrossRef]
- Ouyang, J.; Carcea, I.; Schiavo, J.K.; Jones, K.T.; Rabinowitsch, A.; Kolaric, R.; De Vaca, S.C.; Froemke, R.C.; Carr, K.D. Food restriction induces synaptic incorporation of calcium-permeable AMPA receptors in nucleus accumbens. Eur. J. Neurosci. 2017, 45, 826–836. [Google Scholar] [CrossRef]
- Castro-Fornieles, J.; Bargallo, N.; Lázaro, L.; Andrés, S.; Falcón, C.; Plana, M.T.; Junque, C. Adolescent anorexia nervosa: Cross-sectional and follow-up frontal gray matter disturbances detected with proton magnetic resonance spectroscopy. J. Psychiatr. Res. 2007, 41, 952–958. [Google Scholar] [CrossRef]
- Routtenberg, A.; Kuznesof, A.W. Self-starvation of rats living in activity wheels on a restricted feeding schedule. J. Comp. Physiol. Psychol. 1967, 64, 414–421. [Google Scholar] [CrossRef]
- Carrera, O.; Fraga, Á.; Pellón, R.; Gutiérrez, E. Rodent Model of Activity-Based Anorexia. Curr. Protoc. Neurosci. 2014, 67, 9.47.1–9.47.11. [Google Scholar] [CrossRef]
- Chen, X.; Levy, J.M.; Hou, A.; Winters, C.A.; Azzam, R.; Sousa, A.A.; Leapman, R.D.; Nicoll, R.A.; Reese, T.S. PSD-95 family MAGUKs are essential for anchoring AMPA and NMDA receptor complexes at the postsynaptic density. Proc. Natl. Acad. Sci. USA 2015, 112, E6983–E6992. [Google Scholar] [CrossRef] [Green Version]
- Chapman, R.H.; Stern, J.M. Maternal stress and pituitary–adrenal manipulations during pregnancy in rats: Effects on morphology and sexual behavior of male offspring. J. Comp. Physiol. Psychol. 1978, 92, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 7th ed.; Eselvier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Caffino, L.; Piva, A.; Mottarlini, F.; Di Chio, M.; Giannotti, G.; Chiamulera, C.; Fumagalli, F. Ketamine Self-Administration Elevates alphaCaMKII Autophosphorylation in Mood and Reward-Related Brain Regions in Rats. Mol. Neurobiol. 2018, 55, 5453–5461. [Google Scholar] [CrossRef] [PubMed]
- Caffino, L.; Piva, A.; Giannotti, G.; Di Chio, M.; Mottarlini, F.; Venniro, M.; Yew, D.T.; Chiamulera, C.; Fumagalli, F. Ketamine Self-Administration Reduces the Homeostasis of the Glutamate Synapse in the Rat Brain. Mol. Neurobiol. 2017, 54, 7186–7193. [Google Scholar] [CrossRef] [PubMed]
- Caffino, L.; Verheij, M.M.M.; Roversi, K.; Targa, G.; Mottarlini, F.; Popik, P.; Nikiforuk, A.; Golebiowska, J.; Fumagalli, F.; Homberg, J.R. Hypersensitivity to amphetamine’s psychomotor and reinforcing effects in serotonin transporter knockout rats: Glutamate in the nucleus accumbens. Br. J. Pharm. 2020, 177, 4532–4547. [Google Scholar]
- Naisbitt, S.; Valtschanoff, J.; Allison, D.W.; Sala, C.; Kim, E.; Craig, A.M.; Weinberg, R.J.; Sheng, M. Interaction of the Postsynaptic Density-95/Guanylate Kinase Domain-Associated Protein Complex with a Light Chain of Myosin-V and Dynein. J. Neurosci. 2000, 20, 4524–4534. [Google Scholar] [CrossRef] [Green Version]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Actor-Engel, H.; Sherpa, A.D.; Klingensmith, L.; Chowdhury, T.G.; Aoki, C. NR2A- and NR2B-NMDA receptors and drebrin within postsynaptic spines of the hippocampus correlate with hunger-evoked exercise. Brain Struct. Funct. 2017, 222, 2271–2294. [Google Scholar] [CrossRef]
- Robinette, T.M.; Nicholatos, J.W.; Francisco, A.B.; Brooks, K.E.; Diao, R.Y.; Sorbi, S.; Ricca, V.; Nacmias, B.; Brieño-Enríquez, M.A.; Libert, S. SIRT1 accelerates the progression of activity-based anorexia. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Vickers, C.A.; Stephens, B.; Bowen, J.; Arbuthnott, G.; Grant, S.G.; Ingham, C.A. Neurone specific regulation of dendritic spines in vivo by post synaptic density 95 protein (PSD-95). Brain Res. 2006, 1090, 89–98. [Google Scholar] [CrossRef]
- Müller, B.M.; Kistner, U.; Kindler, S.; Chung, W.J.; Kuhlendahl, S.; Fenster, S.D.; Lau, L.-F.; Veh, R.W.; Huganir, R.L.; Gundelfinger, E.D.; et al. SAP102, a Novel Postsynaptic Protein That Interacts with NMDA Receptor Complexes In Vivo. Neuron 1996, 17, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Foldi, C.J.; Milton, L.K.; Oldfield, B.J. A focus on reward in anorexia nervosa through the lens of the activity-based anorexia rodent model. J. Neuroendocr. 2017, 29, e12479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strober, M.; Freeman, R.; Morrell, W. The long-term course of severe anorexia nervosa in adolescents: Survival analysis of recovery, relapse, and outcome predictors over 10–15 years in a prospective study. Int. J. Eat. Disord. 1997, 22, 339–360. [Google Scholar] [CrossRef]
- Taranis, L.; Meyer, C. Associations between specific components of compulsive exercise and eating-disordered cognitions and behaviors among young women. Int. J. Eat. Disord. 2010, 44, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Reimers, J.M.; Milovanovic, M.; Wolf, M.E. Quantitative analysis of AMPA receptor subunit composition in addiction-related brain regions. Brain Res. 2011, 1367, 223–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.-H.; Takamiya, K.; Petralia, R.S.; Sattler, R.; Yu, S.; Zhou, W.; Kalb, R.G.; Wenthold, R.; Huganir, R. Persistent hippocampal CA1 LTP in mice lacking the C-terminal PDZ ligand of GluR1. Nat. Neurosci. 2005, 8, 985–987. [Google Scholar] [CrossRef]
- Liu, S.J.; Zukin, R.S. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007, 30, 126–134. [Google Scholar] [CrossRef]
- Bellone, C.; Luscher, C. Drug-evoked plasticity: Do addictive drugs reopen a critical period of postnatal synaptic development? Front. Mol. Neurosci. 2012, 5, 75. [Google Scholar] [CrossRef] [Green Version]
- Bergh, C.; Södersten, P. Anorexia nervosa, self–starvation and the reward of stress. Nat. Med. 1996, 2, 21–22. [Google Scholar] [CrossRef]
- Monyer, H.; Burnashev, N.; Laurie, D.J.; Sakmann, B.; Seeburg, P.H. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 1994, 12, 529–540. [Google Scholar] [CrossRef]
- Yuan, T.; Mameli, M.; O’Connor, E.C.; Dey, P.N.; Verpelli, C.; Sala, C.; Pérez-Otaño, I.; Lüscher, C.; Bellone, C. Expression of Cocaine-Evoked Synaptic Plasticity by GluN3A-Containing NMDA Receptors. Neuron 2013, 80, 1025–1038. [Google Scholar] [CrossRef] [Green Version]
- Vega-Villar, M.; Horvitz, J.C.; Nicola, S.M. NMDA receptor-dependent plasticity in the nucleus accumbens connects reward-predictive cues to approach responses. Nat. Commun. 2019, 10, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mottarlini, F.; Bottan, G.; Tarenzi, B.; Colciago, A.; Fumagalli, F.; Caffino, L. Activity-Based Anorexia Dynamically Dysregulates the Glutamatergic Synapse in the Nucleus Accumbens of Female Adolescent Rats. Nutrients 2020, 12, 3661. https://doi.org/10.3390/nu12123661
Mottarlini F, Bottan G, Tarenzi B, Colciago A, Fumagalli F, Caffino L. Activity-Based Anorexia Dynamically Dysregulates the Glutamatergic Synapse in the Nucleus Accumbens of Female Adolescent Rats. Nutrients. 2020; 12(12):3661. https://doi.org/10.3390/nu12123661
Chicago/Turabian StyleMottarlini, Francesca, Giorgia Bottan, Benedetta Tarenzi, Alessandra Colciago, Fabio Fumagalli, and Lucia Caffino. 2020. "Activity-Based Anorexia Dynamically Dysregulates the Glutamatergic Synapse in the Nucleus Accumbens of Female Adolescent Rats" Nutrients 12, no. 12: 3661. https://doi.org/10.3390/nu12123661
APA StyleMottarlini, F., Bottan, G., Tarenzi, B., Colciago, A., Fumagalli, F., & Caffino, L. (2020). Activity-Based Anorexia Dynamically Dysregulates the Glutamatergic Synapse in the Nucleus Accumbens of Female Adolescent Rats. Nutrients, 12(12), 3661. https://doi.org/10.3390/nu12123661