Diabetes-Specific Nutrition Formulas in the Management of Patients with Diabetes and Cardiometabolic Risk
Abstract
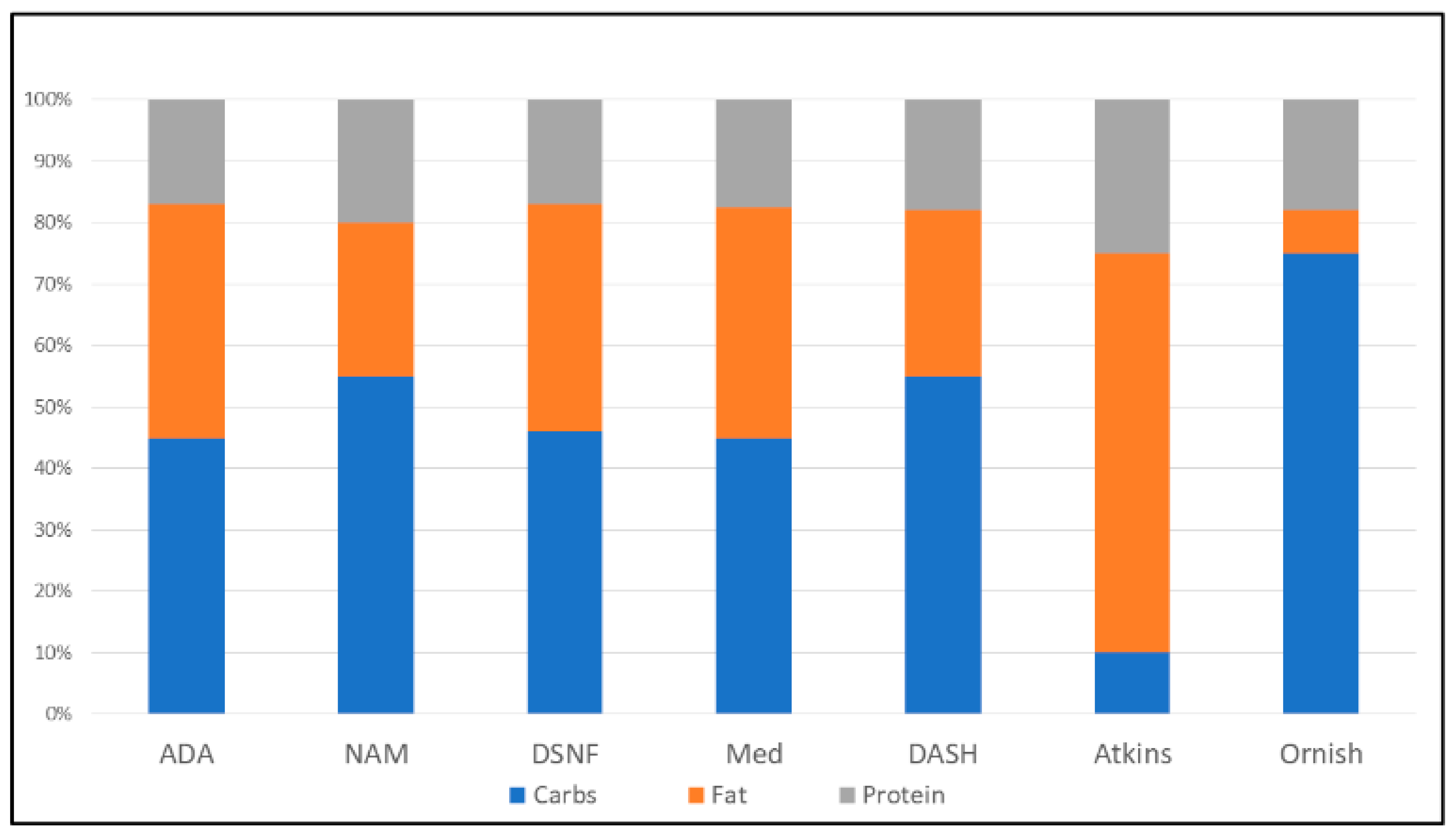
1. Introduction
2. Healthy Eating, Diabetes, and Cardiometabolic Risk
2.1. Carbohydrates
2.2. Fats
2.3. Proteins
2.4. Phytonutrients
2.5. Micronutrients
3. Evidence Base for DSNF, Diabetes, and Cardiometabolic Risk
3.1. Impact of DSNF on Glycemic Status
3.2. Impact of DSNF on Lipid Status
3.3. Impact of DSNF on Hormonal and Inflammatory Markers
3.4. Impact on Blood Pressure
4. Type 1 Diabetes
5. The Economics of DSNF Use
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e563–e595. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Lifestyle management: Standards of Medical Care in Diabetes 2019. Diabetes Care 2019, 42 (Suppl. S1), S46–S60. [Google Scholar] [CrossRef] [PubMed]
- Elia, M.; Ceriello, A.; Laube, H.; Sinclair, A.J.; Engfer, M.; Stratton, R.J. Enteral nutritional support and use of diabetes-specific formulas for patients with diabetes: A systematic review and meta-analysis. Diabetes Care 2005, 28, 2267–2279. [Google Scholar] [CrossRef] [PubMed]
- Mottalib, A.; Abrahamson, M.J.; Pober, D.M.; Polak, R.; Eldib, A.H.; Tomah, S.; Ashrafzadeh, S.; Hamdy, O. Effect of diabetes-specific nutrition formulas on satiety and hunger hormones in patients with type 2 diabetes. Nutr. Diabetes 2019, 9, 1–6. [Google Scholar] [CrossRef]
- Dávila, L.A.; Bermúdez, V.; Aparicio, D.; Céspedes, V.; Escobar, M.C.; Agüero, S.D.; Cisternas, S.; Costa, J.D.A.; Rojas, D.M.; Reyna, N.; et al. Effect of Oral Nutritional Supplements with Sucromalt and Isomaltulose versus Standard Formula on Glycaemic Index, Entero-Insular Axis Peptides and Subjective Appetite in Patients with Type 2 Diabetes: A Randomised Cross-Over Study. Nutrients 2019, 11, 1477. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Farkouh, M.E.; Newman, J.D.; Garvey, W.T. Cardiometabolic-Based Chronic Disease, Adiposity and Dysglycemia Drivers: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 525–538. [Google Scholar] [CrossRef]
- Buranapin, S.; Siangruangsang, S.; Chantapanich, V.; Hengjeerajarus, N. The comparative study of diabetic specific formula and standard formula on postprandial plasma glucose control in type 2 DM patients. J. Med. Assoc. Thai. Chotmaihet Thangphaet 2014, 97, 582–588. [Google Scholar]
- Pohl, M.; Mayr, P.; Mertl-Roetzer, M.; Lauster, F.; Lerch, M.; Eriksen, J.; Haslbeck, M.; Rahlfs, V.W. Glycaemic control in type II diabetic tube-fed patients with a new enteral formula low in carbohydrates and high in monounsaturated fatty acids: A randomised controlled trial. Eur. J. Clin. Nutr. 2005, 59, 1221–1232. [Google Scholar] [CrossRef][Green Version]
- Lansink, M.; Van Laere, K.M.; Vendrig, L.; Rutten, G.E. Lower postprandial glucose responses at baseline and after 4 weeks use of a diabetes-specific formula in diabetes type 2 patients. Diabetes Res. Clin. Pract. 2011, 93, 421–429. [Google Scholar] [CrossRef]
- Vaisman, N.; Lansink, M.; Rouws, C.H.; Van Laere, K.M.; Segal, R.; Niv, E.; Bowling, T.E.; Waitzberg, D.L.; Morley, J.E. Tube feeding with a diabetes-specific feed for 12 weeks improves glycaemic control in type 2 diabetes patients. Clin. Nutr. 2009, 28, 549–555. [Google Scholar] [CrossRef]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition Therapy for Adults With Diabetes or Prediabetes: A Consensus Report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, N.; Vinknes, K.J.; Veierød, M.B.; Retterstol, K. Effects of low-carbohydrate dietsv. low-fat diets on body weight and cardiovascular risk factors: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2016, 115, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Sainsbury, E.; Kizirian, N.V.; Partridge, S.R.; Gill, T.; Colagiuri, S.; Gibson, A.A. Effect of dietary carbohydrate restriction on glycemic control in adults with diabetes: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2018, 139, 239–252. [Google Scholar] [CrossRef]
- Ang, M. Metabolic Response of Slowly Absorbed Carbohydrates in Type 2 Diabetes Mellitus, 1st ed.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Veronese, N.; Solmi, M.; Caruso, M.G.; Giannelli, G.; Osella, A.R.; Evangelos, E.; Maggi, S.; Fontana, L.; Stubbs, B.; Tzoulaki, I. Dietary fiber and health outcomes: An umbrella review of systematic reviews and meta-analyses. Am. J. Clin. Nutr. 2018, 107, 436–444. [Google Scholar] [CrossRef]
- Pereira, M.A.; O’Reilly, E.; Augustsson, K.; Augustsson, K.; Fraser, G.E.; Goldbourt, U.; Heitmann, B.L.; Hallmans, G.; Knekt, P.; Liu, S.; et al. Dietary fiber and risk of coronary heart disease: A pooled analysis of cohort studies. Arch. Intern. Med. 2004, 164, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, M.U.; O’Reilly, E.J.; Heitmann, B.L.; Pereira, M.A.; Bälter, K.; Fraser, G.E.; Goldbourt, U.; Hallmans, G.; Knekt, P.; Liu, S.; et al. Major types of dietary fat and risk of coronary heart disease: A pooled analysis of 11 cohort studies. Am. J. Clin. Nutr. 2009, 89, 1425–1432. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Micha, R.; Wallace, S. Effects on Coronary Heart Disease of Increasing Polyunsaturated Fat in Place of Saturated Fat: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS Med. 2010, 7, e1000252. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Summerbell, C.D.; Thompson, R.; Sills, D.; Roberts, F.G.; Moore, H.; Smith, G.D. Reduced or modified dietary fat for preventing cardiovascular disease. Cochrane Database Syst. Rev. 2011. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Pearson, T.A.; Wan, Y.; Hargrove, R.L.; Moriarty, K.; Fishell, V.; Etherton, T.D. High-monounsaturated fatty acid diets lower both plasma cholesterol and triacylglycerol concentrations. Am. J. Clin. Nutr. 1999, 70, 1009–1015. [Google Scholar] [CrossRef]
- Felig, P.; Marliss, E.; Cahill, G.F., Jr. Plasma amino acid levels and insulin secretion in obesity. N. Engl. J. Med. 1969, 281, 811–816. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Canela, M.; Toledo, E.; Clish, C.B.; Hruby, A.; Liang, L.; Salas-Salvadó, J.; Razquin, C.; Corella, D.; Estruch, R.; Ros, E.; et al. Plasma Branched-Chain Amino Acids and Incident Cardiovascular Disease in the PREDIMED Trial. Clin. Chem. 2016, 62, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.; Sharp, P.; Clifford, M.; Morgan, L. Dietary polyphenols decrease glucose uptake by human intestinal Caco-2 cells. FEBS Lett. 2005, 579, 1653–1657. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.H.; Kim, S.R.; Hwang, I.K.; Ha, T.Y. Hypoglycemic Effects of a Phenolic Acid Fraction of Rice Bran and Ferulic Acid in C57BL/KsJ-db/dbMice. J. Agric. Food Chem. 2007, 55, 9800–9804. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T.; Szkudelska, K. Anti-diabetic effects of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Wallerath, T.; Deckert, G.; Ternes, T.; Anderson, H.; Li, H.; Witte, K.; Förstermann, U. Resveratrol, a Polyphenolic Phytoalexin Present in Red Wine, Enhances Expression and Activity of Endothelial Nitric Oxide Synthase. Circulation 2002, 106, 1652–1658. [Google Scholar] [CrossRef]
- Poli, A.; Barbagallo, C.M.; Cicero, A.F.; Corsini, A.; Manzato, E.; Trimarco, B.; Bernini, F.; Zimetti, F.; Bianchi, A.; Canzone, G.; et al. Nutraceuticals and functional foods for the control of plasma cholesterol levels. An intersociety position paper. Pharmacol. Res. 2018, 134, 51–60. [Google Scholar] [CrossRef]
- Siddiqui, K.; Bawazeer, N.; Joy, S.S. Variation in Macro and Trace Elements in Progression of Type 2 Diabetes. Sci. World J. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Kahaly, G.J. Cardiovascular and Atherogenic Aspects of Subclinical Hypothyroidism. Thyroid 2000, 10, 665–679. [Google Scholar] [CrossRef]
- Kaur, B.; Henry, J. Micronutrient Status in Type 2 Diabetes: A Review. Adv. Food Nutr. Res. 2014, 71, 55–100. [Google Scholar] [CrossRef]
- García, O.P.; Long, K.Z.; Rosado, J.L. Impact of micronutrient deficiencies on obesity. Nutr. Rev. 2009, 67, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Ekpenyong, C.E. Micronutrient Vitamin Deficiencies and Cardiovascular Disease Risk: Advancing Current Understanding. Eur. J. Prev. Med. 2017, 5, 1. [Google Scholar] [CrossRef][Green Version]
- Ekpenyong, C.E. Micronutrient deficiency, a novel nutritional risk factor for insulin resistance and Syndrom X. Arch. Food Nutr. Sci. 2018, 2, 16–30. [Google Scholar] [CrossRef]
- Cvetinovic, N.; Loncar, G.; Isakovic, A.M.; Von Haehling, S.; Doehner, W.; Lainscak, M.; Farkas, J. Micronutrient Depletion in Heart Failure: Common, Clinically Relevant and Treatable. Int. J. Mol. Sci. 2019, 20, 5627. [Google Scholar] [CrossRef] [PubMed]
- Aasheim, E.T.; Hofsø, D.; Hjelmesæth, J.; Birkeland, K.I.; Bøhmer, T. Vitamin status in morbidly obese patients: A cross-sectional study. Am. J. Clin. Nutr. 2008, 87, 362–369. [Google Scholar] [CrossRef]
- Via, M. The Malnutrition of Obesity: Micronutrient Deficiencies That Promote Diabetes. ISRN Endocrinol. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Nix, W.A.; Zirwes, R.; Bangert, V.; Kaiser, R.P.; Schilling, M.; Hostalek, U.; Obeid, R. Vitamin B status in patients with type 2 diabetes mellitus with and without incipient nephropathy. Diabetes Res. Clin. Pract. 2015, 107, 157–165. [Google Scholar] [CrossRef]
- Pflipsen, M.C.; Oh, R.C.; Saguil, A.; Seehusen, D.A.; Seaquist, D.; Topolski, R. The Prevalence of Vitamin B12 Deficiency in Patients with Type 2 Diabetes: A Cross-Sectional Study. J. Am. Board Fam. Med. 2009, 22, 528–534. [Google Scholar] [CrossRef]
- Praveen, D.; Puvvada, R.C.; Vijey, A.M. Association of vitamin C status in diabetes mellitus: Prevalence and predictors of vitamin C deficiency. Future J. Pharm. Sci. 2020, 6, 1–5. [Google Scholar] [CrossRef]
- Daradkeh, G.; Zerie, M.; Othman, M.; Chandra, P.; Jaiosi, A.; Mahmood, L.; Alowainati, B.; Mohammad, I.; Daghash, M. Zinc Status among Type (2) Diabetes Mellitus in the State of Qatar. Public Health Front. 2014, 3, 4–10. [Google Scholar] [CrossRef]
- Gillespie, S.; Bold, M.V.D. Agriculture, Food Systems, and Nutrition: Meeting the Challenge. Glob. Chall. 2017, 1, 1600002. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Marchetti, A.E.; Apovian, C.; Benchimol, A.K.; Bisschop, P.H.; Bolio-Galvis, A.; Hegazi, R.A.; Jenkins, D.; Mendoza, E.; Sanz, M.L.; et al. Diabetes-Specific Nutrition Algorithm: A Transcultural Program to Optimize Diabetes and Prediabetes Care. Curr. Diabetes Rep. 2012, 12, 180–194. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Garber, A.J.; Grunberger, G.; Handelsman, Y.; Garvey, W.T. Dysglycemia-Based Chronic Disease: An American Association of Clinical Endocrinologists Position Statement. Endocr. Pract. 2018, 24, 995–1011. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Hurley, D.L.; Garvey, W.T. Adiposity-Based Chronic Disease as a New Diagnostic Term: The American Association of Clinical Endocrinologists and American College of Endocrinology Position Statement. Endocr. Pract. 2017, 23, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Look AHEAD Research Group; Wing, R.R. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: Four-year results of the Look AHEAD trial. Arch. Intern. Med. 2010, 170, 1566–1575. [Google Scholar] [CrossRef]
- Mottalib, A.; Mohd-Yusof, B.N.; Shehabeldin, M.; Pober, D.M.; Mitri, J.; Hamdy, O. Impact of Diabetes-Specific Nutritional Formulas versus Oatmeal on Postprandial Glucose, Insulin, GLP-1 and Postprandial Lipidemia. Nutrients 2016, 8, 443. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Lu, J.; Ma, X.; Ying, L.; Lu, W.; Zhu, W.; Bao, Y.; Zhou, J. Breakfast replacement with a liquid formula improves glycaemic variability in patients with type 2 diabetes: A randomised clinical trial. Br. J. Nutr. 2019, 121, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.; Weldon, S.M.; Thompson, T.; Crockett, R.; Wang, X.-H. The Effect of Diabetes-Specific Enteral Nutrition Formula on Cardiometabolic Parameters in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2019, 11, 1905. [Google Scholar] [CrossRef]
- Sanz-París, A.; Matía-Martín, P.; Martín-Palmero, Á.; Gómez-Candela, C.; Robles, M.C. Diabetes-specific formulas high in monounsaturated fatty acids and metabolic outcomes in patients with diabetes or hyperglycaemia. A systematic review and meta-analysis. Clin. Nutr. 2020, 39, 3273–3282. [Google Scholar] [CrossRef]
- Sanz-París, A.; Boj-Carceller, D.; Lardiés-Sánchez, B.; Perez-Fernandez, L.; Cruz-Jentoft, A.J. Health-Care Costs, Glycemic Control and Nutritional Status in Malnourished Older Diabetics Treated with a Hypercaloric Diabetes-Specific Enteral Nutritional Formula. Nutrients 2016, 8, 153. [Google Scholar] [CrossRef]
- Krinsley, J.S. Glycemic Variability and Mortality in Critically 111 Patients: The Impact of Diabetes. J. Diabetes Sci. Technol. 2009, 3, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Krinsley, J.S. Glycemic variability: A strong independent predictor of mortality in critically ill patients. Crit. Care Med. 2008, 36, 3008–3013. [Google Scholar] [CrossRef] [PubMed]
- Mesejo, A.; Montejo-González, J.C.; Vaquerizo-Alonso, C.; Lobo-Tamer, G.; Zabarte-Martinez, M.; Herrero-Meseguer, J.I.; Acosta, J.; Blesa-Malpica, A.; Martinez-Lozano, F. Diabetes-specific enteral nutrition formula in hyperglycemic, mechanically ventilated, critically ill patients: A prospective, open-label, blind-randomized, multicenter study. Crit. Care 2015, 19, 390. [Google Scholar] [CrossRef] [PubMed]
- Mohan, V.; Kalpana, N.; Lakshmipriya, N.; Anitha, P.; Gayathri, R.; Vijayalakshmi, P.; Krishnaswamy, K.; Unnikrishnan, R.; Anjana, R.M.; Vasudevan, S. A Pilot Study Evaluating the Effects of Diabetes Specific Nutrition Supplement and Lifestyle Intervention on Glycemic Control in Overweight and Obese Asian Indian Adults with Type 2 Diabetes Mellitus. J. Assoc. Phys. India 2019, 67, 25–30. [Google Scholar]
- Doola, R.; Deane, A.M.; Tolcher, D.M.; Presneill, J.J.; Barrett, H.L.; Forbes, J.M.; Todd, A.S.; Okano, S.; Sturgess, D.J. The effect of a low carbohydrate formula on glycaemia in critically ill enterally-fed adult patients with hyperglycaemia: A blinded randomised feasibility trial. Clin. Nutr. ESPEN 2019, 31, 80–87. [Google Scholar] [CrossRef]
- Mustad, V.A.; Hegazi, R.A.; Hustead, D.S.; Budiman, E.S.; Rueda, R.; Maki, K.; Powers, M.; Mechanick, J.I.; Bergenstal, R.M.; Hamdy, O. Use of a diabetes-specific nutritional shake to replace a daily breakfast and afternoon snack improves glycemic responses assessed by continuous glucose monitoring in people with type 2 diabetes: A randomized clinical pilot study. BMJ Open Diabetes Res. Care 2020, 8, e001258. [Google Scholar] [CrossRef]
- Yip, I.; Go, V.L.W.; Deshields, S.; Saltsman, P.; Bellman, M.; Thames, G.; Murray, S.; Wang, H.-J.; Elashoff, R.; Heber, D. Liquid Meal Replacements and Glycemic Control in Obese Type 2 Diabetes Patients. Obes. Res. 2001, 9, 341S–347S. [Google Scholar] [CrossRef]
- Mottalib, A.; Salsberg, V.; Mohd-Yusof, B.N.; Mohamed, W.; Carolan, P.; Pober, D.M.; Mitri, J.; Hamdy, O. Effects of nutrition therapy on HbA1c and cardiovascular disease risk factors in overweight and obese patients with type 2 diabetes. Nutr. J. 2018, 17, 42. [Google Scholar] [CrossRef]
- Lee, H.; Lee, I.S.; Choue, R. Obesity, inflammation and diet. Pediatr. Gastroenterol. Hepatol. Nutr. 2013, 16, 143–152. [Google Scholar] [CrossRef]
- Selvin, E.; Paynter, N.P.; Erlinger, T.P. The Effect of Weight Loss on C-Reactive Protein: A Systematic Review. Arch. Intern. Med. 2007, 167, 31–39. [Google Scholar] [CrossRef]
- Stenvers, D.J.; Schouten, L.J.; Jurgens, J.; Endert, E.; Kalsbeek, A.; Fliers, E.; Bisschop, P.H. Breakfast replacement with a low-glycaemic response liquid formula in patients with type 2 diabetes: A randomised clinical trial. Br. J. Nutr. 2014, 112, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Mechanick, J.I.; Zhao, S.; Garvey, W.T. The Adipokine-Cardiovascular-Lifestyle Network: Translation to Clinical Practice. J. Am. Coll. Cardiol. 2016, 68, 1785–1803. [Google Scholar] [CrossRef] [PubMed]
- The SPRINT Research Group A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N. Engl. J. Med. 2015, 373, 2103–2116. [CrossRef] [PubMed]
- Chee, W.S.S.; Singh, H.K.G.; Hamdy, O.; I Mechanick, J.; Lee, V.K.M.; Barua, A.; Ali, S.Z.M.; Hussein, Z. Structured lifestyle intervention based on a trans-cultural diabetes-specific nutrition algorithm (tDNA) in individuals with type 2 diabetes: A randomized controlled trial. BMJ Open Diabetes Res. Care 2017, 5, e000384. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, Y.; Chen, X.; Chen, Y.; Feng, Y.; Zhang, X.; Pan, Y.; Hu, T.; Xu, J.; Du, L.; et al. An integrated intervention program to control diabetes in overweight Chinese women and men with type 2 diabetes. Asia Pac. J. Clin. Nutr. 2008, 17, 514–524. [Google Scholar]
- The Look AHEAD Research Group Look AHEAD (Action for Health in Diabetes): Design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control. Clin. Trials 2003, 24, 610–628. [CrossRef]
- The Look AHEAD Research Group. Eight-year weight losses with an intensive lifestyle intervention: The look AHEAD study. Obesity 2014, 22, 5–13. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care 2017, 40 (Suppl. S1), 1–142. [Google Scholar]
- The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group. Risk Factors for Cardiovascular Disease in Type 1 Diabetes. Diabetes 2016, 65, 1370–1379. [Google Scholar] [CrossRef]
- Peters, A.L.; Davidson, M.B.; Isaac, R.M. Lack of glucose elevation after simulated tube feeding with a low-carbohydrate, high-fat enteral formula in patients with type I diabetes. Am. J. Med. 1989, 87, 178–182. [Google Scholar] [CrossRef]
- Peters, A.L.; Davidson, M.B. Effects of Various Enteral Feeding Products on Postprandial Blood Glucose Response in Patients with Type I Diabetes. J. Parenter. Enter. Nutr. 1992, 16, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Crespillo, M.d.C.; Olveira, G.; De Adana, M.S.R.; Rojo-Martínez, G.; García-Alemán, J.; Olvera, P.; Soriguer, F.; Muñoz, A. Metabolic effects of an enteral nutrition formula for diabetes: Comparison with standard formulas in patients with type 1 diabetes. Clin. Nutr. 2003, 22, 483–487. [Google Scholar] [CrossRef]
- Han, Y.-Y.; Lai, S.-R.; Partridge, J.S.; Wang, M.Y.; Sulo, S.; Tsao, F.-W.; Hegazi, R. The clinical and economic impact of the use of diabetes-specific enteral formula on ICU patients with type 2 diabetes. Clin. Nutr. 2017, 36, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Matía-Martín, P.; Agudo, F.R.; Medina, J.A.L.; Paris, A.S.; Santabalbina, F.T.; Pascual, J.R.D.; Penabad, L.L.; Barriuso, R.S.; GluceNut Study Group. Effectiveness of an oral diabetes-specific supplement on nutritional status, metabolic control, quality or life, and functional status in elderly patients. A multicentre study. Clin. Nutr. 2019, 38, 1253–1261. [Google Scholar] [CrossRef]
| Minerals | Impact on Dysglycemia-Based Chronic Disease (DBCD) Progression | Impact on Adiposity-Based Chronic Disease (ABCD) Progression |
|---|---|---|
| Calcium | Affects β-cell secretory function, insulin release, T2D complications. | |
| Chromium | Involved in carbohydrate metabolism and glucose homeostasis, cofactor for insulin action, component of glucose tolerance factor (GTF). | Supplements have been studied for their effects on cholesterol, heart disease risk, but results are unclear. Low levels linked to increased CVD risks. |
| Cobalt | Influences glycemic control, gluconeogenesis, tissue glucose uptake, GLUT-1 expression | Excessive cobalt levels cause toxicity that may lead to heart failure but overexposure is currently rare. |
| Copper | Affects glucose tolerance/intolerance, insulin response, and increased glucose via insulin-like activity. | Lipogenesis, hypercholesterolemia, atherosclerosis |
| Iodine | Correlated with thyroid stimulating hormone (TSH), which affects insulin resistance and β-cell function. | Hypothyroidism produces abnormal lipid profiles, elevated LDL-c and TC levels and raising the risk of atherosclerosis. It also weakens myocardial contractility and can cause cardiac arrhythmias. |
| Iron | May induce diabetes via oxidative damage to β cells, impairment of hepatic insulin extraction, and suppression of hepatic glucose production by insulin interference. | Iron deficiency may result in left ventricular dysfunction, especially when the hemoglobin level is less than 5 g/dL |
| Magnesium | Cofactor of many enzymes in carbohydrate metabolism. Involved in insulin metabolism, secretion, binding, and activity. Improves insulin resistance. | CVD risk, normotension state, rate and rhythm, arterial health. Low magnesium linked to CVD risk factors: hypertension, atherosclerosis with calcification |
| Manganese | Manganese-activated enzyme essential for the metabolism of carbohydrates, amino acids, and cholesterol. Needed for normal insulin production and secretion. Antioxidant. Inverse relationship with futureT2D | Component of potent antioxidant enzyme, manganese superoxide dismutase (MnSOD). Neutralizes the reactive oxygen species (ROS) in mitochondria. MnSOD also protects cells from inflammation. |
| Selenium | Antioxidant. Mimics insulin activity in models. Prevents development of diabetic complications. | In deficiency, lipid peroxides may collect in the heart, especially during ischemia, damage cell membranes, and impair calcium transport with intra-cellular accumulation |
| Vanadium | Affects glucose transport, glycolysis, glucose oxidation, insulin sensitivity, insulin signaling, and glycogen synthesis. | Facilitates lipid and amino acid metabolism. |
| Zinc | Cofactor in glucose metabolism. Required for insulin storage and cellular binding. | Cofactor for intracellular enzymes involved in lipid metabolism |
| Micronutrient | Deficiency Prevalence | Deficiency Prevalence |
|---|---|---|
| Obesity | T2D | |
| B1 Thiamine | 15–29% | 17–79% |
| B6 Pyridoxine | 0–11% | 58–63% |
| B9 Folic acid | 3–4% | 22% |
| B12 Cobalamin | 3–8% | 22% |
| B7 Biotin | NA | NA |
| Chromium | NA | NA |
| Selenium | 58% | NA |
| Vitamin A | 17% | NA |
| Vitamin C | 35–45% | 13–55% |
| Vitamin D | 80–90% | 85–91% |
| Vitamin E | 0% | 0% |
| Zinc | 14–30% | 19% |
| Clinical Scenario (Reference) | Cardiometabolic Risk(s) | Design | Population | Findings Intervention vs. Control | Meal Replacement(s) |
|---|---|---|---|---|---|
| [47] Outpatient. Weight loss. | CMBCD/Cardiovascular ABCD/Obesity DBCD/Diabetes, T2D | RCT | Overweight and obese patients. N = 5145 | ↓ body weight. | SlimFast (SlimFast Foods), Glucerna (Abbott Nutrition), OPTIFAST (Novartis Nutrition) and HMR (HMR, Inc., Boston, MA USA). |
| [48] Outpatient. Weight loss and glycemic control. | CMBCD/Cardiovascular ABCD/Obesity DBCD/Diabetes, T2D | RCT, 3 arms | Overweight and obese patients. A1C 8.7 +/− 1.5 N = 108 | ↓ A1C, body weight, body fat %, waist circumference. All p = 0.001 | Glucerna, (Abbott Nutrition): Carb-26 g, Fat-7 g, Prot-10 g per serving Ultra Glucose Control (Metagenics) carb-27 g, fat-7 g, prot-15 g |
| [4] Outpatient. Glycemic control | DBCD, T2D | RCT, 2 arms | Patients with T2D. N = 123 | Improved outcomes: SDBG (p = 0·005), CV (p = 0·002), MAGE (p = 0·016) and AUCpp (p < 0.001), SBP (p < 0.046) | Glucerna SR (Abbott Nutrition) carb-31 g, fat-8 g, prot-11 g per serving |
| [49] Inpatients and outpatients. DSNF oral and tube feeding vs. non-DSNF standard care | DBCD/Diabetes | Meta-analysis, 19 RCTs +4 non-RTC | Patients with T1D, T2D, or stress DM. N = 605 | ↓ PG, PPG, AUC-G, and insulin requirement | Various diabetes-specific formulas (containing high proportions of monounsaturated fatty acids, fructose, and fiber |
| [50] Varied settings. DSNF vs. standard enteral nutrition formula. | CMBCD/Cardiovascular ABCD/Obesity DBCD/Diabetes, T2D | Meta-analysis 4 RCTs +1 parallel design | Patients with T2D +/− complication. N = 269 | ↓ PPG, A1C ↑ HDL-c All p ≤ 0.01 | Various diabetes-specific formulas with average macronutrient proportions of carb-37–55%, fat-30–45%, prot-15–19% |
| [51] Varied settings. High MUFA vs. standard formula. | DBCD/T2D, T1D | Meta-analysis18 RCTs | Patients with T2D, T1D, or stress DM. Enteral nutrition. N = 845 | ↓ PG, PPG, AUC-G, A1C, and insulin requirement vs. baseline. Individual results all p < 0.05 | Various diabetes-specific formulas with MUFAs 20% of total energy or fat 40% of total energy |
| [52] Community or nursing home settings. Malnourished older patients. 1 year pre- and post-DSNF oral nutrition. | CMBCD/CVD ABCD DBCD/T2D | 1-year retrospective, 1-year prospective observational study | Patients with T2D. N = 93 | ↓hospital admissions (−54.7%, p < 0.001), hospital days (−64.1%, p < 0.001), emergency visits (57.7%, p < 0.001), healthcare costs (−65.6%, p < 0.001) year to year. | Glucerna® 1.5 Cal (Abbott Nutrition) carb-35%, fat-45%, prot-20% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mechanick, J.I.; Marchetti, A.; Hegazi, R.; Hamdy, O. Diabetes-Specific Nutrition Formulas in the Management of Patients with Diabetes and Cardiometabolic Risk. Nutrients 2020, 12, 3616. https://doi.org/10.3390/nu12123616
Mechanick JI, Marchetti A, Hegazi R, Hamdy O. Diabetes-Specific Nutrition Formulas in the Management of Patients with Diabetes and Cardiometabolic Risk. Nutrients. 2020; 12(12):3616. https://doi.org/10.3390/nu12123616
Chicago/Turabian StyleMechanick, Jeffrey I., Albert Marchetti, Refaat Hegazi, and Osama Hamdy. 2020. "Diabetes-Specific Nutrition Formulas in the Management of Patients with Diabetes and Cardiometabolic Risk" Nutrients 12, no. 12: 3616. https://doi.org/10.3390/nu12123616
APA StyleMechanick, J. I., Marchetti, A., Hegazi, R., & Hamdy, O. (2020). Diabetes-Specific Nutrition Formulas in the Management of Patients with Diabetes and Cardiometabolic Risk. Nutrients, 12(12), 3616. https://doi.org/10.3390/nu12123616





