Timing and Frequency of Daily Energy Intake in Adults with Prediabetes and Overweight or Obesity and Their Associations with Body Fat
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Examination
2.3. Dietary Intake
2.4. Classification of Valid Reporters
2.5. Statistical Analyses
3. Results
3.1. Characteristics
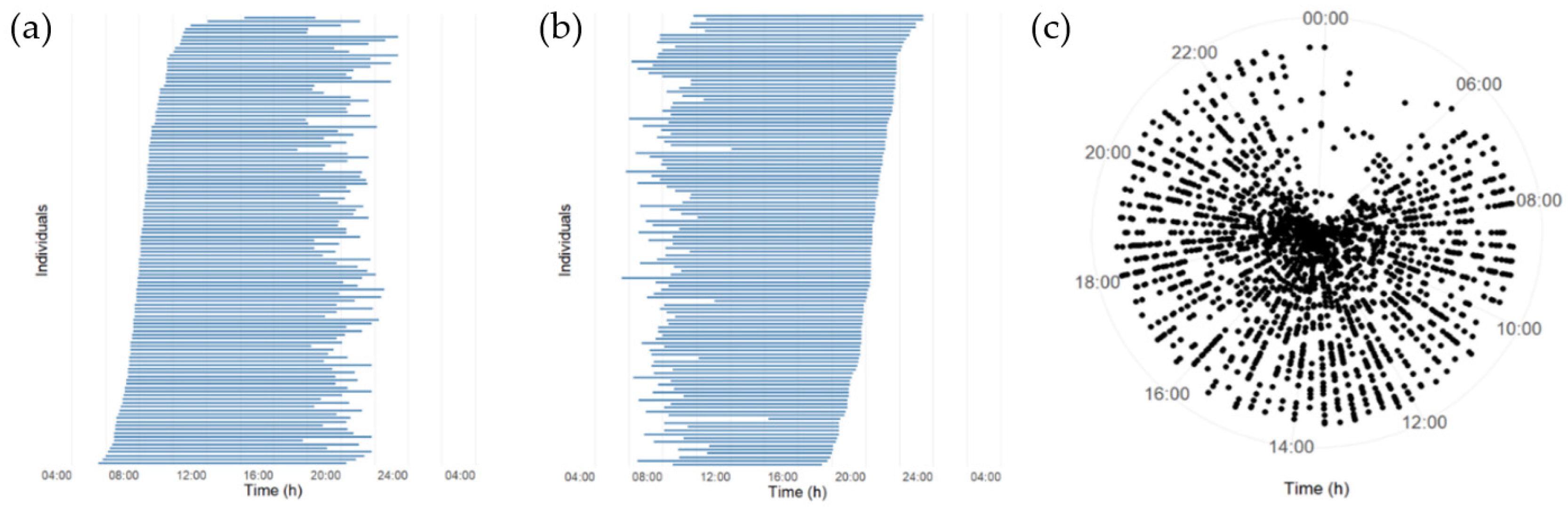
3.2. Daily Eating Patterns
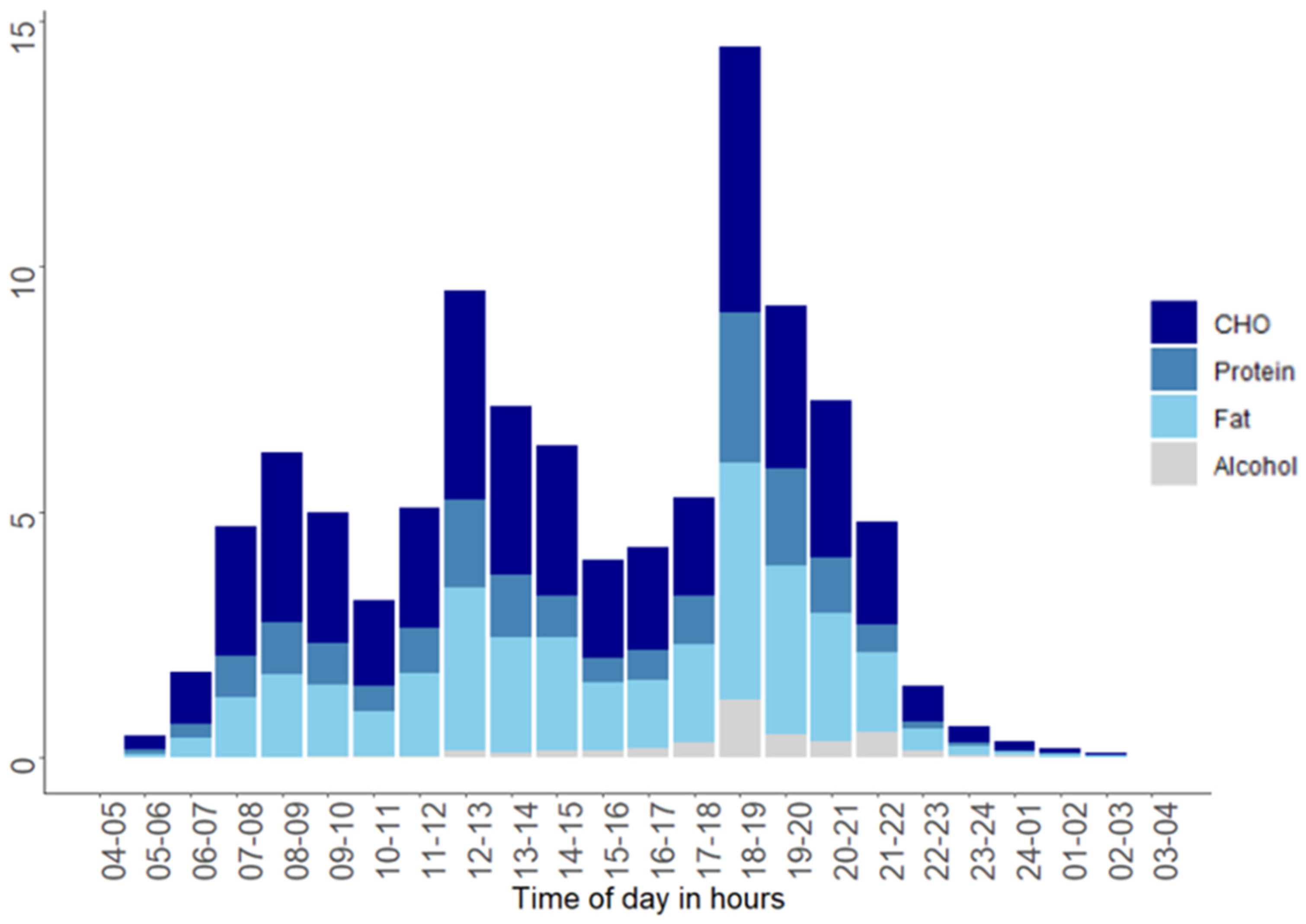
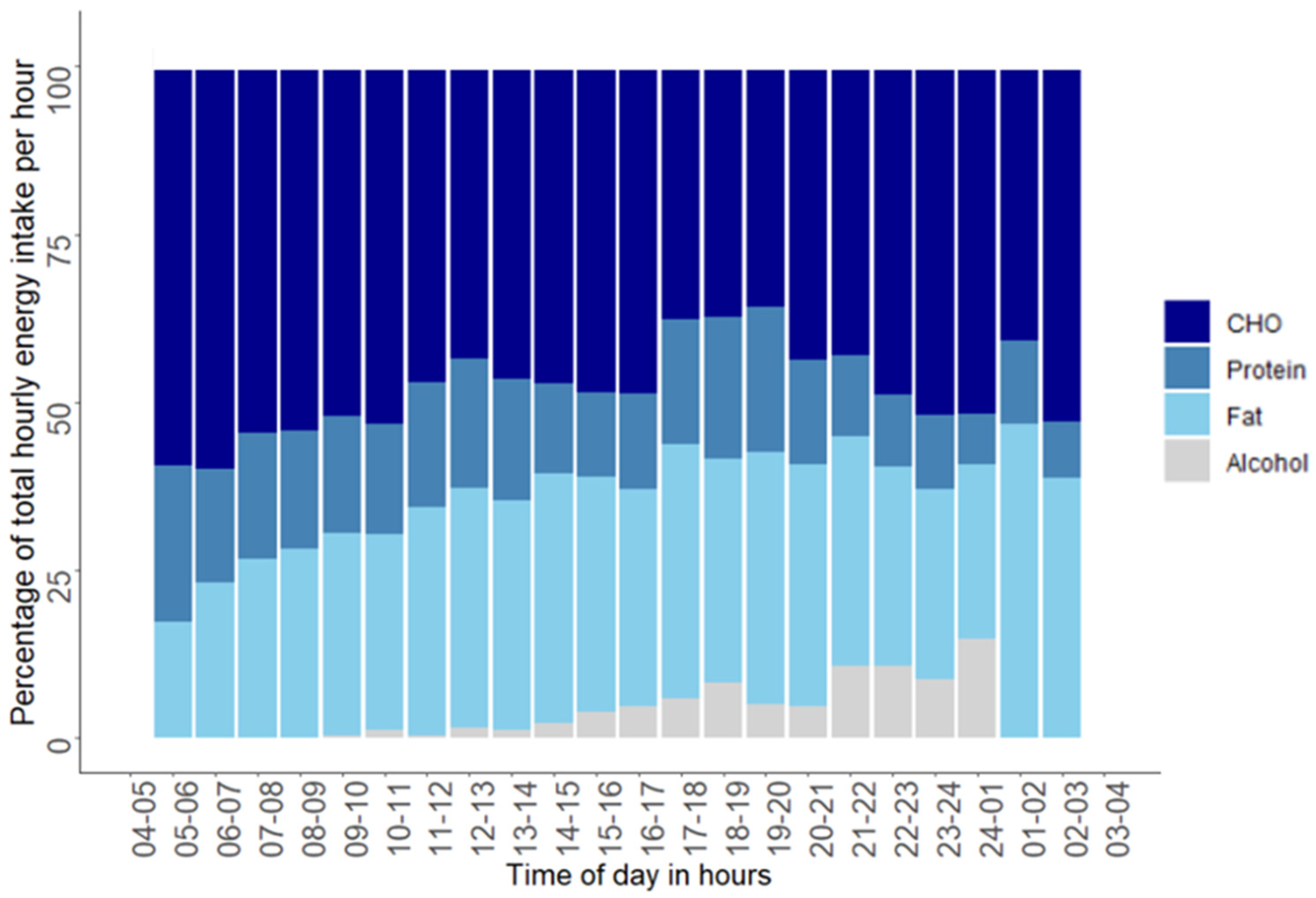
3.3. Diurnal Variation in Intake of Energy and Macronutrients
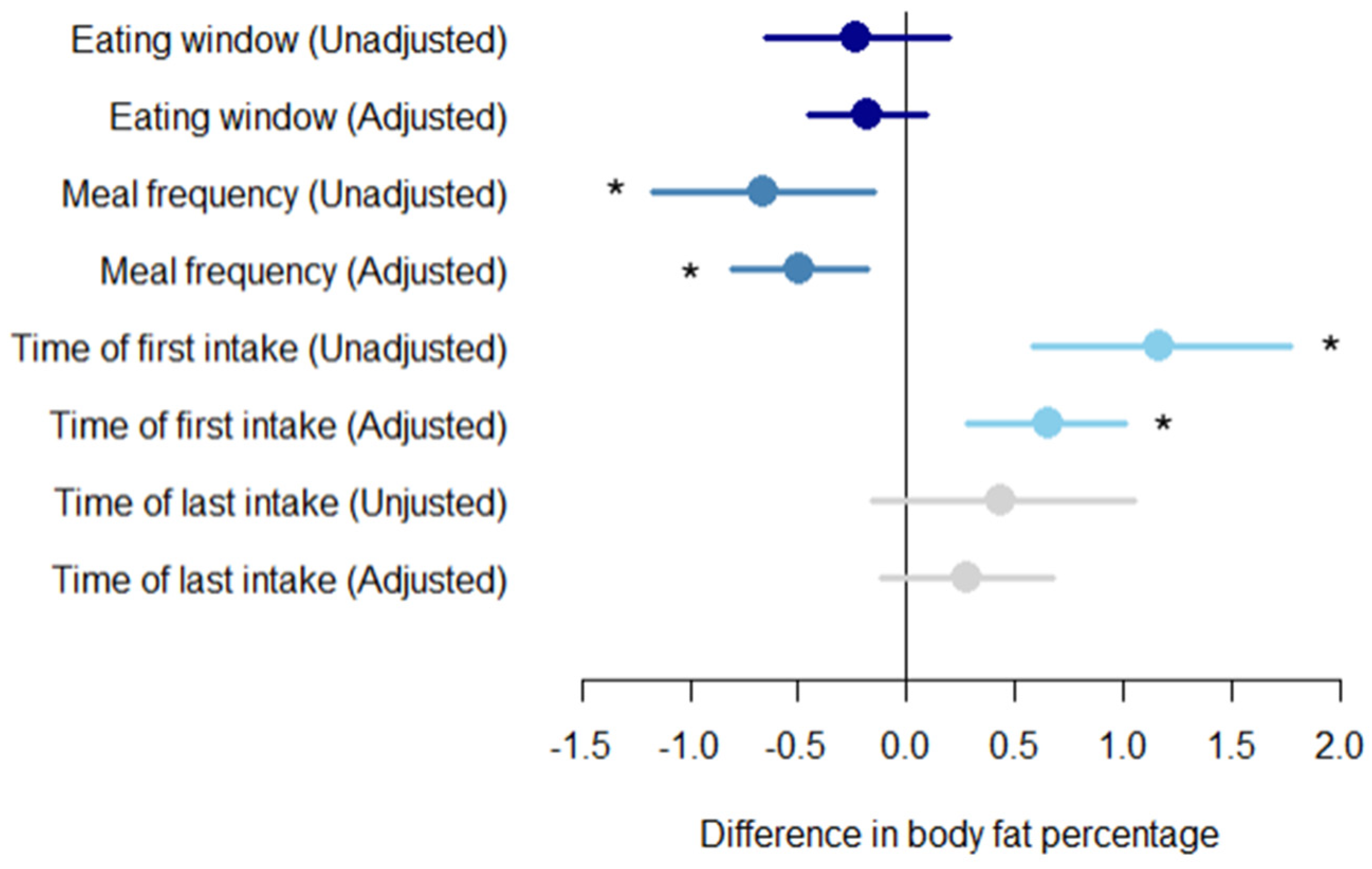
3.4. Relationship between Daily Eating Patterns and Body Fat Percentage
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ballico, E. On the x-rank of a points of the tangent developable of a curve in a projective space. Int. J. Pure Apllied Math. 2014, 97, 253–262. [Google Scholar] [CrossRef]
- Levesque, R.J.R. Obesity and Overweight. Encycl. Adolesc. 2018, 2561–2565. [Google Scholar] [CrossRef]
- Arroyo, K.; Herron, D.M. The Epidemiology of Obesity. Bariatr. Endosc. 2012, 1–9. [Google Scholar] [CrossRef]
- Anderson, J.W.; Konz, E.C.; Frederich, R.C.; Wood, C.L. Long-term weight-loss maintenance: A meta-analysis of US studies. Am. J. Clin. Nutr. 2001, 74, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Ministers, N.C.O.M.N.C.O. Nordic Nutrition Recommendations 2012. Nord. Nutr. Recomm. 2012 2008, 5, 1–3. [Google Scholar] [CrossRef]
- Slavin, J. Dietary Guidelines. Nutr. Today 2012, 47, 245–251. [Google Scholar] [CrossRef]
- American Diabetes Association. Nutrition Recommendations and Interventions for Diabetes: A position statement of the American Diabetes Association. Diabetes Care 2007, 31, S61–S78. [Google Scholar] [CrossRef]
- Gill, S.; Panda, S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab. 2015, 22, 789–798. [Google Scholar] [CrossRef]
- Melkani, G.C.; Panda, S. Time-restricted feeding for prevention and treatment of cardiometabolic disorders. J. Physiol. 2017, 595, 3691–3700. [Google Scholar] [CrossRef]
- Rothschild, J.; Hoddy, K.K.; Jambazian, P.; Varady, K.A. Time-restricted feeding and risk of metabolic disease: A review of human and animal studies. Nutr. Rev. 2014, 72, 308–318. [Google Scholar] [CrossRef]
- Speechly, D.; Buffenstein, R. Greater Appetite Control Associated with an Increased Frequency of Eating in Lean Males. Appetite 1999, 33, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Howarth, N.C.; Huang, T.T.-K.; Roberts, S.B.; Lin, B.-H.; A McCrory, M. Eating patterns and dietary composition in relation to BMI in younger and older adults. Int. J. Obes. 2006, 31, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Gómez-Abellán, P.; Alburquerque-Béjar, J.J.; Lee, Y.-C.; Ordovás, J.M.; Scheer, F.A.J.L. Timing of food intake predicts weight loss effectiveness. Int. J. Obes. 2013, 37, 604–611. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.-P.; Ard, J.; American Heart Association Obesity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; Stroke Council; Baskin, M.L.; Chiuve, S.E.; Johnson, H.M.; Kris-Etherton, P.; et al. Meal Timing and Frequency: Implications for Cardiovascular Disease Prevention: A Scientific Statement From the American Heart Association. Circulation 2017, 135, e96–e121. [Google Scholar] [CrossRef]
- Færch, K.; Blond, M.B.; Bruhn, L.; Amadid, H.; Vistisen, D.; Clemmensen, K.K.B.; Vainø, C.T.R.; Pedersen, C.; Tvermosegaard, M.; Dejgaard, T.F.; et al. The effects of dapagliflozin, metformin or exercise on glycaemic variability in overweight or obese individuals with prediabetes (the PRE-D Trial): A multi-arm, randomised, controlled trial. Diabetologia 2020, 1–14. [Google Scholar] [CrossRef]
- Færch, K.; Amadid, H.; Nielsen, L.B.; Ried-Larsen, M.; Karstoft, K.; Persson, F.; Jørgensen, M.E. Protocol for a randomised controlled trial of the effect of dapagliflozin, metformin and exercise on glycaemic variability, body composition and cardiovascular risk in prediabetes (the PRE-D Trial). BMJ Open 2017, 7, e013802. [Google Scholar] [CrossRef]
- Li, C.; Ford, E.S.; Zhao, G.; Balluz, L.S.; Giles, W.H. Estimates of body composition with dual-energy X-ray absorptiometry in adults. Am. J. Clin. Nutr. 2009, 90, 1457–1465. [Google Scholar] [CrossRef]
- Lau, C.J.; Færch, K.; Glumer, C.; Toft, U.; Tetens, I.; Borch-Johnsen, K.; Jørgensen, T. Evaluation of dietary intake in a Danish population: The Inter99 study. Scand. J. Nutr. 2004, 48, 136–143. [Google Scholar] [CrossRef]
- The Danish Veterinary and Food Administration. The Danish Veterinary and Food Administration, Ministry of Environment and Food, Danish Dietary Recommendations n.d. Available online: https://altomkost.dk/english/#c41067 (accessed on 3 November 2020).
- Dietitian Net Pro. 2016. n.d. Available online: http://www.kostdata.se/en/dietitian-net/dietitian-net-pro (accessed on 23 January 2020).
- McHill, A.W.; Czeisler, C.A.; Phillips, A.J.; Keating, L.; Barger, L.K.; Garaulet, M.; Scheer, F.A.J.L.; Klerman, E.B. Caloric and Macronutrient Intake Differ with Circadian Phase and between Lean and Overweight Young Adults. Nutrients 2019, 11, 587. [Google Scholar] [CrossRef]
- Black, A.E. Critical evaluation of energy intake using the Goldberg cut-off for energy intake:basal metabolic rate. A practical guide to its calculation, use and limitations. Int. J. Obes. 2000, 24, 1119–1130. [Google Scholar] [CrossRef]
- Henry, C.J.K. Basal metabolic rate studies in humans: Measurement and development of new equations. Public Health Nutr. 2005, 8, 1133–1152. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations; World Health Organization; United Nations University. Human Energy Requirements. Report of a Joint FAO/WHO/UNU Expert Consultation. Available online: http://www.fao.org/3/y5686e/y5686e00.htm (accessed on 8 February 2019).
- Kant, A.K. Eating patterns of US adults: Meals, snacks, and time of eating. Physiol. Behav. 2018, 193, 270–278. [Google Scholar] [CrossRef] [PubMed]
- McHill, A.W.; Phillips, A.J.; A Czeisler, C.; Keating, L.; Yee, K.; Barger, L.K.; Garaulet, M.; Scheer, F.A.; Klerman, E.B. Later circadian timing of food intake is associated with increased body fat. Am. J. Clin. Nutr. 2017, 106, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Anton, S.D.; Lee, S.; Donahoo, W.T.; McLaren, C.; Manini, T.M.; Leeuwenburgh, C.; Pahor, M. The Effects of Time Restricted Feeding on Overweight, Older Adults: A Pilot Study. Nutrients 2019, 11, 1500. [Google Scholar] [CrossRef] [PubMed]
- Antoni, R.; Robertson, T.M.; Robertson, M.D.; Johnston, J.D. A pilot feasibility study exploring the effects of a moderate time-restricted feeding intervention on energy intake, adiposity and metabolic physiology in free-living human subjects. J. Nutr. Sci. 2018, 7. [Google Scholar] [CrossRef]
- LeCheminant, J.D.; Christenson, E.; Bailey, B.W.; Tucker, L.A. Restricting night-time eating reduces daily energy intake in healthy young men: A short-term cross-over study. Br. J. Nutr. 2013, 110, 2108–2113. [Google Scholar] [CrossRef]
- Aljuraiban, G.S.; Chan, Q.; Griep, L.M.O.; Brown, I.J.; Daviglus, M.L.; Stamler, J.; Van Horn, L.; Elliott, P.; Frost, G.S. The Impact of Eating Frequency and Time of Intake on Nutrient Quality and Body Mass Index: The INTERMAP Study, a Population-Based Study. J. Acad. Nutr. Diet. 2015, 115, 528–536.e1. [Google Scholar] [CrossRef]
- Ritchie, L.D. Less frequent eating predicts greater BMI and waist circumference in female adolescents. Am. J. Clin. Nutr. 2012, 95, 290–296. [Google Scholar] [CrossRef]
- Forslund, H.B.; Klingström, S.; Hagberg, H.; Löndahl, M.; Torgerson, J.S.; Lindroos, A.K. Should snacks be recommended in obesity treatment? A 1-year randomized clinical trial. Eur. J. Clin. Nutr. 2008, 62, 1308–1317. [Google Scholar] [CrossRef][Green Version]
- Scheer, F.A.; Morris, C.J.; Shea, S.A. The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity 2013, 21, 421–423. [Google Scholar] [CrossRef]
- De Castro, J.M. The Time of Day of Food Intake Influences Overall Intake in Humans. J. Nutr. 2004, 134, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. High Caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity 2013, 21, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Arble, D.M.; Bass, J.; Laposky, A.D.; Vitaterna, M.H.; Turek, F.W. Circadian Timing of Food Intake Contributes to Weight Gain. Obesity 2009, 17, 2100–2102. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.J.; Baron, K.G.; Zee, P.C. Meal timing influences daily caloric intake in healthy adults. Nutr. Res. 2014, 34, 930–935. [Google Scholar] [CrossRef]
- Bandín, C.; Scheer, F.A.J.L.; Luque, A.J.; Ávila-Gandía, V.; Zamora, S.; A Madrid, J.; Gomezabellan, P.; Garaulet, M. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: A randomized, crossover trial. Int. J. Obes. 2015, 39, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Lissner, L.; Heitmann, B.L.; Bengtsson, C. Population studies of diet and obesity. Br. J. Nutr. 2000, 83, S21–S24. [Google Scholar] [CrossRef] [PubMed]
- Tooze, J.A.; Krebs-Smith, S.M.; Troiano, R.P.; Subar, A.F. The accuracy of the Goldberg method for classifying misreporters of energy intake on a food frequency questionnaire and 24-h recalls: comparison with doubly labeled water. Eur. J. Clin. Nutr. 2012, 66, 569–576. [Google Scholar] [CrossRef]
- Poslusna, K.; Ruprich, J.; De Vries, J.H.M.; Jakubikova, M.; Veer, P.V. Misreporting of energy and micronutrient intake estimated by food records and 24 h recalls, control and adjustment methods in practice. Br. J. Nutr. 2009, 101, S73–S85. [Google Scholar] [CrossRef]
- Fyfe, C.L.; Stewart, J.; Murison, S.D.; Jackson, D.M.; Rance, K.; Speakman, J.R.; Horgan, G.W.; Johnstone, A.M. Evaluating energy intake measurement in free-living subjects: When to record and for how long? Public Health Nutr. 2010, 13, 172–180. [Google Scholar] [CrossRef]
- Booth, A.O.; Lowis, C.; Dean, M.; Hunter, S.J.; McKinley, M.C. Diet and physical activity in the self-management of type 2 diabetes: Barriers and facilitators identified by patients and health professionals. Prim. Health Care Res. Dev. 2013, 14, 293–306. [Google Scholar] [CrossRef]
| All (n = 119) | Women (n = 66) | Men (n = 53) | |
|---|---|---|---|
| Age (years) | 57.8 (9.3) | 57.9 (8.9) | 57.7 (9.7) |
| Height (m) | 1.71 (0.09) | 1.65 (0.06) | 1.80 (0.07) |
| Weight (kg) | 93.3 (17.8) | 86.0 (16.2) | 102.3 (15.5) |
| BMI (kg/m2) | 31.5 (5.0) | 31.4 (5.8) | 31.6 (3.9) |
| Body fat (%) | 38.0 (7.4) | 43.3 (4.5) | 31.4 (4.4) |
| HbA1c (mmol/mol) | 41 (2) | 41 (2) | 41 (2) |
| Dietary intake | |||
| Daily energy intake (kJ/day) | 8246 (2578) | 7402 (2301) | 9299 (2523) |
| CHO (g) | 226 (78) | 205 (70) | 252 (78) |
| CHO (E%) | 46.6 % | 47.1 % | 46.1 % |
| Fat (g) | 75 (31) | 70 (31) | 81 (30) |
| Fat (E%) | 33.7 % | 35.0 % | 32.2 % |
| Protein (g) | 85 (23) | 77 (18) | 96 (25) |
| Protein (E%) | 17.5 % | 17.7 % | 17.6 % |
| Alcohol (g) | 11 (18) | 5 (7) | 18 (23) |
| Alcohol (E%) | 3.8 % | 2.0 % | 5.6 % |
| Daily eating patterns | |||
| Daily eating window (hours) | 12.3 (1.8) | 12.2 (1.6) | 12.3 (2.1) |
| Number of meals (meals/day) | 5.6 (1.5) | 5.6 (1.3) | 5.7 (1.8) |
| Time of first intake (hours) | 8.23 (1.3) | 8.36 (1.1) | 8.07 (1.4) |
| Time of last intake (hours) | 20.41 (1.3) | 20.46 (1.3) | 20.35 (1.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mogensen, C.S.; Færch, K.; Bruhn, L.; Amadid, H.; Tetens, I.; Quist, J.S.; Clemmensen, K.K.B. Timing and Frequency of Daily Energy Intake in Adults with Prediabetes and Overweight or Obesity and Their Associations with Body Fat. Nutrients 2020, 12, 3484. https://doi.org/10.3390/nu12113484
Mogensen CS, Færch K, Bruhn L, Amadid H, Tetens I, Quist JS, Clemmensen KKB. Timing and Frequency of Daily Energy Intake in Adults with Prediabetes and Overweight or Obesity and Their Associations with Body Fat. Nutrients. 2020; 12(11):3484. https://doi.org/10.3390/nu12113484
Chicago/Turabian StyleMogensen, Christina Sonne, Kristine Færch, Lea Bruhn, Hanan Amadid, Inge Tetens, Jonas Salling Quist, and Kim Katrine Bjerring Clemmensen. 2020. "Timing and Frequency of Daily Energy Intake in Adults with Prediabetes and Overweight or Obesity and Their Associations with Body Fat" Nutrients 12, no. 11: 3484. https://doi.org/10.3390/nu12113484
APA StyleMogensen, C. S., Færch, K., Bruhn, L., Amadid, H., Tetens, I., Quist, J. S., & Clemmensen, K. K. B. (2020). Timing and Frequency of Daily Energy Intake in Adults with Prediabetes and Overweight or Obesity and Their Associations with Body Fat. Nutrients, 12(11), 3484. https://doi.org/10.3390/nu12113484





