Whey Versus Casein as a Protein Source during the Weaning Period: Impact on Growth and Later Adiposity and Glucose Homeostasis in a Rat Model of Intrauterine Growth Restriction
Abstract
1. Introduction
2. Material and Methods
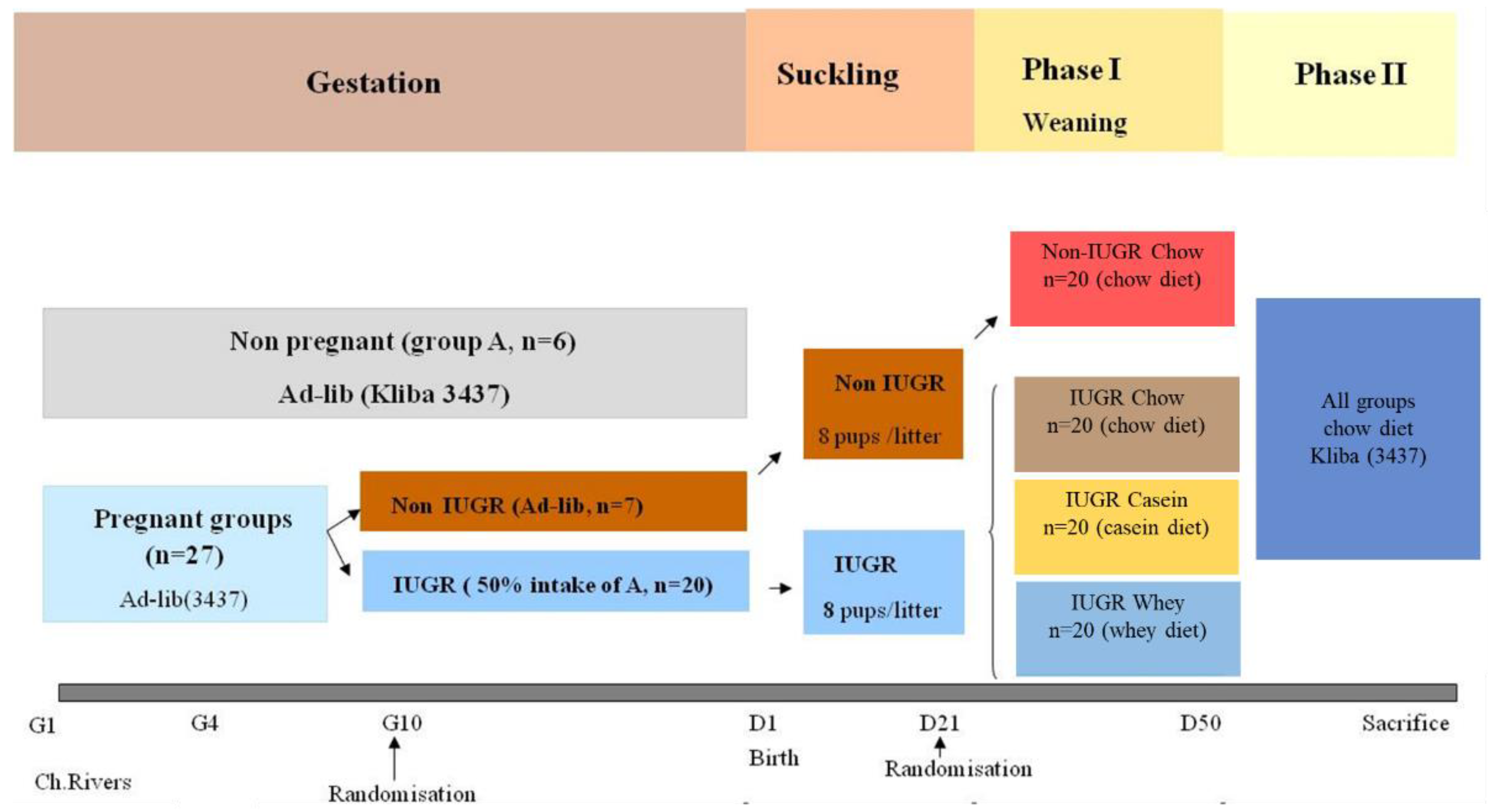
2.1. Animals and Diets
2.2. Food Intake, Body Weight, and Body Composition
2.3. Blood and Tissue Collection
2.4. Blood Assays
2.5. Statistical Analysis
3. Results
3.1. Birth Weight and Time of Catch-Up Growth
3.2. Weaning and Post-Weaning Growth, Body Composition, and Energy Intake
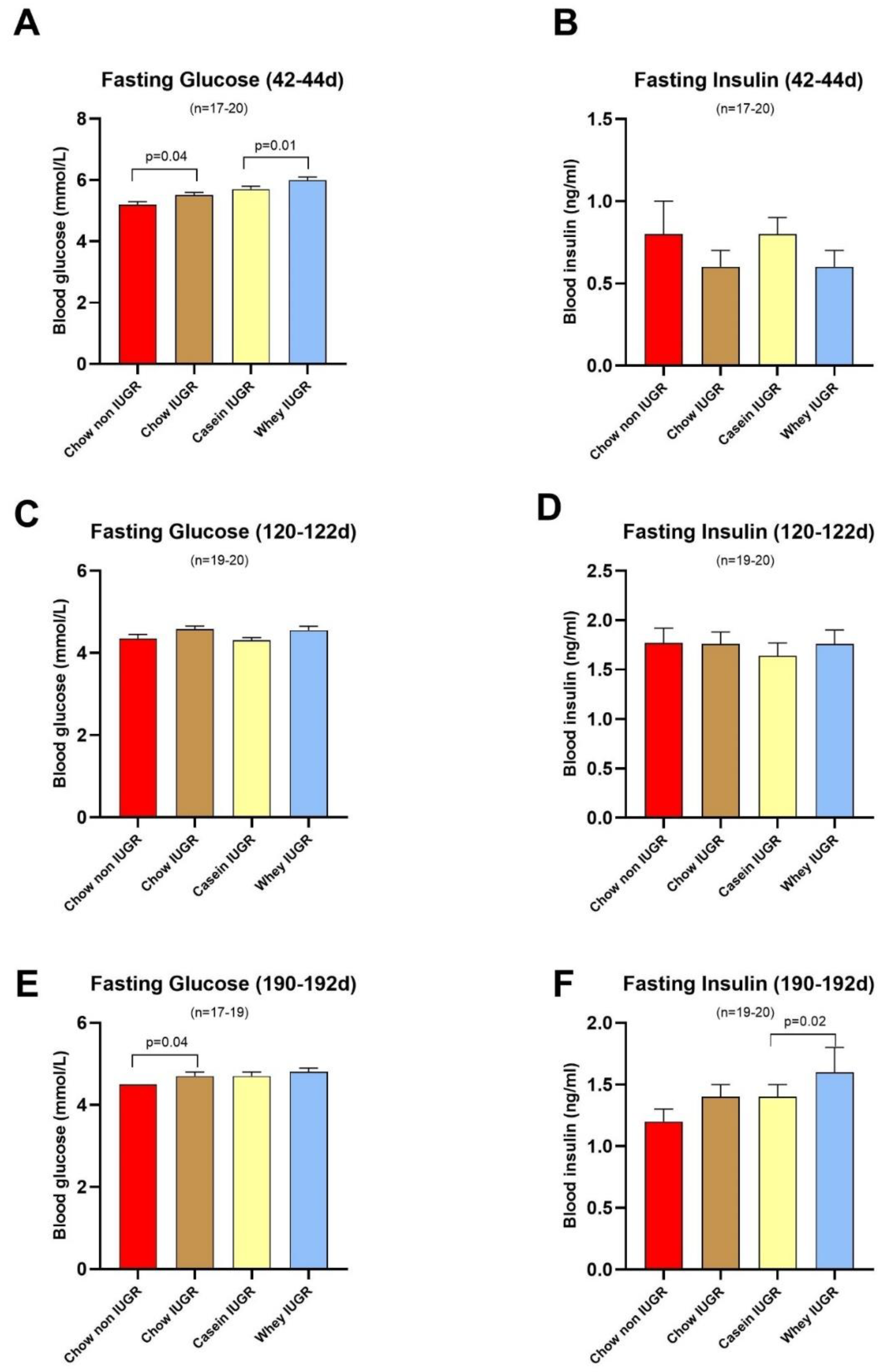
3.3. Blood Glucose and Insulin
3.4. Evolution of the HOMA-IR Index
3.5. Plasma Lipids and Leptin
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Barker, D.; Godfrey, K.; Gluckman, P.; Harding, J.; Owens, J.; Robinson, J. Fetal nutrition and cardiovascular disease in adult life. Lancet 1993, 341, 938–941. [Google Scholar] [CrossRef]
- Barker, D.J.; Osmond, C.; Forsén, T.J.; Kajantie, E.; Eriksson, J.G. Trajectories of Growth among Children Who Have Coronary Events as Adults. N. Engl. J. Med. 2005, 353, 1802–1809. [Google Scholar] [CrossRef]
- Eriksson, J.G.; Forsen, T.J.; Osmond, C.; Barker, D.J. Pathways of Infant and Childhood Growth That Lead to Type 2 Diabetes. Diabetes Care 2003, 26, 3006–3010. [Google Scholar] [CrossRef] [PubMed]
- Forsén, T.J.; Eriksson, J.G.; Osmond, C.; Barker, D.J.P. The infant growth of boys who later develop coronary heart disease. Ann. Med. 2004, 36, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.N.; Barker, D.J.; Clark, P.M.; Cox, L.J.; Fall, C.; Osmond, C.; Winter, P.D. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 1991, 303, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Hofman, P.L.; Cutfield, W.S.; Robinson, E.M.; Bergman, R.N.; Menon, R.K.; Sperling, M.A.; Gluckman, P.D. Insulin resistance in short children with intrauterine growth retardation. J. Clin. Endocrinol. Metab. 1997, 82, 402–406. [Google Scholar] [CrossRef]
- Jaquet, D.; Gaboriau, A.; Czernichow, P.; Levy-Marchal, C. Insulin resistance early in adulthood in subjects born with intrauterine growth retardation. J. Clin. Endocrinol. Metab. 2000, 85, 1401–1406. [Google Scholar] [CrossRef]
- Kaijser, M.; Bonamy, A.-K.E.; Akre, O.; Cnattingius, S.; Granath, F.; Norman, M.; Ekbom, A. Perinatal Risk Factors for Diabetes in Later Life. Diabetes 2009, 58, 523–526. [Google Scholar] [CrossRef]
- McCance, D.R.; Pettitt, D.J.; Hanson, R.L.; Jacobsson, L.T.H.; Knowler, W.C.; Bennett, P.H. Birth weight and non-insulin dependent diabetes: Thrifty genotype, thrifty phenotype, or surviving small baby genotype? BMJ 1994, 308, 942–945. [Google Scholar] [CrossRef]
- Mi, J.; Law, C.; Zhang, K.-L.; Osmond, C.; Stein, C.; Barker, D. Effects of Infant Birthweight and Maternal Body Mass Index in Pregnancy on Components of the Insulin Resistance Syndrome in China. Ann. Intern. Med. 2000, 132, 253–260. [Google Scholar] [CrossRef]
- Okosun, I.S.; Liao, Y.; Rotimi, C.N.; Dever, G.E.; Cooper, R.S. Impact of birth weight on ethnic variations in subcutaneous and central adiposity in American children aged 5–11 years. A study from the Third National Health and Nutrition Examination Survey. Int. J. Obes. 2000, 24, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Nobili, V.; Marcellini, M.; Marchesini, G.; Vanni, E.; Manco, M.; Villani, A.; Bugianesi, E. Intrauterine Growth Retardation, Insulin Resistance, and Nonalcoholic Fatty Liver Disease in Children. Diabetes Care 2007, 30, 2638–2640. [Google Scholar] [CrossRef]
- Finken, M.J.J.; Keijzer-Veen, M.G.; Dekker, F.W.; Frölich, M.; Hille, E.T.M.; Romijn, J.A.; Wit, J.M. Preterm birth and later insulin resistance: Effects of birth weight and postnatal growth in a population based longitudinal study from birth into adult life. Diabetologia 2006, 49, 478–485. [Google Scholar] [CrossRef]
- Lacroix, M.; Bos, C.; Léonil, J.; Airinei, G.; Luengo, C.; Daré, S.; Benamouzig, R.; Fouillet, H.; Fauquant, J.; Tomé, D.; et al. Compared with casein or total milk protein, digestion of milk soluble proteins is too rapid to sustain the anabolic postprandial amino acid requirement. Am. J. Clin. Nutr. 2006, 84, 1070–1079. [Google Scholar] [CrossRef]
- Lönnerdal, B. Nutritional and physiologic significance of human milk proteins. Am. J. Clin. Nutr. 2003, 77, 1537S–1543S. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, K.; E LeBlanc, R.; Loh, D.; Schwartz, G.J.; Yu, Y.-H. Increasing Dietary Leucine Intake Reduces Diet-Induced Obesity and Improves Glucose and Cholesterol Metabolism in Mice via Multimechanisms. Diabetes 2007, 56, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.K.; Walker, D.A. Potential Importance of Leucine in Treatment of Obesity and the Metabolic Syndrome. J. Nutr. 2006, 136 (Suppl. 1), 319S–323S. [Google Scholar] [CrossRef]
- Eller, L.K.; A Reimer, R. A High Calcium, Skim Milk Powder Diet Results in a Lower Fat Mass in Male, Energy-Restricted, Obese Rats More than a Low Calcium, Casein, or Soy Protein Diet. J. Nutr. 2010, 140, 1234–1241. [Google Scholar] [CrossRef]
- Hoppe, C.; Mølgaard, C.; Dalum, C.; Vaag, A.; Michaelsen, K.F. Differential effects of casein versus whey on fasting plasma levels of insulin, IGF-1 and IGF-1/IGFBP-3: Results from a randomized 7-day supplementation study in prepubertal boys. Eur. J. Clin. Nutr. 2009, 63, 1076–1083. [Google Scholar] [CrossRef]
- Dangin, M.; Boirie, Y.; Garcia-Rodenas, C.; Gachon, P.; Fauquant, J.; Callier, P.; Ballèvre, O.; Beaufrère, B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am. J. Physiol. Metab. 2001, 280, E340–E348. [Google Scholar] [CrossRef] [PubMed]
- Tessari, P.; Kiwanuka, E.; Cristini, M.; Zaramella, M.; Enslen, M.; Zurlo, C.; Garcia-Rodenas, C. Slow versus fast proteins in the stimulation of beta-cell response and the activation of the entero-insular axis in type 2 diabetes. Diabetes Metab. Res. Rev. 2007, 23, 378–385. [Google Scholar] [CrossRef]
- Shahkhalili, Y.; Moulin, J.; Zbinden, I.; Aprikian, O.; Macé, K. Comparison of two models of intrauterine growth restriction for early catch-up growth and later development of glucose intolerance and obesity in rats. Am. J. Physiol. Integr. Comp. Physiol. 2010, 298, R141–R146. [Google Scholar] [CrossRef] [PubMed]
- Vickers, M.H.; Breier, B.H.; Cutfield, W.S.; Hofman, P.L.; Gluckman, P.D. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am. J. Physiol. Metab. 2000, 279, E83–E87. [Google Scholar] [CrossRef]
- Holemans, K.; Verhaeghe, J.; Dequeker, J.; Vanassche, F. Insulin sensitivity in adult female rats subjected to malnutrition during the perinatal period. J. Soc. Gynecol. Investig. 1996, 3, 71–77. [Google Scholar] [CrossRef]
- Hall, W.L.; Millward, D.J.; Long, S.J.; Morgan, L.M. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br. J. Nutr. 2003, 89, 239–248. [Google Scholar] [CrossRef]
- Pichon, L.; Potier, M.-C.; Tomé, D.; Mikogami, T.; Laplaize, B.; Martin-Rouas, C.; Fromentin, G. High-protein diets containing different milk protein fractions differently influence energy intake and adiposity in the rat. Br. J. Nutr. 2008, 99, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.H.; Moore, S.E. Dietary Proteins in the Regulation of Food Intake and Body Weight in Humans. J. Nutr. 2004, 134, 974S–979S. [Google Scholar] [CrossRef]
- Corring, T.; Beaufrere, B.; Maubois, J.L. The wonders of whey—catch the power. In Proceedings of the 4th International Whey Congress, Elmhirst, IL, USA, 11–14 September 2005. [Google Scholar]
- Pedersen, N.L.-R.; Nagain-Domaine, C.; Mahé, S.; Chariot, J.; Rozé, C.; Tomé, D. Caseinomacropeptide specifically stimulates exocrine pancreatic secretion in the anesthetized rat. Peptides 2000, 21, 1527–1535. [Google Scholar] [CrossRef]
- Hayes, M.R.; Covasa, M. Gastric distension enhances CCK-induced Fos-like immunoreactivity in the dorsal hindbrain by activating 5-HT3 receptors. Brain Res. 2006, 1088, 120–130. [Google Scholar] [CrossRef]
- Cota, D.; Yoon, A.; Peng, G.; Brandenburg, Y.; Zollo, O.; Xu, W.; Rego, E.; Ruggero, D. Hypothalamic mTOR Signaling Regulates Food Intake. Science 2006, 312, 927–930. [Google Scholar] [CrossRef]
- Velazquez, M.; Smith, C.; Smyth, N.; Osmond, C.; Fleming, T. Advanced maternal age causes adverse programming of mouse blastocysts leading to altered growth and impaired cardiometabolic health in post-natal life. Hum. Reprod. 2016, 31, 1970–1980. [Google Scholar] [CrossRef]
- The HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef]
- Lowe, W.L.; Scholtens, D.M.; Lowe, L.P.; Kuang, A.; Nodzenski, M.; Talbot, O.; Catalano, P.M.; Linder, B.; Brickman, W.J.; Clayton, P.; et al. Association of Gestational Diabetes with Maternal Disorders of Glucose Metabolism and Childhood Adiposity. JAMA 2018, 320, 1005–1016. [Google Scholar] [CrossRef]
- Lu, H.-Q.; Hu, R. Lasting Effects of Intrauterine Exposure to Preeclampsia on Offspring and the Underlying Mechanism. Am. J. Perinatol. Rep. 2019, 9, e275–e291. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.; Eriksson, J.G.; Broekman, B.F.P. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef]
- Fernandez-Twinn, D.S.; Gascoin, G.; Musial, B.; Carr, S.; Duque-Guimaraes, D.; Blackmore, H.L.; Alfaradhi, M.Z.; Loche, E.; Sferruzzi-Perri, A.N.; Fowden, A.L.; et al. Exercise rescues obese mothers’ insulin sensitivity, placental hypoxia and male offspring insulin sensitivity. Sci. Rep. 2017, 7, srep44650. [Google Scholar] [CrossRef] [PubMed]
- Kusuyama, J.; Alves-Wagner, A.B.; Makarewicz, N.S.; Goodyear, L.J. Effects of maternal and paternal exercise on offspring metabolism. Nat. Metab. 2020, 2, 858–872. [Google Scholar] [CrossRef]
- Berghänel, A.; Heistermann, M.; Schülke, O.; Ostner, J. Prenatal stress accelerates offspring growth to compensate for reduced maternal investment across mammals. Proc. Natl. Acad. Sci. USA 2017, 114, E10658–E10666. [Google Scholar] [CrossRef] [PubMed]
- Dearden, L.; Bouret, S.G.; Ozanne, S.E. Sex and gender differences in developmental programming of metabolism. Mol. Metab. 2018, 15, 8–19. [Google Scholar] [CrossRef] [PubMed]




| Diet Component | Kliba 3437 | Casein Diet | Whey Diet |
|---|---|---|---|
| Gross energy (kcal/100 g) | 382 | 365 | 365 |
| Protein (% energy) | 19.4 | 20 | 20 |
| Fat (% energy) | 11 | 16 | 16 |
| Carbohydrate (% energy) | 57 | 64 | 64 |
| Fiber (% energy) | 4.7 | 4.8 | 4.8 |
| Amino acids (%protein) | |||
| Aspartame | - | 7.5 | 11.5 |
| Threonine | 3.5 | 4.8 | 5.1 |
| Serine | - | 4.8 | 5.1 |
| Glutamine | - | 22.8 | 18.1 |
| Proline | - | 10.9 | 5.2 |
| Glycine | 2.1 | 2.0 | |
| Alanine | - | 3.2 | 5.1 |
| Valine | - | 6.8 | 5.7 |
| Cysteine | - | 0.4 | 2.7 |
| Methionine | 2.2 | 2.8 | 2.3 |
| Isoleucine | - | 5.8 | 5.7 |
| Leucine | - | 10.4 | 13.1 |
| Tyrosine | - | 5.9 | 4.0 |
| Phenylalanine | - | 5.0 | 3.8 |
| Lysine | 5.4 | 8.4 | 10.3 |
| Histidine | - | 2.9 | 2.2 |
| Arginine | 5.6 | 4.0 | 2.8 |
| Tryptophan | 1.2 | 1.4 | 2.2 |
| Bootstrap Mean | Approximate | Approximate Lower | Approximate Upper | ||
|---|---|---|---|---|---|
| Age (Day) | Standard Error | Confidence Limit | Confidence Limit | ||
| Litter | IUGR Chow | 9.1 | 3.06 | 6.4 | 18.4 |
| IUGR Casein | 11.1 | 3.32 | 7.4 | 20.4 | |
| IUGR Whey | 10.5 | 3.22 | 6.5 | 19.2 | |
| Pup | IUGR Chow | 10.6 | 3.29 | 7.2 | 20.1 |
| IUGR Casein | 13.4 | 2.76 | 9.1 | 19.9 | |
| IUGR Whey | 12.7 | 2.76 | 8.7 | 19.5 | |
| Litter IUGR | 11.2 | 3.31 | 7.2 | 20.2 | |
| Pups IUGR | 12.8 | 2.84 | 8.6 | 19.7 | |
| Epidydimal | Retroperitoneal | Kidney | Liver | Pancreas | |
|---|---|---|---|---|---|
| Non IUGR Chow | 9.72 (0.73) | 7.35(0.8) | 3.34 (0.1) a | 17.7 (0.84) | 1.22 (0.04) |
| IUGR Chow | 10.9 (1.25) | 9.44 (0.74) | 3.2 (0.09) b | 18.02 (0.48) | 1.17 (0.06) a |
| IUGR Casein | 9.55 (0.72) | 8.86 (0.58) | 3.22 (0.09) | 16.53 (0.42) | 1.34 (0.05) b |
| IUGR Whey | 10.07 (0.79) | 10.56 (1.24) | 3.08 (0.07) | 17.21 (0.5) | 1.17 (0.06) a |
| Leptin | FFA | TG | Total Cholesterol | |
|---|---|---|---|---|
| ng/mL | µmol/L | mmol/L | mmol/L | |
| Non IUGR Chow | 5.75 | 360.5 (32.4) | 0.99 (0.11) | 2.47 (0.12) |
| IUGR Chow | 5.87 | 363 (19.0) | 1.13 (0.12) | 2.7 (0.13) |
| IUGR Casein | 7.08 | 419.75 (33.1) | 0.96 (0.14) | 2.54 (0.09) |
| IUGR Whey | 6.85 | 419 (26.4) | 1.04 (0.12) | 2.8 (0.18) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahkhalili, Y.; Blancher-Budin, F.; Monnard, C.; Moulin, J.; Sanchez-Garcia, J.; Macé, K. Whey Versus Casein as a Protein Source during the Weaning Period: Impact on Growth and Later Adiposity and Glucose Homeostasis in a Rat Model of Intrauterine Growth Restriction. Nutrients 2020, 12, 3399. https://doi.org/10.3390/nu12113399
Shahkhalili Y, Blancher-Budin F, Monnard C, Moulin J, Sanchez-Garcia J, Macé K. Whey Versus Casein as a Protein Source during the Weaning Period: Impact on Growth and Later Adiposity and Glucose Homeostasis in a Rat Model of Intrauterine Growth Restriction. Nutrients. 2020; 12(11):3399. https://doi.org/10.3390/nu12113399
Chicago/Turabian StyleShahkhalili, Yasaman, Florence Blancher-Budin, Cathriona Monnard, Julie Moulin, José Sanchez-Garcia, and Katherine Macé. 2020. "Whey Versus Casein as a Protein Source during the Weaning Period: Impact on Growth and Later Adiposity and Glucose Homeostasis in a Rat Model of Intrauterine Growth Restriction" Nutrients 12, no. 11: 3399. https://doi.org/10.3390/nu12113399
APA StyleShahkhalili, Y., Blancher-Budin, F., Monnard, C., Moulin, J., Sanchez-Garcia, J., & Macé, K. (2020). Whey Versus Casein as a Protein Source during the Weaning Period: Impact on Growth and Later Adiposity and Glucose Homeostasis in a Rat Model of Intrauterine Growth Restriction. Nutrients, 12(11), 3399. https://doi.org/10.3390/nu12113399





