Estimation of Sodium and Potassium Intake: Current Limitations and Future Perspectives
Abstract
1. Introduction
2. Dietary Assessment Tools
2.1. Estimation of Average Population Intake
2.2. Estimation of Individual Intake
2.3. Dietary Assessment Tools vs. Urine-Based Methods
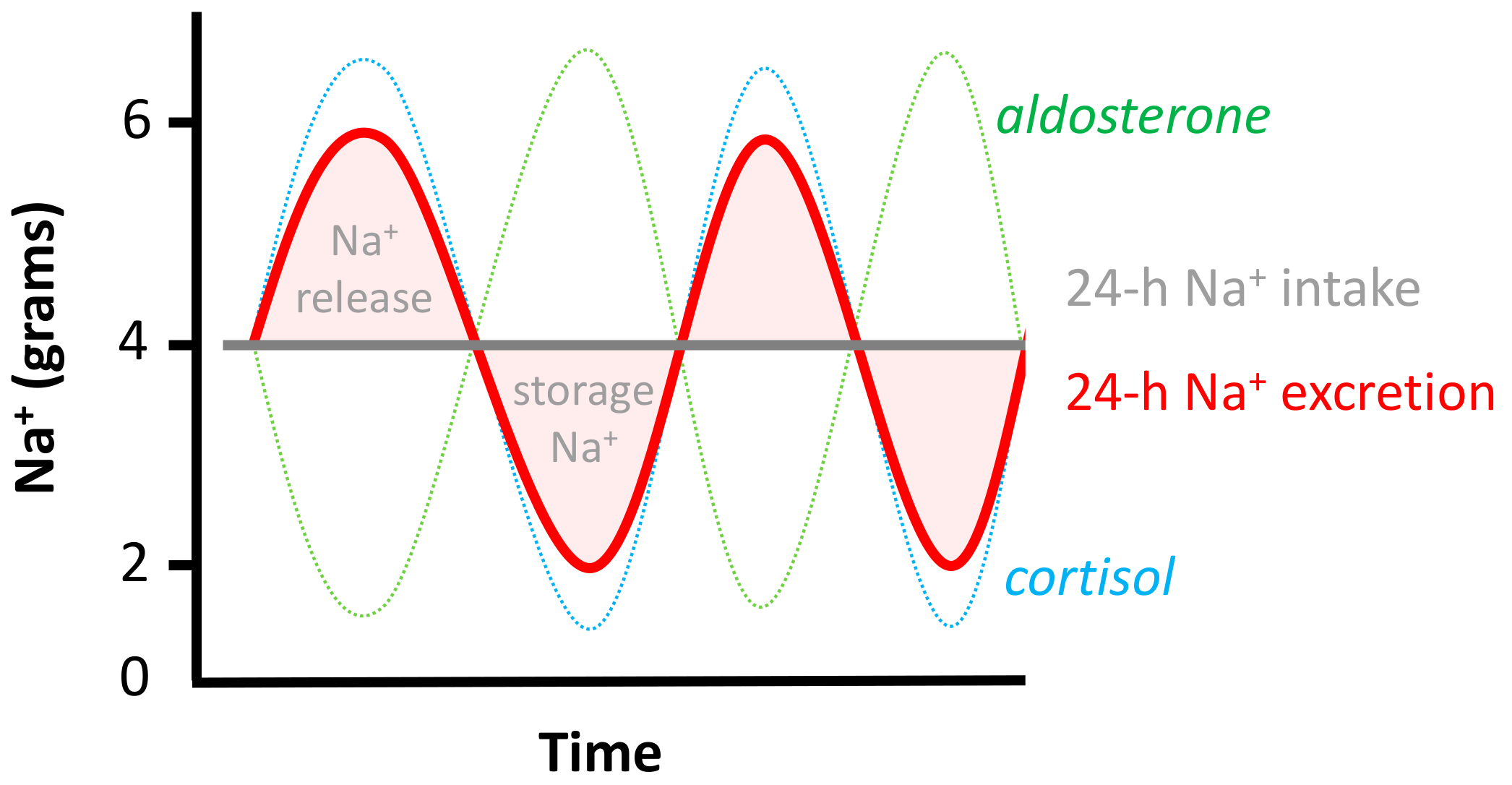
3. Twenty-Four Hour Urine Collections
3.1. Estimation of Average Population Intake
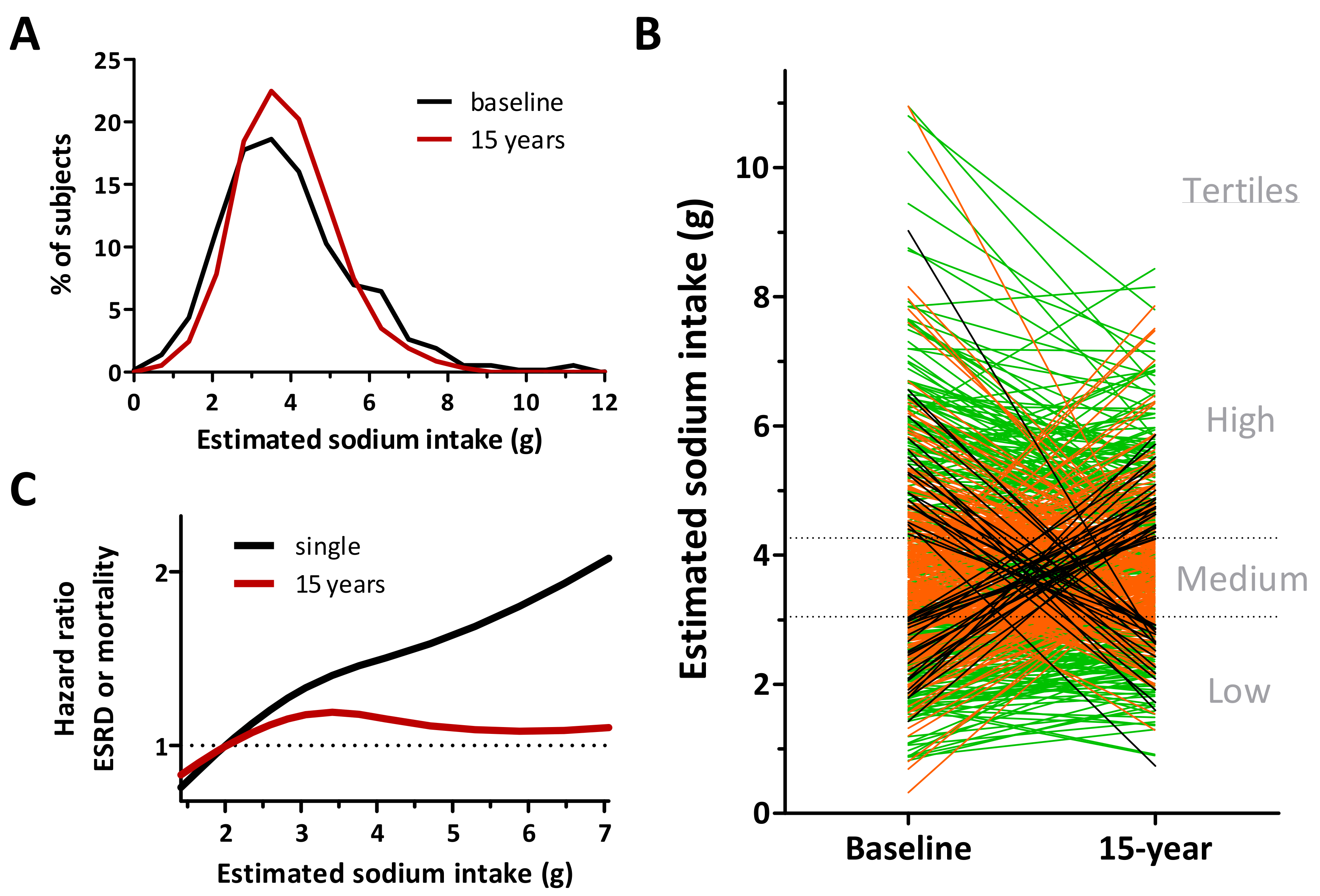
3.2. Estimation of Individual Intake
4. Spot Urine Based Equations
4.1. Estimation of Average Population Intake
4.2. Estimation of Individual Intake
5. CKD Patients
6. Practical Implications
- Dietary assessment tools are insufficiently validated for estimation of average population and individual-level dietary sodium and potassium intake.
- Spot urine based equations have substantial limitations when estimating average population sodium and potassium intake, and should not be used for estimation of individual-level intake.
- A single 24-h urine collection can be used to estimate short-term average population sodium or potassium intake.
- Multiple 24-h urine collections are needed to estimate individual-level sodium and potassium intake. For estimation of individual-level intake on the long term, this set of measurements should be repeated.
- The use of spot urine samples and 24-h urine collections for estimation of sodium and potassium intake has been insufficiently validated in CKD patients.
7. Potential Future Methods
7.1. Repeated Spot Urine Based Equation
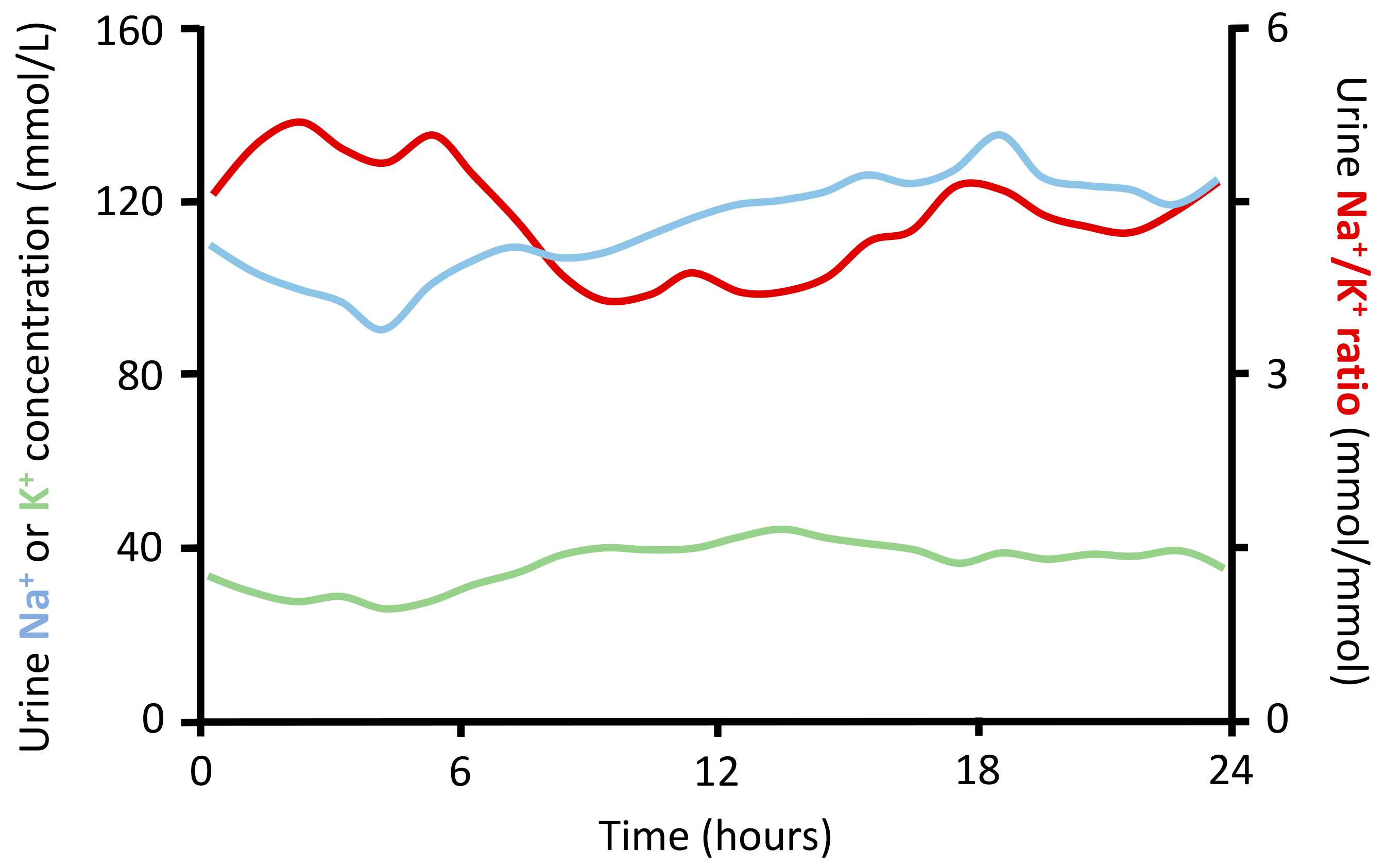
7.2. Urinary Sodium to Potassium Ratio
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- He, F.J.; Tan, M.; Ma, Y.; MacGregor, G.A. Salt Reduction to Prevent Hypertension and Cardiovascular Disease. J. Am. Coll. Cardiol. 2020, 75, 632–647. [Google Scholar] [CrossRef] [PubMed]
- Aburto, N.J.; Ziolkovska, A.; Hooper, L.; Elliott, P.; Cappuccio, F.P.; Meerpohl, J.J. Effect of lower sodium intake on health: Systematic review and meta-analyses. BMJ 2013, 346, f1326. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Borrelli, S.; Provenzano, M.; De Stefano, T.; Vita, C.; Chiodini, P.; Minutolo, R.; De Nicola, L.; Conte, G. Dietary Salt Restriction in Chronic Kidney Disease: A Meta-Analysis of Randomized Clinical Trials. Nutrients 2018, 10, 732. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.R.T.; Santos, J.A.; McKenzie, B.; Trieu, K.; Johnson, C.; McLean, R.M.; Arcand, J.; Campbell, N.R.C.; Webster, J. The Science of Salt: Updating the evidence on global estimates of salt intake. J. Clin. Hypertens. 2019, 21, 710–721. [Google Scholar] [CrossRef]
- WHO. Guideline: Sodium Intake for Adults and Children; World Health Organization (WHO): Geneva, Switzerland, 2012. [Google Scholar]
- Campbell, N.R.C.; He, F.J.; Tan, M.; Cappuccio, F.P.; Neal, B.; Woodward, M.; Cogswell, M.E.; McLean, R.; Arcand, J.; MacGregor, G.A.; et al. The International Consortium for Quality Research on Dietary Sodium/Salt (TRUE) position statement on the use of 24-hour, spot, and short duration (<24 hours) timed urine collections to assess dietary sodium intake. J. Clin. Hypertens. 2019, 21, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Aburto, N.J.; Hanson, S.; Gutierrez, H.; Hooper, L.; Elliott, P.; Cappuccio, F.P. Effect of increased potassium intake on cardiovascular risk factors and disease: Systematic review and meta-analyses. BMJ 2013, 346, f1378. [Google Scholar] [CrossRef] [PubMed]
- Kieneker, L.M.; Bakker, S.J.L.; De Boer, R.A.; Navis, G.J.; Gansevoort, R.T.; Joosten, M.M. Low potassium excretion but not high sodium excretion is associated with increased risk of developing chronic kidney disease. Kidney Int. 2016, 90, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Kieneker, L.M.; Gansevoort, R.T.; De Boer, R.; Brouwers, F.P.; Feskens, E.J.; Geleijnse, J.M.; Navis, G.; Bakker, S.J.; Joosten, M.M.; for The PREVEND Study Group. Urinary potassium excretion and risk of cardiovascular events. Am. J. Clin. Nutr. 2016, 103, 1204–1212. [Google Scholar] [CrossRef]
- Leonberg-Yoo, A.K.; Tighiouart, H.; Levey, A.S.; Beck, G.J.; Sarnak, M.J. Urine Potassium Excretion, Kidney Failure, and Mortality in CKD. Am. J. Kidney Dis. 2017, 69, 341–349. [Google Scholar] [CrossRef]
- WHO. Guideline: Potassium Intake for Adults and Children; World Health Organization (WHO): Geneva, Switzerland, 2012. [Google Scholar]
- Perin, M.S.; Gallani, M.C.B.J.; Andrechuk, C.R.S.; São-João, T.M.; Rhéaume, C.; Cornélio, M.E. What methods have been used to estimate salt intake? A systematic review. Int. J. Food Sci. Nutr. 2019, 71, 22–35. [Google Scholar] [CrossRef]
- Shim, J.-S.; Oh, K.; Kim, H.C. Dietary assessment methods in epidemiologic studies. Epidem. Health 2014, 36, e2014009. [Google Scholar] [CrossRef] [PubMed]
- McLean, R.M.; Farmer, V.L.; Nettleton, A.; Cameron, C.M.; Cook, N.R.; Campbell, N.R.C.; TRUE Consortium (in Ternational Consortium for Quality Research on Dietary, Sodium/Salt). Assessment of dietary sodium intake using a food frequency questionnaire and 24-hour urinary sodium excretion: A systematic literature review. J. Clin. Hypertens. 2017, 19, 1214–1230. [Google Scholar] [CrossRef] [PubMed]
- McLean, R.M.; Farmer, V.L.; Nettleton, A.; Cameron, C.M.; Cook, N.R.; Woodward, M.; Campbell, N.R.C.; TRUE Consortium (in Ternational Consortium for Quality Research on Dietary, Sodium/Salt). Twenty-Four-Hour Diet recall and Diet records compared with 24-hour urinary excretion to predict an individual’s sodium consumption: A Systematic Review. J. Clin. Hypertens. 2018, 20, 1360–1376. [Google Scholar] [CrossRef] [PubMed]
- Gemming, L.; Jiang, Y.; Swinburn, B.; Utter, J.; Ni Mhurchu, C. Under-reporting remains a key limitation of self-reported dietary intake: An analysis of the 2008/09 New Zealand Adult Nutrition Survey. Eur. J. Clin. Nutr. 2013, 68, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Freedman, L.S.; Commins, J.M.; Moler, J.E.; Willett, W.; Tinker, L.F.; Subar, A.F.; Spiegelman, D.; Rhodes, D.; Potischman, N.; Neuhouser, M.L.; et al. Pooled Results From 5 Validation Studies of Dietary Self-Report Instruments Using Recovery Biomarkers for Potassium and Sodium Intake. Am. J. Epidem. 2015, 181, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.M.; Pérez-Rodrigo, C.; López-Sobaler, A.M. Dietary assessment methods: Dietary records. Nutr. Hospit. 2015, 31, 38–45. [Google Scholar]
- Berger, I.; Wu, S.; Masson, P.; Kelly, P.J.; Duthie, F.A.; Whiteley, W.; Parker, D.; Gillespie, D.; Webster, A.C. Cognition in chronic kidney disease: A systematic review and meta-analysis. BMC Med. 2016, 14, 206. [Google Scholar] [CrossRef]
- Fraser, S.D.; Roderick, P.J.; Casey, M.; Taal, M.W.; Yuen, H.M.; Nutbeam, D. Prevalence and associations of limited health literacy in chronic kidney disease: A systematic review. Nephrol. Dial. Transplant. 2012, 28, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Zhou, L.; Stamler, J.; Chan, Q.; Van Horn, L.; Daviglus, M.L.; Dyer, A.R.; Elliott, P.; Ueshima, H.; Miura, K.; et al. Agreement between 24-h dietary recalls and 24-h urine collections for estimating sodium intake in China, Japan, UK, USA. J. Hypertens. 2019, 37, 814–819. [Google Scholar] [CrossRef]
- Cogswell, M.E.; Loria, C.M.; Terry, A.L.; Zhao, L.; Wang, C.-Y.; Chen, T.-C.; Wright, J.D.; Pfeiffer, C.M.; Merritt, R.; Moy, C.S.; et al. Estimated 24-Hour Urinary Sodium and Potassium Excretion in US Adults. JAMA 2018, 319, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Birukov, A.; Rakova, N.; Lerchl, K.; Engberink, R.H.G.O.; Johannes, B.; Wabel, P.; Moissl, U.; Rauh, M.; Luft, F.C.; Titze, J. Ultra-long-term human salt balance studies reveal interrelations between sodium, potassium, and chloride intake and excretion. Am. J. Clin. Nutr. 2016, 104, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Lerchl, K.; Rakova, N.; Dahlmann, A.; Rauh, M.; Goller, U.; Basner, M.; Dinges, D.F.; Beck, L.; Agureev, A.; Larina, I.; et al. Agreement Between 24-Hour Salt Ingestion and Sodium Excretion in a Controlled Environment. Hypertension 2015, 66, 850–857. [Google Scholar] [CrossRef]
- Jakobsen, J.; Ovesen, L.; Fagt, S.; Pedersen, A.N. Para-aminobenzoic acid used as a marker for completeness of 24-hour urine: Assessment of control limits for a specific HPLC method. Eur. J. Clin. Nutr. 1997, 51, 514–519. [Google Scholar] [CrossRef] [PubMed]
- John, K.A.; Cogswell, M.E.; Campbell, N.R.; Nowson, C.A.; Legetic, B.; Hennis, A.J.M.; Patel, S.M. Accuracy and Usefulness of Select Methods for Assessing Complete Collection of 24-Hour Urine: A Systematic Review. J. Clin. Hypertens. 2016, 18, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Sasaki, S.; Okubo, S.; Hayashi, M.; Tsugane, S. Blood pressure change in a free-living population-based dietary modification study in Japan. J. Hypertens. 2006, 24, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Charlton, K.; Schutte, A.; Wepener, L.; Corso, B.; Kowal, P.; Ware, L. Correcting for Intra-Individual Variability in Sodium Excretion in Spot Urine Samples Does Not Improve the Ability to Predict 24 h Urinary Sodium Excretion. Nutrients 2020, 12, 2026. [Google Scholar] [CrossRef]
- Rakova, N.; Jüttner, K.; Dahlmann, A.; Schröder, A.; Linz, P.; Kopp, C.; Rauh, M.; Goller, U.; Beck, L.; Agureev, A.; et al. Long-Term Space Flight Simulation Reveals Infradian Rhythmicity in Human Na+ Balance. Cell Metab. 2013, 17, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Engberink, R.H.G.O.; Hoek, T.C.V.D.; Van Noordenne, N.D.; Born, B.-J.H.V.D.; Peters-Sengers, H.; Vogt, L. Use of a Single Baseline Versus Multiyear 24-Hour Urine Collection for Estimation of Long-Term Sodium Intake and Associated Cardiovascular and Renal Risk. Circulation 2017, 136, 917–926. [Google Scholar] [CrossRef]
- He, F.J.; Ma, Y.; Campbell, N.R.; MacGregor, G.A.; Cogswell, M.E.; Cook, N.R. Formulas to Estimate Dietary Sodium Intake from Spot Urine Alter Sodium-Mortality Relationship. Hypertension 2019, 74, 572–580. [Google Scholar] [CrossRef]
- Tanaka, T.; Okamura, T.; Miura, K.; Kadowaki, T.; Ueshima, H.; Nakagawa, H.; Hashimoto, T. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J. Hum. Hypertens. 2002, 16, 97–103. [Google Scholar] [CrossRef]
- Brown, I.J.; Dyer, A.R.; Chan, Q.; Cogswell, M.E.; Ueshima, H.; Stamler, J.; Elliott, P.; on behalf of the INTERSALT Co-Operative Research Group. Estimating 24-Hour Urinary Sodium Excretion from Casual Urinary Sodium Concentrations in Western Populations. Am. J. Epidemiol. 2013, 177, 1180–1192. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, Y.; Song, N.; Zhang, X.; Teng, J.; Zou, J.; Ding, X. Estimating 24-Hour Urinary Sodium Excretion from Spot Urine Samples in Chronic Kidney Disease Patients. J. Ren. Nutr. 2020, 30, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, M.E.; Wang, C.-Y.; Chen, T.-C.; Pfeiffer, C.M.; Elliott, P.; Gillespie, C.D.; Carriquiry, A.L.; Sempos, C.T.; Liu, K.; Perrine, C.G.; et al. Validity of predictive equations for 24-h urinary sodium excretion in adults aged 18–39 y. Am. J. Clin. Nutr. 2013, 98, 1502–1513. [Google Scholar] [CrossRef] [PubMed]
- Mage, D.T.; Allen, R.H.; Kodali, A. Creatinine corrections for estimating children’s and adult’s pesticide intake doses in equilibrium with urinary pesticide and creatinine concentrations. J. Expo. Sci. Environ. Epidem. 2007, 18, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Itoh, K.; Uezono, K.; Sasaki, H. A simple method for estimating 24 h urinary sodium and potassium excretion from second morning voiding urine specimen in adults. Clin. Exp. Pharmacol. Physiol. 1993, 20, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Toft, U.; Cerqueira, C.; Andreasen, A.H.; Thuesen, B.H.; Laurberg, P.; Ovesen, L.; Perrild, H.; Jørgensen, T. Estimating salt intake in a Caucasian population: Can spot urine substitute 24-hour urine samples? Eur. J. Prev. Cardiol. 2013, 21, 1300–1307. [Google Scholar] [CrossRef]
- Peng, Y.; Li, W.; Wang, Y.; Chen, H.; Bo, J.; Wang, X.; Liu, L. Validation and Assessment of Three Methods to Estimate 24-h Urinary Sodium Excretion from Spot Urine Samples in Chinese Adults. PLoS ONE 2016, 11, e0149655. [Google Scholar] [CrossRef] [PubMed]
- Mente, A.; O’Donnell, M.J.; Dagenais, G.; Wielgosz, A.; Lear, S.; McQueen, M.J.; Jiang, Y.; Xingyu, W.; Jian, B.; Calik, K.B.T.; et al. Validation and comparison of three formulae to estimate sodium and potassium excretion from a single morning fasting urine compared to 24-h measures in 11 countries. J. Hypertens. 2014, 32, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Du, X.; Bai, Y.; Fang, L.; Liu, M.; Ji, N.; Zhong, J.; Yu, M.; Wu, J. Assessment and validation of spot urine in estimating the 24-h urinary sodium, potassium, and sodium/potassium ratio in Chinese adults. J. Hum. Hypertens. 2019, 34, 184–192. [Google Scholar] [CrossRef]
- Ji, C.; Sykes, L.; Paul, C.; Dary, O.; Legetic, B.; Campbell, N.R.C.; Cappuccio, F. Systematic review of studies comparing 24-hour and spot urine collections for estimating population salt intake. Rev. Panam. Salud Públ. 2012, 32, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Crino, M.; Wu, J.H.Y.; Woodward, M.; Barzi, F.; Land, M.-A.; McLean, R.; Webster, J.; Enkhtungalag, B.; Neal, B. Mean population salt intake estimated from 24-h urine samples and spot urine samples: A systematic review and meta-analysis. Int. J. Epidem. 2016, 45, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Mercado, C.I.; Cogswell, M.; Loria, C.M.; Liu, K.; Allen, N.; Gillespie, C.; Wang, C.-Y.; De Boer, I.H.; Wright, J. Validity of predictive equations for 24-h urinary potassium excretion based on timing of spot urine collection among adults: The MESA and CARDIA Urinary Sodium Study and NHANES Urinary Sodium Calibration Study. Am. J. Clin. Nutr. 2018, 108, 532–547. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Tian, Y.; Fu, J.-J.; Jiang, Y.-Y.; Bai, Y.-M.; Zhang, Z.-H.; Hu, X.-H.; Lian, H.-W.; Guo, M.; Yang, Z.-X.; et al. Validation of spot urine in predicting 24-h sodium excretion at the individual level. Am. J. Clin. Nutr. 2017, 105, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, H.; Kim, T.Y.; Ryu, H.; Ju, D.L.; Jang, M.; Oh, K.-H.; Ahn, C.; Han, S.N. Dietary Assessment of Korean Non-dialysis Chronic Kidney Disease Patients with or without Diabetes. J. Korean Med. Sci. 2020, 35, e181. [Google Scholar] [CrossRef] [PubMed]
- Soi, V.; Yee, J. Sodium Homeostasis in Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2017, 24, 325–331. [Google Scholar] [CrossRef]
- Kimura, G.; Dohi, Y.; Fukuda, M. Salt sensitivity and circadian rhythm of blood pressure: The keys to connect CKD with cardiovasucular events. Hypertens. Res. 2010, 33, 515–520. [Google Scholar] [CrossRef]
- Dougher, C.; Rifkin, D.; Anderson, C.A.; Smits, G.; Persky, M.S.; Block, G.; Ix, J.H. Spot urine sodium measurements do not accurately estimate dietary sodium intake in chronic kidney disease. Am. J. Clin. Nutr. 2016, 104, 298–305. [Google Scholar] [CrossRef]
- Imai, E.; Yasuda, Y.; Horio, M.; Shibata, K.; Kato, S.; Mizutani, Y.; Imai, J.; Hayashi, M.; Kamiya, H.; Oiso, Y.; et al. Validation of the equations for estimating daily sodium excretion from spot urine in patients with chronic kidney disease. Clin. Exp. Nephrol. 2011, 15, 861–867. [Google Scholar] [CrossRef]
- Kang, S.S.; Kang, E.H.; Kim, S.O.; Lee, M.S.; Hong, C.D.; Kim, S.B. Use of mean spot urine sodium concentrations to estimate daily sodium intake in patients with chronic kidney disease. Nutrients 2012, 28, 256–261. [Google Scholar] [CrossRef]
- Nerbass, F.B.; Pecoits-Filho, R.; McIntyre, N.J.; McIntyre, C.W.; Taal, M.W. Development of a Formula for Estimation of Sodium Intake from Spot Urine in People with Chronic Kidney Disease. Nephron Clin. Pr. 2014, 128, 61–66. [Google Scholar] [CrossRef]
- Okada, T.; Hayashi, A.; Nakao, T. Is the estimation of daily urinary sodium excretion in patients with chronic kidney disease sufficiently accurate in clinical practice? Clin. Exp. Nephrol. 2012, 16, 975–976. [Google Scholar] [CrossRef] [PubMed]
- Uechi, K.; Asakura, K.; Ri, Y.; Masayasu, S.; Sasaki, S. Advantage of multiple spot urine collections for estimating daily sodium excretion. J. Hypertens. 2016, 34, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-X.; Cuevas, C.A.; Su, X.-T.; Wu, P.; Gao, Z.-X.; Lin, D.-H.; McCormick, J.A.; Yang, C.-L.; Wang, W.-H.; Ellison, D.H. Potassium intake modulates the thiazide-sensitive sodium-chloride cotransporter (NCC) activity via the Kir4.1 potassium channel. Kidney Int. 2018, 93, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Iwahori, T.; Ueshima, H.; Torii, S.; Saito, Y.; Kondo, K.; Tanaka-Mizuno, S.; Arima, H.; Miura, K. Diurnal variation of urinary sodium-to-potassium ratio in free-living Japanese individuals. Hypertens. Res. 2017, 40, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Nazeri, P.; Bahadoran, Z.; Khalili-Moghadam, S.; Azizi, F. Dietary Sodium to Potassium Ratio and the Incidence of Chronic Kidney Disease in Adults: A Longitudinal Follow-Up Study. Prev. Nutr. Food Sci. 2018, 23, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, T.; Kuklina, E.V.; Flanders, W.D.; Hong, Y.; Gillespie, C.; Chang, M.-H.; Gwinn, M.; Dowling, N.; Khoury, M.J.; et al. Sodium and Potassium Intake and Mortality Among US Adults. Arch. Intern. Med. 2011, 171, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Averill, M.M.; Young, R.L.; Frazier-Wood, A.C.; Kurlak, E.O.; Kramer, H.; Steffen, L.M.; McClelland, R.L.; Delaney, J.A.; Drewnowski, A. Spot Urine Sodium-to-Potassium Ratio Is a Predictor of Stroke. Stroke 2019, 50, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Iwahori, T.; Ueshima, H.; Miyagawa, N.; Ohgami, N.; Yamashita, H.; Ohkubo, T.; Murakami, Y.; Shiga, T.; Miura, K. Six random specimens of daytime casual urine on different days are sufficient to estimate daily sodium/potassium ratio in comparison to 7-day 24-h urine collections. Hypertens. Res. 2014, 37, 765–771. [Google Scholar] [CrossRef]
- Iwahori, T.; Ueshima, H.; Torii, S.; Saito, Y.; Fujiyoshi, A.; Ohkubo, T.; Miura, K. Four to seven random casual urine specimens are sufficient to estimate 24-h urinary sodium/potassium ratio in individuals with high blood pressure. J. Hum. Hypertens. 2015, 30, 328–334. [Google Scholar] [CrossRef]
| FFQ | 24-h Diet Recall | Diet Record | |
|---|---|---|---|
| Costs | low | moderate | moderate to high |
| Time and burden | low | low to moderate | high |
| Precision | low | moderate | high |
| Timeframe | weeks or months | one day, but can be repeated over weeks or months | days |
| Biases | recall bias, information bias | recall bias | social desirability, selective reporting |
| Age | Sex | Height | Weight | BMI | Spot Na+ | Spot K+ | Spot Cr | Spot Ur | 24-h uV | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Method | Equation Variables | Region | Men | Women | Age | BMI | eGFR | |||||||||
| INTERSALT [33] | ■ | North America and Europe | 2841 | 2852 | 20–59 | 25.8 | N/A | |||||||||
| CKDSALT [34] | China | 2939 | 2296 | 54 | 24.2 | 56.7 | ||||||||||
| Kawasaki [37] | Japan | 78 | 81 | 34 | 22.1 * | N/A | ||||||||||
| Tanaka [32] | Japan | 295 | 296 | 40 | 22.4 | N/A | ||||||||||
| Toft [38] | Denmark | 102 | 371 | 51 | 25.5 | N/A | ||||||||||
| Mage [36] | United States | 483 | 246 | N/A | N/A | N/A | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ginos, B.N.R.; Engberink, R.H.G.O. Estimation of Sodium and Potassium Intake: Current Limitations and Future Perspectives. Nutrients 2020, 12, 3275. https://doi.org/10.3390/nu12113275
Ginos BNR, Engberink RHGO. Estimation of Sodium and Potassium Intake: Current Limitations and Future Perspectives. Nutrients. 2020; 12(11):3275. https://doi.org/10.3390/nu12113275
Chicago/Turabian StyleGinos, Bigina N.R., and Rik H.G. Olde Engberink. 2020. "Estimation of Sodium and Potassium Intake: Current Limitations and Future Perspectives" Nutrients 12, no. 11: 3275. https://doi.org/10.3390/nu12113275
APA StyleGinos, B. N. R., & Engberink, R. H. G. O. (2020). Estimation of Sodium and Potassium Intake: Current Limitations and Future Perspectives. Nutrients, 12(11), 3275. https://doi.org/10.3390/nu12113275





