Changes in Erythrocyte Omega-3 Fatty Acids in German Employees upon Dietary Advice by Corporate Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting and Participants
2.2. Occupational Health Check-Up
2.3. Omega-3 Fatty acid Intake Specific Questionnaire
2.4. Analysis of Blood Parameters
2.5. Variable Definitions
2.6. Statistical Assessment
3. Results
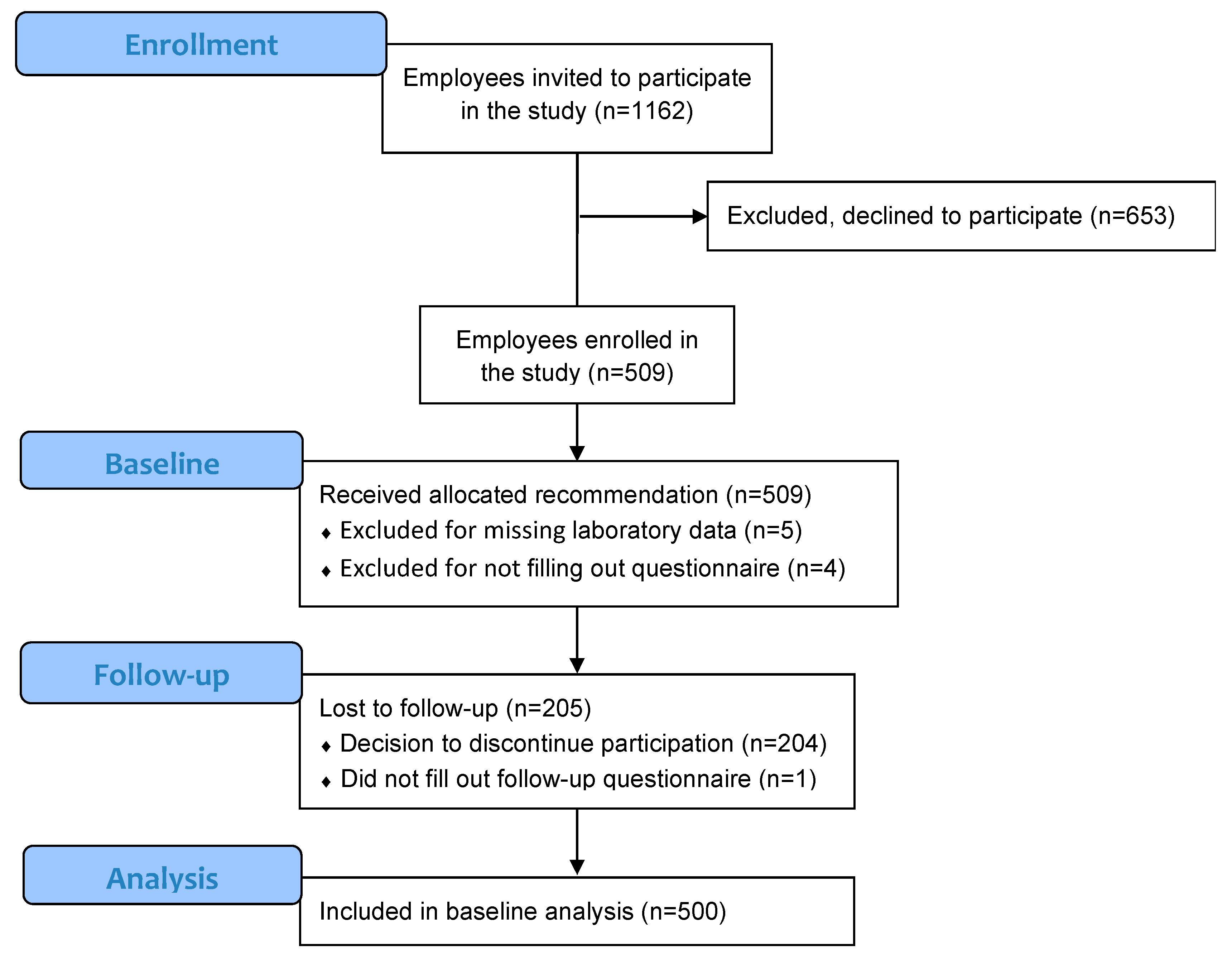
3.1. Baseline
3.1.1. Study Participants
3.1.2. Questionnaires
3.1.3. Blood Parameters
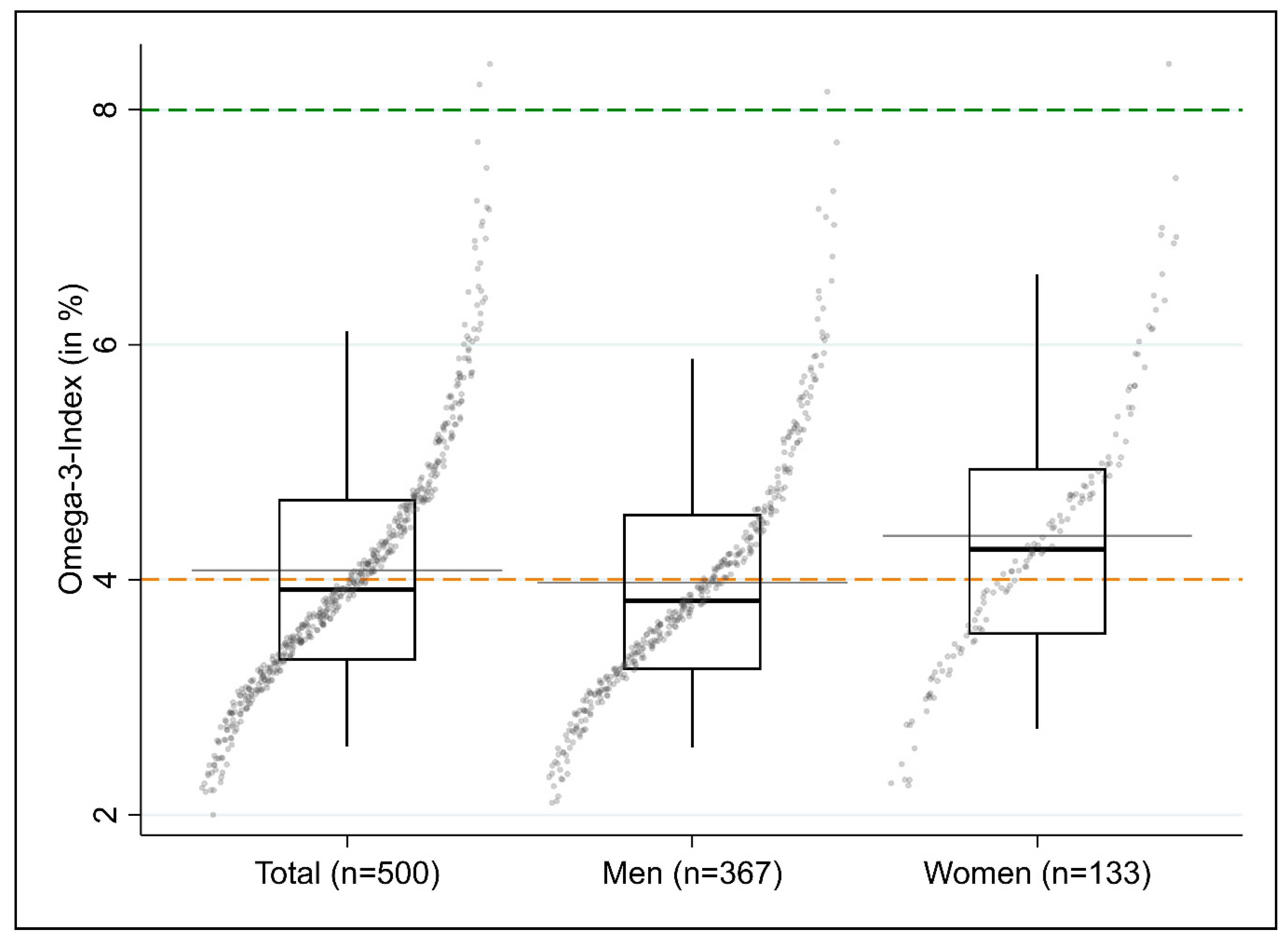
3.1.4. Omega-3 Index
3.2. Follow-Up
3.2.1. General Characteristics of Participants in the Follow-Up
3.2.2. Changes in Food Consumption between Baseline and Follow-Up
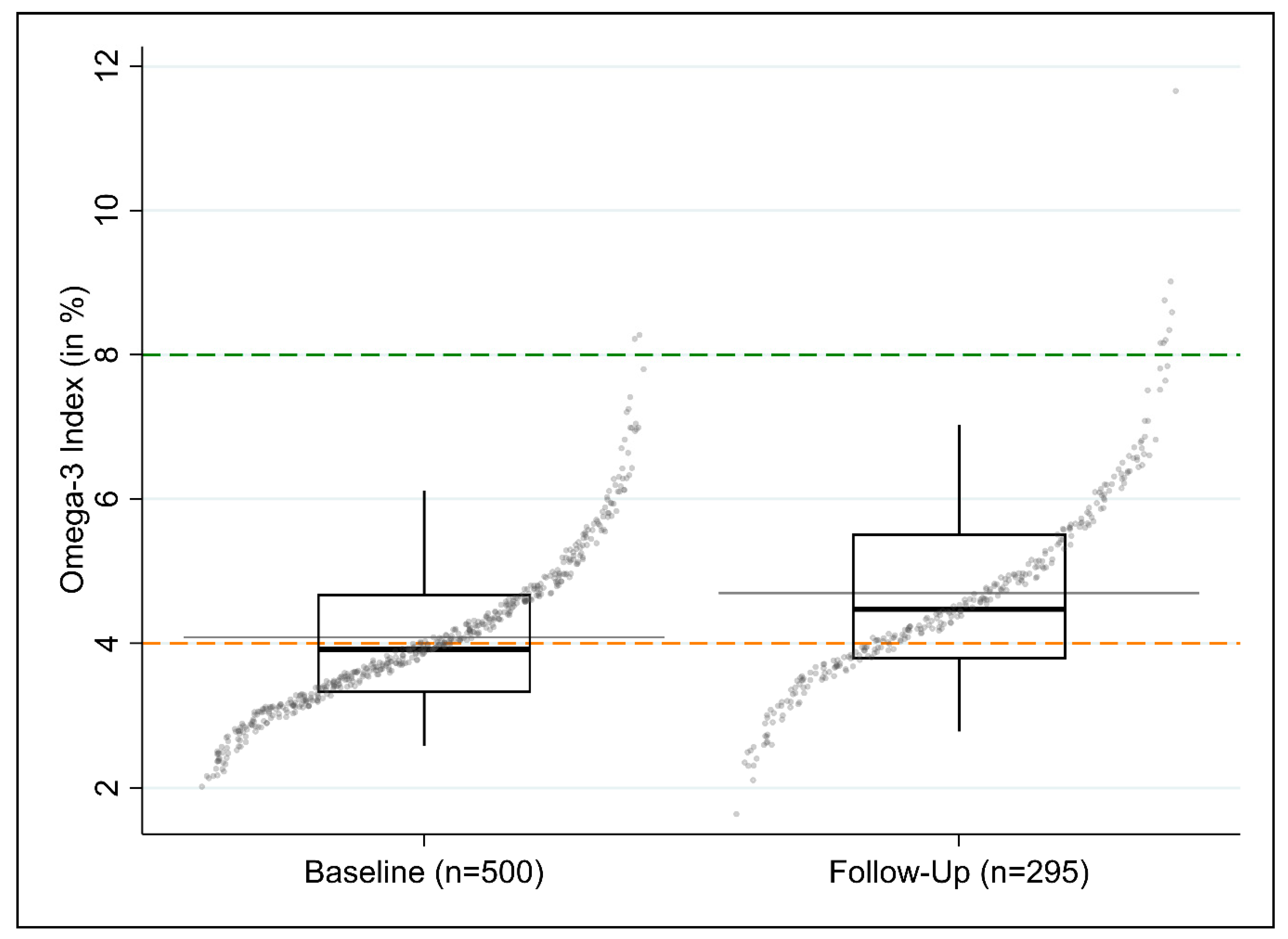
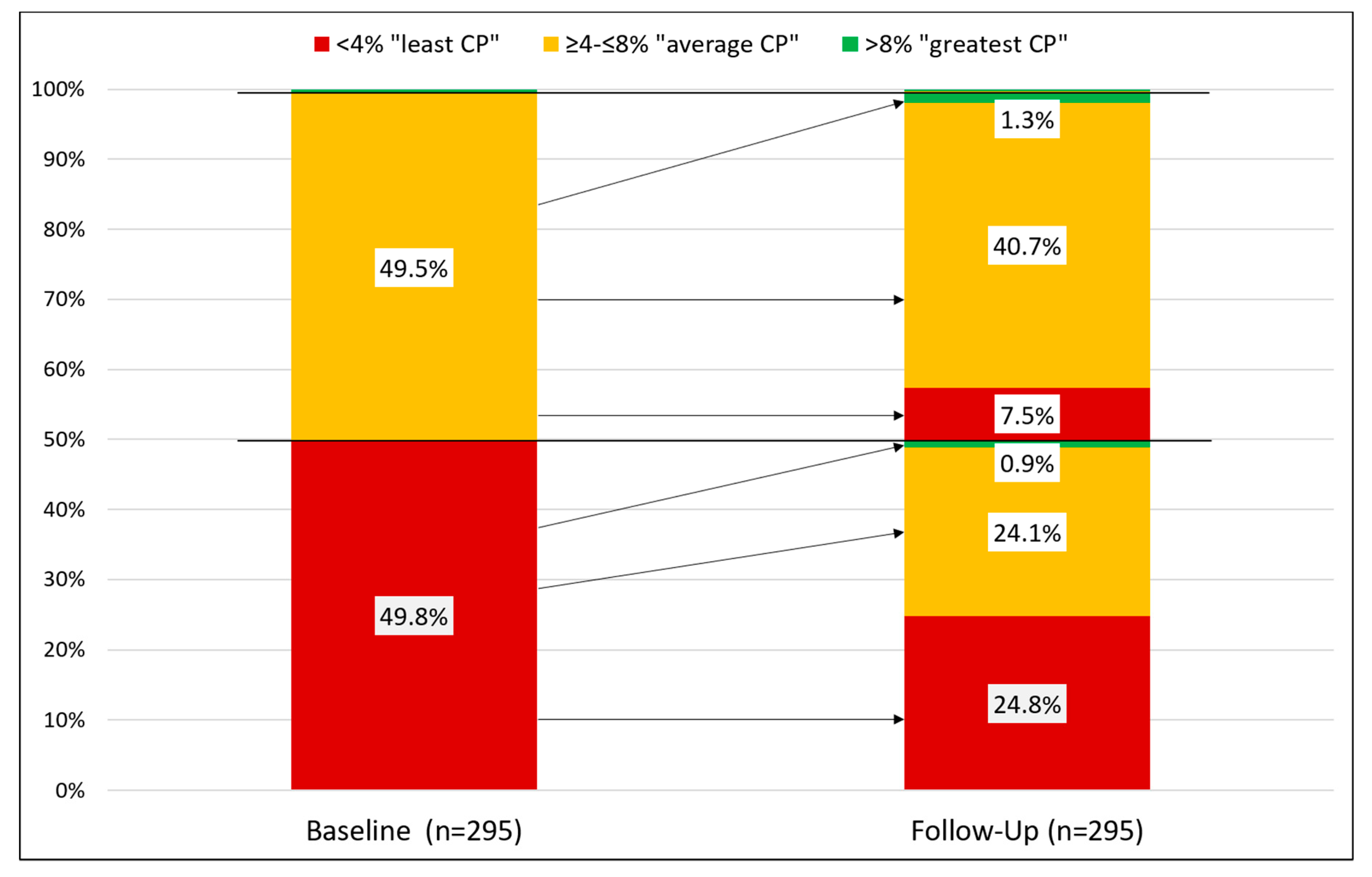
3.2.3. Changes in O3I between Baseline and Follow-Up
4. Discussion
4.1. Low Omega-3 Fatty Acid Intake in the Southern German Working Population
4.2. Low Baseline O3I Reveals Typical Characteristics
4.3. Moderate Effects of Dietary Recommendations
4.4. Omega-3 Fatty Acid Status for Employee Health Campaigns
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Unit | n | Total | Men | Women | |
|---|---|---|---|---|---|
| Large blood count | Mean (SD) or Median (IQR) | ||||
| Erythrocytes | 1012/L | 500 | 4.9 (IQR: 4.6–5.2) | 5.0 (IQR: 4.8–5.3) | 4.5 (IQR: 4.3–4.7) |
| Hemoglobin | g/L | 500 | 147 (SD: 12) | 151 (SD: 10) | 135 (SD: 10) |
| Hematocrit | L/L | 500 | 0.43 (SD: 0.03) | 0.44 (SD: 0.03) | 0.40 (SD: 0.03) |
| Mean cellular volume (MCV) | fL | 500 | 88.1 (IQR: 86.2–90.5) | 87.8 (IQR: 85.7–90.1) | 89 (IQR: 87.2–90.9) |
| Mean corpuscular hemoglobin (MCH) | pg | 500 | 30.1 (IQR: 29.2–30.9) | 30.1 (IQR: 29.3–31) | 30 (IQR: 29–30.6) |
| Mean corpuscular hemoglobin concentration (MCHC) | g/L | 500 | 340 (SD: 9) | 342 (SD: 9) | 335 (SD: 8) |
| Leucocytes | nL | 500 | 6.3 (IQR: 5.4–7.4) | 6.3 (IQR: 5.5–7.4) | 6.3 (IQR: 5.2–7.7) |
| Thrombocytes | nL | 500 | 254 (IQR: 220.5–293) | 249 (IQR: 215–285) | 272 (IQR: 237–319.5) |
| Neutrophilic granulocytes (relative) | % | 462 | 57.0 (SD: 8.5) | 56.9 (SD: 8.3) | 57.3 (SD: 8.9) |
| Neutrophilic granulocytes (absolute) | nL | 462 | 3.6 (IQR: 2.8–4.5) | 3.6 (IQR: 2.8–4.4) | 3.6 (IQR: 2.7–4.7) |
| Eosinophilic granulocytes (relative) | % | 461 | 2.0 (IQR: 1.3–3.1) | 2.1 (IQR: 1.3–3.4) | 1.8 (IQR: 1.2–2.6) |
| Eosinophilic granulocytes (absolute) | nL | 461 | 0.12 (IQR: 0.08–0.20) | 0.13 (IQR: 0.08–0.21) | 0.12 (IQR: 0.07–0.15) |
| Basophilic granulocytes (relative) | % | 461 | 0.7 (IQR: 0.5–0.9) | 0.7 (IQR: 0.5–0.9) | 0.7 (IQR: 0.5–0.9) |
| Basophilic granulocytes (absolute) | nL | 461 | 0.04 (IQR: 0.03–0.06) | 0.04 (IQR: 0.03–0.06) | 0.04 (IQR: 0.03–0.06) |
| Monocytes (relative) | % | 461 | 8.4 (IQR: 7.3–9.7) | 8.8 (IQR: 7.6–10) | 7.6 (IQR: 6.5–8.7) |
| Lymphocytes (relative) | % | 461 | 30.9 (SD: 7.5) | 30.5 (SD: 7.4) | 32.0 (SD: 7.8) |
| Liver enzymes | |||||
| Aspartate aminotransferase (ASAT) | U/L | 499 | 26 (IQR: 22–31) | 28 (IQR: 24–32) | 22 (IQR: 19.3–25.8) |
| Alanine aminotransferase (ALAT) | U/L | 499 | 27 (IQR: 21–38) | 31 (IQR: 24–42) | 18 (IQR: 15–23) |
| γ-glutamyl transferase (GGT) | U/L | 499 | 23 (IQR: 17–35) | 26 (IQR: 20–39) | 15 (IQR: 12–22) |
| Uric acid | mg/dL | 499 | 5.5 (IQR: 4.5–6.3) | 5.9 (IQR: 5.2–6.7) | 4.1 (IQR: 3.6–4.8) |
| Creatinine | mg/dL | 500 | 0.92 (IQR: 0.82–1.02) | 0.97 (IQR: 0.88–1.05) | 0.79 (IQR: 0.75–0.83) |
| Triglycerides | mg/dL | 495 | 120 (IQR: 82–171) | 131 (IQR: 93.8–186.5) | 94 (IQR: 68–128) |
| Total cholesterol | mg/dL | 499 | 205 (IQR: 183–230) | 204.5 (IQR: 183–229) | 207 (IQR: 180.5–233) |
| HDL/LDL cholesterol ratio | - | 482 | 0.44 (IQR: 0.34–0.57) | 0.40 (IQR: 0.32–0.49) | 0.58 (IQR: 0.45–0.69) |
| Serum glucose | mg/dL | 498 | 95 (IQR: 88–102) | 96 (IQR: 89–103) | 91 (IQR: 86–97) |
| Glycated hemoglobin (HbA1c) | % | 497 | 5.2 (IQR: 5.1–5.4) | 5.3 (IQR: 5.1–5.4) | 5.2 (IQR: 5.0–5.4) |
| C-reactive protein (CRP) | mg/L | 493 | 1.1 (IQR: 0.6–2.3) | 1.1 (IQR: 0.6–2.2) | 1.0 (IQR: 0.6–2.3) |
| Basal thyroid-stimulating hormone (TSH) | mU/L | 495 | 1.3 (IQR: 0.9–1.6) | 1.3 (IQR: 0.9–1.6) | 1.2 (IQR: 0.9–1.6) |
References
- Mason, R.P.; Jacob, R.F.; Shrivastava, S.; Sherratt, S.C.R.; Chattopadhyay, A. Eicosapentaenoic acid reduces membrane fluidity, inhibits cholesterol domain formation, and normalizes bilayer width in atherosclerotic-like model membranes. Biochim. Biophys. Acta 2016, 1858, 3131–3140. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Docosahexaenoic Acid. Ann. Nutr. Metab. 2016, 69 (Suppl. 1), 7–21. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Ecker, J. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Harayama, T.; Shimizu, T. Roles of polyunsaturated fatty acids, from mediators to membranes. J. Lipid Res. 2020. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. The Differential Effects of Eicosapentaenoic Acid and Docosahexaenoic Acid on Cardiometabolic Risk Factors: A Systematic Review. Int. J. Mol. Sci. 2018, 19, 532. [Google Scholar] [CrossRef]
- Hibbeln, J.R.; Spiller, P.; Brenna, J.T.; Golding, J.; Holub, B.J.; Harris, W.S.; Kris-Etherton, P.; Lands, B.; Connor, S.L.; Myers, G.; et al. Relationships between seafood consumption during pregnancy and childhood and neurocognitive development: Two systematic reviews. Prostaglandins Leukot. Essent. Fatty Acids 2019, 151, 14–36. [Google Scholar] [CrossRef]
- Ding, D.; Li, Y.H.; Xiao, M.L.; Dong, H.L.; Lin, J.S.; Chen, G.D.; Chen, Z.Y.; Tang, X.Y.; Chen, Y.M. Erythrocyte Membrane Polyunsaturated Fatty Acids Are Associated with Incidence of Metabolic Syndrome in Middle-Aged and Elderly People-An 8.8-Year Prospective Study. J. Nutr. 2020, 150, 1488–1498. [Google Scholar] [CrossRef]
- Burdge, G.C.; Jones, A.E.; Wootton, S.A. Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic acid metabolism in young men. Br. J. Nutr. 2002, 88, 355–363. [Google Scholar] [CrossRef]
- Burdge, G.C.; Wootton, S.A. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br. J. Nutr. 2002, 88, 411–420. [Google Scholar] [CrossRef]
- Ameur, A.; Enroth, S.; Johansson, A.; Zaboli, G.; Igl, W.; Johansson, A.C.; Rivas, M.A.; Daly, M.J.; Schmitz, G.; Hicks, A.A.; et al. Genetic adaptation of fatty-acid metabolism: A human-specific haplotype increasing the biosynthesis of long-chain omega-3 and omega-6 fatty acids. Am. J. Hum. Genet. 2012, 90, 809–820. [Google Scholar] [CrossRef]
- Vannice, G.; Rasmussen, H. Position of the academy of nutrition and dietetics: Dietary fatty acids for healthy adults. J. Acad Nutr. Diet. 2014, 114, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.D.; Drevon, C.A.; Harris, B.; Sinclair, A.; Spector, A. Recommendations for Intake of Polyunsaturated Fatty Acids in Healthy Adults; International Society for the Study of Fatty Acids and Lipids (ISSFAL): Washington, DC, USA, 2004. [Google Scholar]
- Harris, W.S.; Von Schacky, C. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S. The omega-3 index as a risk factor for coronary heart disease. Am. J. Clin. Nutr. 2008, 87, 1997S–2002S. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Pottala, J.V.; Varvel, S.A.; Borowski, J.J.; Ward, J.N.; McConnell, J.P. Erythrocyte omega-3 fatty acids increase and linoleic acid decreases with age: Observations from 160,000 patients. Prostaglandins Leukot. Essent. Fatty Acids 2013, 88, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Stark, K.D.; Van Elswyk, M.E.; Higgins, M.R.; Weatherford, C.A.; Salem, N., Jr. Global survey of the omega-3 fatty acids, docosahexaenoic acid and eicosapentaenoic acid in the blood stream of healthy adults. Prog. Lipid Res. 2016, 63, 132–152. [Google Scholar] [CrossRef] [PubMed]
- Kohler, A.; Heinrich, J.; von Schacky, C. Bioavailability of Dietary Omega-3 Fatty Acids Added to a Variety of Sausages in Healthy Individuals. Nutrients 2017, 9, 629. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.E.; Jackson, K.H.; Tintle, N.L.; Shearer, G.C.; Bernasconi, A.; Masson, S.; Latini, R.; Heydari, B.; Kwong, R.Y.; Flock, M.; et al. Predicting the effects of supplemental EPA and DHA on the omega-3 index. Am. J. Clin. Nutr. 2019, 110, 1034–1040. [Google Scholar] [CrossRef]
- de Groot, R.H.M.; Meyer, B.J. ISSFAL Official Statement Number 6: The importance of measuring blood omega-3 long chain polyunsaturated fatty acid levels in research. Prostaglandins Leukot. Essent. Fatty Acids 2019, 157, 102029. [Google Scholar] [CrossRef]
- Von Schacky, C. Omega-3 index and cardiovascular health. Nutrients 2014, 6, 799–814. [Google Scholar] [CrossRef]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; Summerbell, C.D.; Worthington, H.V.; Song, F.; et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2020, 3, CD003177. [Google Scholar] [CrossRef]
- Skulas-Ray, A.C.; Wilson, P.W.F.; Harris, W.S.; Brinton, E.A.; Kris-Etherton, P.M.; Richter, C.K.; Jacobson, T.A.; Engler, M.B.; Miller, M.; Robinson, J.G.; et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory From the American Heart Association. Circulation 2019, 140, e673–e691. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Marine Omega-3 (N-3) Fatty Acids for Cardiovascular Health: An Update for 2020. Int. J. Mol. Sci. 2020, 21, 1362. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, F.B.; Manson, J.E. Marine Omega-3 Supplementation and Cardiovascular Disease: An Updated Meta-Analysis of 13 Randomized Controlled Trials Involving 127 477 Participants. J. Am. Heart Assoc. 2019, 8, e013543. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhong, W.F.; Liu, S.; Kraus, V.B.; Zhang, Y.J.; Gao, X.; Lv, Y.B.; Shen, D.; Zhang, X.R.; Zhang, P.D.; et al. Associations of habitual fish oil supplementation with cardiovascular outcomes and all cause mortality: Evidence from a large population based cohort study. BMJ 2020, 368, m456. [Google Scholar] [CrossRef] [PubMed]
- GOED Global Organization for EPA and DHA. Global EPA and DHA Omega-3 Ingredient Market. Report 2017–2018; Global Organization for EPA and DHA: Salt Lake City, UT, USA, 2019. [Google Scholar]
- West, A.L.; Miles, E.A.; Lillycrop, K.A.; Han, L.; Sayanova, O.; Napier, J.A.; Calder, P.C.; Burdge, G.C. Postprandial incorporation of EPA and DHA from transgenic Camelina sativa oil into blood lipids is equivalent to that from fish oil in healthy humans. Br. J. Nutr. 2019, 121, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Kleber, M.E.; Delgado, G.E.; Lorkowski, S.; Marz, W.; von Schacky, C. Omega-3 fatty acids and mortality in patients referred for coronary angiography. The Ludwigshafen Risk and Cardiovascular Health Study. Atherosclerosis 2016, 252, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Oberlinner, C.; Humpert, P.M.; Nawroth, P.P.; Zober, A.; Morcos, M. Metabolic syndrome in a large chemical company: Prevalence in a screened worksite sample. Acta Diabetol. 2008, 45, 31–35. [Google Scholar] [CrossRef]
- Claus, M.; Schuster, M.; Webendorfer, S.; Groneberg, D.A.; Jahner, J.; Schiffmann, D. Prevalence of back pain in employees of a German chemical company: Results of a large cross-sectional study. J. Occup. Med. Toxicol. 2019, 14, 16. [Google Scholar] [CrossRef]
- Schuster, M.; Oberlinner, C.; Claus, M. Shift-specific associations between age, chronotype and sleep duration. Chronobiol. Int. 2019, 36, 784–795. [Google Scholar] [CrossRef]
- Haftenberger, M.; Heuer, T.; Heidemann, C.; Kube, F.; Krems, C.; Mensink, G.B. Relative validation of a food frequency questionnaire for national health and nutrition monitoring. Nutr. J. 2010, 9, 36. [Google Scholar] [CrossRef]
- Engeset, D.; Braaten, T.; Teucher, B.; Kuhn, T.; Bueno-de-Mesquita, H.B.; Leenders, M.; Agudo, A.; Bergmann, M.M.; Valanou, E.; Naska, A.; et al. Fish consumption and mortality in the European Prospective Investigation into Cancer and Nutrition cohort. Eur. J. Epidemiol. 2015, 30, 57–70. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture Agricultural Research Service. Food Data Central. Available online: https://fdc.nal.usda.gov/ (accessed on 8 August 2020).
- Kleiner, A.C.; Cladis, D.P.; Santerre, C.R. A comparison of actual versus stated label amounts of EPA and DHA in commercial omega-3 dietary supplements in the United States. J. Sci. Food Agric. 2015, 95, 1260–1267. [Google Scholar] [CrossRef]
- MRI Max Rubner Institut. Ergebnisbericht Teil 2, Nationale Verzehrsstudie II (Report part 2, National Food Intake Study II); Lebensmittel, B.F.E.U., Ed.; Bundesministeriums für Ernährung, Landwirtschaft und Verbraucherschutz: Berlin, Germany, 2008; p. 308. [Google Scholar]
- Thompson, M.; Hein, N.; Hanson, C.; Smith, L.M.; Anderson-Berry, A.; Richter, C.K.; Stessy Bisselou, K.; Kusi Appiah, A.; Kris-Etherton, P.; Skulas-Ray, A.C.; et al. Omega-3 Fatty Acid Intake by Age, Gender, and Pregnancy Status in the United States: National Health and Nutrition Examination Survey 2003(–)2014. Nutrients 2019, 11, 177. [Google Scholar] [CrossRef] [PubMed]
- Kantor, E.D.; Rehm, C.D.; Du, M.; White, E.; Giovannucci, E.L. Trends in Dietary Supplement Use Among US Adults From 1999–2012. JAMA 2016, 316, 1464–1474. [Google Scholar] [CrossRef]
- Jackson, K.H.; Polreis, J.M.; Tintle, N.L.; Kris-Etherton, P.M.; Harris, W.S. Association of reported fish intake and supplementation status with the omega-3 index. Prostaglandins Leukot. Essent. Fatty Acids 2019, 142, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Thuppal, S.V.; von Schacky, C.; Harris, W.S.; Sherif, K.D.; Denby, N.; Steinbaum, S.R.; Haycock, B.; Bailey, R.L. Discrepancy between Knowledge and Perceptions of Dietary Omega-3 Fatty Acid Intake Compared with the Omega-3 Index. Nutrients 2017, 9, 930. [Google Scholar] [CrossRef] [PubMed]
- Gellert, S.; Schuchardt, J.P.; Hahn, A. Low long chain omega-3 fatty acid status in middle-aged women. Prostaglandins Leukot. Essent. Fatty Acids 2017, 117, 54–59. [Google Scholar] [CrossRef]
- Kleber, M.E.; Delgado, G.E.; Lorkowski, S.; Marz, W.; von Schacky, C. Data on gender and subgroup specific analyses of omega-3 fatty acids in the Ludwigshafen Risk and Cardiovascular Health Study. Data Brief. 2016, 8, 1311–1321. [Google Scholar] [CrossRef]
- Sarter, B.; Kelsey, K.S.; Schwartz, T.A.; Harris, W.S. Blood docosahexaenoic acid and eicosapentaenoic acid in vegans: Associations with age and gender and effects of an algal-derived omega-3 fatty acid supplement. Clin. Nutr. 2015, 34, 212–218. [Google Scholar] [CrossRef]
- Yong, M.; Germann, C.; Lang, S.; Oberlinner, C. Primary selection into shift work and change of cardiovascular risk profile. Scand. J. Work Environ. Health 2015, 41, 259–267. [Google Scholar] [CrossRef][Green Version]
- Danaei, G.; Ding, E.L.; Mozaffarian, D.; Taylor, B.; Rehm, J.; Murray, C.J.; Ezzati, M. The preventable causes of death in the United States: Comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS. Med. 2009, 6, e1000058. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, S.L.; French, C.B.; Baggerly, C.A.; Harris, W.S. Cross-sectional study of the combined associations of dietary and supplemental eicosapentaenoic acid+docosahexaenoic acid on Omega-3 Index. Nutr. Res. 2019, 71, 43–55. [Google Scholar] [CrossRef]
- 2015–2020 Dietary Guidelines for Americans, 8th ed.; U.S. Department of Health and Human Services and U.S. Department of Agriculture: Washington, DC, USA, 2015.
- Sparkes, C.; Sinclair, A.J.; Gibson, R.A.; Else, P.L.; Meyer, B.J. High Variability in Erythrocyte, Plasma and Whole Blood EPA and DHA Levels in Response to Supplementation. Nutrients 2020, 12, 1017. [Google Scholar] [CrossRef]
- Flock, M.R.; Skulas-Ray, A.C.; Harris, W.S.; Etherton, T.D.; Fleming, J.A.; Kris-Etherton, P.M. Determinants of erythrocyte omega-3 fatty acid content in response to fish oil supplementation: A dose-response randomized controlled trial. J. Am. Heart Assoc. 2013, 2, e000513. [Google Scholar] [CrossRef]
- Harris, W.S.; Pottala, J.V.; Sands, S.A.; Jones, P.G. Comparison of the effects of fish and fish-oil capsules on the n 3 fatty acid content of blood cells and plasma phospholipids. Am. J. Clin. Nutr. 2007, 86, 1621–1625. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Tintle, N.L.; Etherton, M.R.; Vasan, R.S. Erythrocyte long-chain omega-3 fatty acid levels are inversely associated with mortality and with incident cardiovascular disease: The Framingham Heart Study. J. Clin. Lipidol. 2018, 12, 718–727.e716. [Google Scholar] [CrossRef]
- Shojima, Y.; Ueno, Y.; Tanaka, R.; Yamashiro, K.; Miyamoto, N.; Hira, K.; Kurita, N.; Nakajima, S.; Urabe, T.; Hattori, N. Eicosapentaenoic-to-Arachidonic Acid Ratio Predicts Mortality and Recurrent Vascular Events in Ischemic Stroke Patients. J. Atheroscler. Thromb. 2020, 52373. [Google Scholar] [CrossRef] [PubMed]
- Bittner, D.O.; Goeller, M.; Zopf, Y.; Achenbach, S.; Marwan, M. Early-onset coronary atherosclerosis in patients with low levels of omega-3 fatty acids. Eur. J. Clin. Nutr. 2020, 74, 651–656. [Google Scholar] [CrossRef]
- Guo, X.F.; Li, X.; Shi, M.; Li, D. n-3 Polyunsaturated Fatty Acids and Metabolic Syndrome Risk: A Meta-Analysis. Nutrients 2017, 9, 703. [Google Scholar] [CrossRef]
- Baghai, T.C.; Varallo-Bedarida, G.; Born, C.; Hafner, S.; Schule, C.; Eser, D.; Rupprecht, R.; Bondy, B.; von Schacky, C. Major depressive disorder is associated with cardiovascular risk factors and low Omega-3 Index. J. Clin. Psychiatry 2011, 72, 1242–1247. [Google Scholar] [CrossRef]

| Omega-3 Employee Study (Baseline) | Company Population on 1st January 2019 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | Total | Male | Female | |||||||
| n | % a | n | % a | n | % a | N | % a | n | % a | n | % a | |
| Total | 500 | 100 | 367 | 100 | 133 | 100 | 32,921 | 100 | 25,909 | 100 | 7012 | 100 |
| Age (in years) | ||||||||||||
| <35 | 100 | 20.0 | 66 | 18.0 | 34 | 25.6 | 6561 | 19.9 | 4787 | 18.5 | 1774 | 25.4 |
| 35–39 | 52 | 10.4 | 33 | 9.0 | 19 | 14.3 | 3238 | 9.8 | 2348 | 9.1 | 890 | 12.7 |
| 40–44 | 69 | 13.8 | 52 | 14.2 | 17 | 12.8 | 3542 | 10.8 | 2615 | 10.1 | 927 | 13.2 |
| 45–49 | 76 | 15.2 | 55 | 15.0 | 21 | 15.8 | 4905 | 14.9 | 3695 | 14.3 | 1210 | 17.3 |
| 50–54 | 101 | 20.2 | 79 | 21.5 | 22 | 16.5 | 6299 | 19.1 | 5125 | 19.8 | 1174 | 16.7 |
| ≥55 | 102 | 20.4 | 82 | 22.3 | 20 | 15.0 | 8376 | 25.4 | 7339 | 28.3 | 1037 | 14.8 |
| Gender ** | ||||||||||||
| Male | 367 | 73.4 | - | - | - | - | 25,909 | 78.7 | - | - | - | - |
| Female | 133 | 26.6 | - | - | - | - | 7012 | 21.3 | - | - | - | - |
| Occupational status ***,b | ||||||||||||
| Manual worker | 109 | 21.8 | 106 | 28.9 | 3 | 2.3 | 10,337 | 31.4 | 9762 | 37.7 | 575 | 8.2 |
| Skilled/supervisory worker | 250 | 50.0 | 158 | 43.1 | 92 | 69.2 | 13,917 | 42.3 | 9653 | 37.3 | 4264 | 60.8 |
| Managerial staff | 140 | 28.0 | 103 | 28.1 | 37 | 27.8 | 8667 | 26.4 | 6494 | 25.1 | 2173 | 31.0 |
| Working time system ***,c | ||||||||||||
| Day work | 415 | 83.0 | 285 | 77.7 | 130 | 97.7 | 24,997 | 75.9 | 18,195 | 70.2 | 6802 | 97.0 |
| Shift work | 85 | 17.0 | 82 | 22.3 | 3 | 2.3 | 7915 | 24.0 | 7710 | 29.8 | 205 | 2.9 |
| Body mass index (in kg/m2) | ||||||||||||
| Normal weight/underweight (<25) | 203 | 40.6 | 115 | 31.3 | 88 | 66.2 | ||||||
| Overweight (25–30) | 220 | 44.0 | 195 | 53.1 | 25 | 18.8 | ||||||
| Obesity (≥30) | 74 | 14.8 | 57 | 15.5 | 17 | 12.8 | ||||||
| Smoking status | ||||||||||||
| Smoker (cigarette/e-cigarette) | 60 | 12.0 | 49 | 13.3 | 11 | 8.3 | ||||||
| Former smoker | 117 | 23.4 | 94 | 25.6 | 23 | 17.3 | ||||||
| Nonsmoker | 320 | 64.0 | 224 | 61.0 | 96 | 72.2 | ||||||
| Baseline (n = 500) | Follow-Up (n = 295) | Baseline (n = 295) | Predicted within Difference between Baseline and Follow-up and 95%-CI † | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Fish | EPA + DHA | n | Fish | EPA + DHA | N | Fish | EPA + DHA | Fish | EPA + DHA | |
| (%) a | (g/d) b | (mg/d) c | (%) a | (g/d) b | (mg/d) c | (%) a | (g/d) b | (mg/d) c | (g/d) | (mg/d) | |
| Lean fish | 493 (82.2) | 8.2 (19.7) | 24.5 (59.2) | 290 (85.1) | 8.7 (9.5) | 26.1 (28.4) | 291 (82.4) | 6.8 (6.6) | 20.5 (20.0) | 1.3 (0.1–2.5) | 4.0 (0.4–7.6) |
| Fatty fish | 492 (79.2) | 12.2 (15.6) | 183.4 (233.7) | 291 (84.8) | 16.0 (19.9) | 240.2 (298.2) | 291 (79.0) | 11.9 (15.2) | 178.7 (SD: 227.3) | 3.9 (1.6–6.2) | 58.7 (24.3–93.1) |
| Omega-3 fatty acid supplements | 485 (6.8) | - | 14.7 (53.7) | 291 (22.0) | - | 46.9 (87.6) | 288 (7.8) | - | 16.8 (57.0) | - | 30.3 (21.1–39.4) |
| Total | 20.4 (28.6) | 220.7 (269.3) | 24.8 (24.5) | 314.3 (317.8) | 18.8 (19.2) | 215.3 (249.8) | 5.3 (2.5–8.1) | 94.1 (56.5–131.7) | |||
| Omega-3 Index | |||||||
|---|---|---|---|---|---|---|---|
| Continuous | Categorical | Multivariable Linear Regression Analysis | |||||
| n | Mean (SD) | Median (IQR) | <4% (Least CP) % a | 4%–8% (Average CP) % a | >8% (Largest CP) % a | Coeff. (95% CI) | |
| Total | 500 | 4.1 (1.1) | 3.9 (3.3–4.7) | 53.6 | 46.0 | 0.4 | n = 484 |
| Age (in years) | |||||||
| <35 | 100 | 4.0 (1.0) | 3.8 (3.2–4.5) | 60.0 | 40.0 | 0.0 | −0.01 (−0.33; 0.32) |
| 35–39 | 52 | 4.1 (0.9) | 3.8 (3.3–4.6) | 57.7 | 42.3 | 0.0 | 0.04 (−0.33; 0.42) |
| 40–44 | 69 | 4.0 (1.0) | 3.8 (3.2–4.6) | 58.0 | 42.0 | 0.0 | Reference |
| 45–49 | 76 | 4.0 (1.0) | 4.0 (3.3–4.7) | 54.0 | 46.1 | 0.0 | 0.09 (−0.25; 0.43) |
| 50–54 | 101 | 4.2 (1.3) | 3.9 (3.4–4.8) | 52.5 | 45.5 | 2.0 | 0.42 (0.09; 0.75) |
| ≥55 | 102 | 4.2 (1.1) | 4.2 (3.3–4.8) | 43.1 | 56.9 | 0.0 | 0.35 (0.03; 0.68) |
| Gender *** | |||||||
| Male | 367 | 4.0 (1.0) | 3.8 (3.2–4.6) | 58.9 | 40.9 | 0.3 | Reference |
| Female | 133 | 4.4 (1.2) | 4.3 (3.5–5.0) | 39.1 | 60.2 | 0.8 | 0.51 (0.27; 0.74) |
| Occupational status ***,a | |||||||
| Manual worker | 109 | 3.9 (1.0) | 3.8 (3.2–4.3) | 57.8 | 42.2 | 0.0 | Reference |
| Skilled/supervisory worker | 250 | 3.9 (1.0) | 3.8 (3.2–4.5) | 59.2 | 40.8 | 0.0 | −0.23 (−0.50; 0.04) |
| Managerial staff | 140 | 4.5 (1.2) | 4.3 (3.7–5.2) | 40.0 | 58.6 | 1.4 | 0.33 (−0.01; 0.66) |
| Working time system b | |||||||
| Day work | 415 | 4.1 (1.1) | 4.0 (3.3–4.7) | 51.6 | 48.0 | 0.5 | Reference |
| Shift work | 85 | 3.9 (1.0) | 3.7 (3.2–4.3) | 63.5 | 36.5 | 0.0 | 0.08 (−0.21; 0.37) |
| Body mass index (in kg/m2) * | |||||||
| Normal weight/underweight (<25) | 203 | 4.2 (1.2) | 4.1 (3.4–4.9) | 48.3 | 50.7 | 1.0 | Reference |
| Overweight (25–30) | 220 | 4.0 (1.0) | 3.9 (3.3–4.5) | 56.8 | 43.2 | 0.0 | 0.04 (−0.18; 0.27) |
| Obesity (≥30) | 74 | 3.9 (1.1) | 3.8 (3.1–4.6) | 59.5 | 40.5 | 0.0 | −0.16 (−0.46; 0.14) |
| Smoking status *** | |||||||
| Nonsmoker | 320 | 4.2 (1.1) | 4.0 (3.4–4.8) | 50.6 | 48.8 | 0.6 | Reference |
| Former smoker | 117 | 4.1 (1.1) | 3.9 (3.4–4.6) | 52.1 | 47.9 | 0.0 | 0.01 (−0.22; 0.25) |
| Smoker (cigarette/e-cigarette) | 60 | 3.6 (0.8) | 3.5 (3.0–4.1) | 73.3 | 26.7 | 0.0 | −0.32 (−0.63; −0.01) |
| Vegetarian diet ** | |||||||
| Strict (no fish) | 5 | 2.6 (0.3) | 2.7 (2.3–2.9) | 100.0 | 0.0 | 0.0 | −1.62 (−2.54; 0.70) |
| Predominantly | 27 | 4.2 (0.9) | 4.3 (3.4–4.7) | 37.0 | 63.0 | 0.0 | −0.05 (−0.45; 0.36) |
| No | 466 | 4.1 (1.1) | 3.9 (3.3–4.7) | 53.9 | 45.7 | 0.4 | Reference |
| Eating in company restaurants ** | |||||||
| Never | 168 | 3.8 (1.0) | 3.7 (3.1–4.4) | 62.5 | 37.5 | 0.0 | Reference |
| One time per month | 43 | 4.0 (1.2) | 3.7 (3.1–4.7) | 67.4 | 32.6 | 0.0 | 0.16 (−0.20; 0.51) |
| 2–3 times per month | 35 | 4.1 (1.1) | 4.0 (3.2–4.6) | 54.3 | 45.7 | 0.0 | 0.24 (−0.15; 0.63) |
| 1 time per week | 41 | 4.2 (0.9) | 4.1 (3.6–4.7) | 46.3 | 53.7 | 0.0 | 0.28 (−0.09; 0.65) |
| Several times per week | 103 | 4.3 (1.1) | 4.1 (3.5–4.9) | 45.6 | 53.4 | 1.0 | 0.27 (−0.02; 0.56) |
| Daily (Monday-Friday) | 100 | 4.3 (1.1) | 4.1 (3.5–4.8) | 44.0 | 55.0 | 1.0 | 0.25 (−0.05; 0.55) |
| Omega-3-Index (in %) | ||||||
|---|---|---|---|---|---|---|
| Continuous | Categorical | |||||
| n | Mean (SD) | Median (IQR) | <4% (least CP) % a | 4%–8% (average CP) % a | >8% (largest CP) % a | |
| Consumption of lean fish (g/d) *** | ||||||
| Yes | 411 | 4.2 (1.0) | 4.0 (3.5–4.7) | 49.2 | 50.4 | 0.5 |
| No (=0 g/day) | 82 | 3.5 (1.0) | 3.3 (2.7–4.0) | 78.1 | 22.0 | 0.0 |
| Consumption of fatty fish (g/d) *** | ||||||
| Yes | 396 | 4.3 (1.0) | 4.1 (3.5–4.8) | 46.7 | 52.8 | 0.5 |
| No (=0 g/d) | 96 | 3.3 (0.9) | 3.2 (2.7–3.8) | 83.3 | 16.7 | 0.0 |
| Omega-3 supplementation *** | ||||||
| Yes | 34 | 4.9 (1.4) | 4.5 (3.9–5.8) | 35.3 | 61.8 | 2.9 |
| No | 451 | 4.0 (1.0) | 3.9 (3.3–4.7) | 55.4 | 44.4 | 0.2 |
| Consumption of fatty fish in canteens of the company ***,a | ||||||
| Never | 312 | 3.9 (1.0) | 3.7 (3.2–4.4) | 61.5 | 38.5 | 0.0 |
| One time per month | 118 | 4.4 (1.0) | 4.2 (3.5–5.0) | 40.7 | 59.3 | 0.0 |
| 2–3 times per month | 46 | 4.5 (1.3) | 4.3 (3.6–5.0) | 39.1 | 56.5 | 4.4 |
| 1 time per week | 19 | 4.5 (1.0) | 4.8 (3.7–5.2) | 31.6 | 68.4 | 0.0 |
| Estimated within Group Change of O3I between Baseline and Follow-up Coeff. (95%-CI) | Estimated between Group Differences of Changes in O3I between Baseline and Follow-up Coeff. (95%-CI) | |
|---|---|---|
| Variables at baseline | ||
| Total | 0.55 (0.42; 0.68) | - |
| Age (in years) | ||
| <35 | 0.38 (0.09; 0.67) | −0.26 (−0.71; 0.20) |
| 35–39 | 0.38 (−0.08; 0.85) | −0.26 (−0.84; 0.33) |
| 40–44 | 0.64 (0.29; 0.99) | Reference |
| 45–49 | 0.70 (0.39; 1.01) | 0.06 (−0.41; 0.53) |
| 50–54 | 0.47 (0.19; 0.75) | −0.17 (−0.62; 0.28) |
| ≥55 | 0.70 (0.39; 1.01) | 0.06 (−0.41; 0.53) |
| Gender | ||
| Male | 0.55 (0.39; 0.71) | Reference |
| Female | 0.54 (0.31; 0.78) | 0 (−0.29; 0.28) |
| Occupational status a | ||
| Manual worker | 0.23 (−0.11; 0.57) | Reference |
| Skilled/supervisory worker | 0.47 (0.30; 0.65) | 0.24 (−0.14; 0.62) |
| Managerial staff | 0.82 (0.59; 1.05) | 0.59 (0.18; 1.00) |
| Working time system b | ||
| Day work | 0.55 (0.41; 0.69) | Reference |
| Shift work | 0.52 (0.11; 0.94) | −0.03 (−0.47; 0.41) |
| Body mass index (in kg/m2) | ||
| Normal weight/underweight (<25) | 0.61 (0.41; 0.80) | Reference |
| Overweight (25–30) | 0.49 (0.28; 0.70) | −0.11 (−0.40; 0.17) |
| Obesity (≥30) | 0.51 (0.17; 0.85) | −0.09 (−0.49; 0.30) |
| Smoking status | ||
| Nonsmoker | 0.56 (0.40; 0.71) | Reference |
| Former smoker | 0.31 (0.02; 0.59) | −0.25 (−0.57; 0.07) |
| Smoker | 1.00 (0.56; 1.44) | 0.45 (−0.02; 0.91) |
| Vegetarian diet | ||
| Strict (no fish) | 0.27 (−0.85; 1.39) | −0.28 (−1.41; 0.85) |
| Predominantly | 0.36 (−0.16; 0.89) | −0.19 (−0.73; 0.35) |
| No | 0.55 (0.42; 0.68) | Reference |
| Eating in company restaurants | ||
| Never | 0.55 (0.29; 0.80) | Reference |
| Once per month | 0.33 (−0.12; 0.77) | −0.22 (−0.73; 0.29) |
| 2–3 times per month | 0.36 (−0.13; 0.86) | −0.18 (−0.74; 0.37) |
| 1 time per week | 0.40 (−0.07; 0.86) | −0.15 (−0.68; 0.38) |
| Several times per week | 0.60 (0.33; 0.88) | 0.06 (−0.31; 0.43) |
| Daily (Monday to Friday) | 0.68 (0.41; 0.95) | 0.13 (−0.24; 0.51) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rein, D.; Claus, M.; Frosch, W.; März, W.; Lorkowski, S.; Webendoerfer, S.; Schreiner, T. Changes in Erythrocyte Omega-3 Fatty Acids in German Employees upon Dietary Advice by Corporate Health. Nutrients 2020, 12, 3267. https://doi.org/10.3390/nu12113267
Rein D, Claus M, Frosch W, März W, Lorkowski S, Webendoerfer S, Schreiner T. Changes in Erythrocyte Omega-3 Fatty Acids in German Employees upon Dietary Advice by Corporate Health. Nutrients. 2020; 12(11):3267. https://doi.org/10.3390/nu12113267
Chicago/Turabian StyleRein, Dietrich, Matthias Claus, Wolfgang Frosch, Winfried März, Stefan Lorkowski, Stefan Webendoerfer, and Thorsten Schreiner. 2020. "Changes in Erythrocyte Omega-3 Fatty Acids in German Employees upon Dietary Advice by Corporate Health" Nutrients 12, no. 11: 3267. https://doi.org/10.3390/nu12113267
APA StyleRein, D., Claus, M., Frosch, W., März, W., Lorkowski, S., Webendoerfer, S., & Schreiner, T. (2020). Changes in Erythrocyte Omega-3 Fatty Acids in German Employees upon Dietary Advice by Corporate Health. Nutrients, 12(11), 3267. https://doi.org/10.3390/nu12113267






