Iodine Intake in Norwegian Women and Men: The Population-Based Tromsø Study 2015–2016
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.1.1. Twenty-Four-Hour Urine Collection
2.1.2. Questionnaires and Measurements
2.2. Urine Analysis
2.3. Ethics
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Iodine Excretion and Estimated Iodine Intake
3.3. Estimated Iodine Intake from Diet and Supplements (FFQ)
3.4. Sources of Iodine
3.5. Supplements
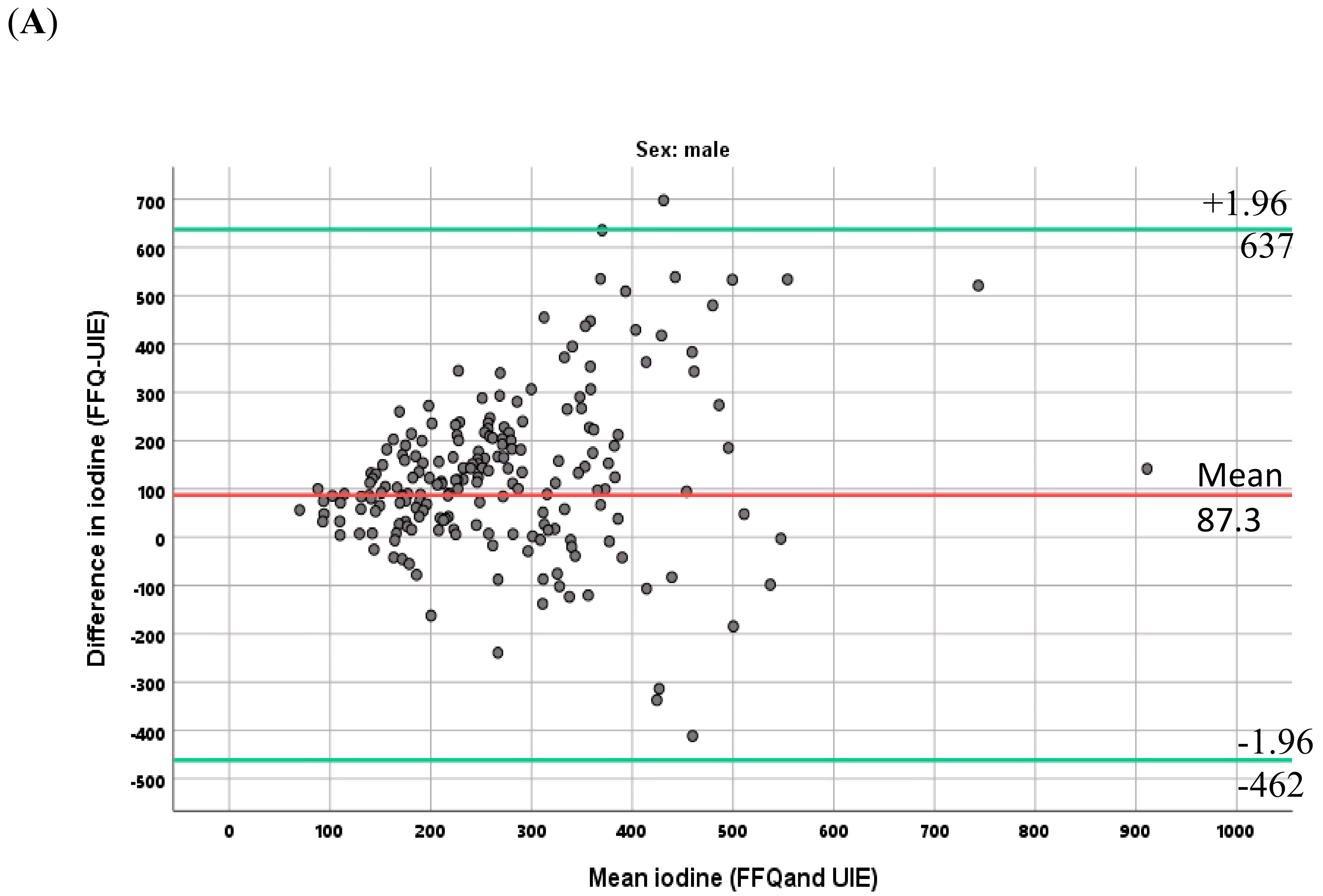
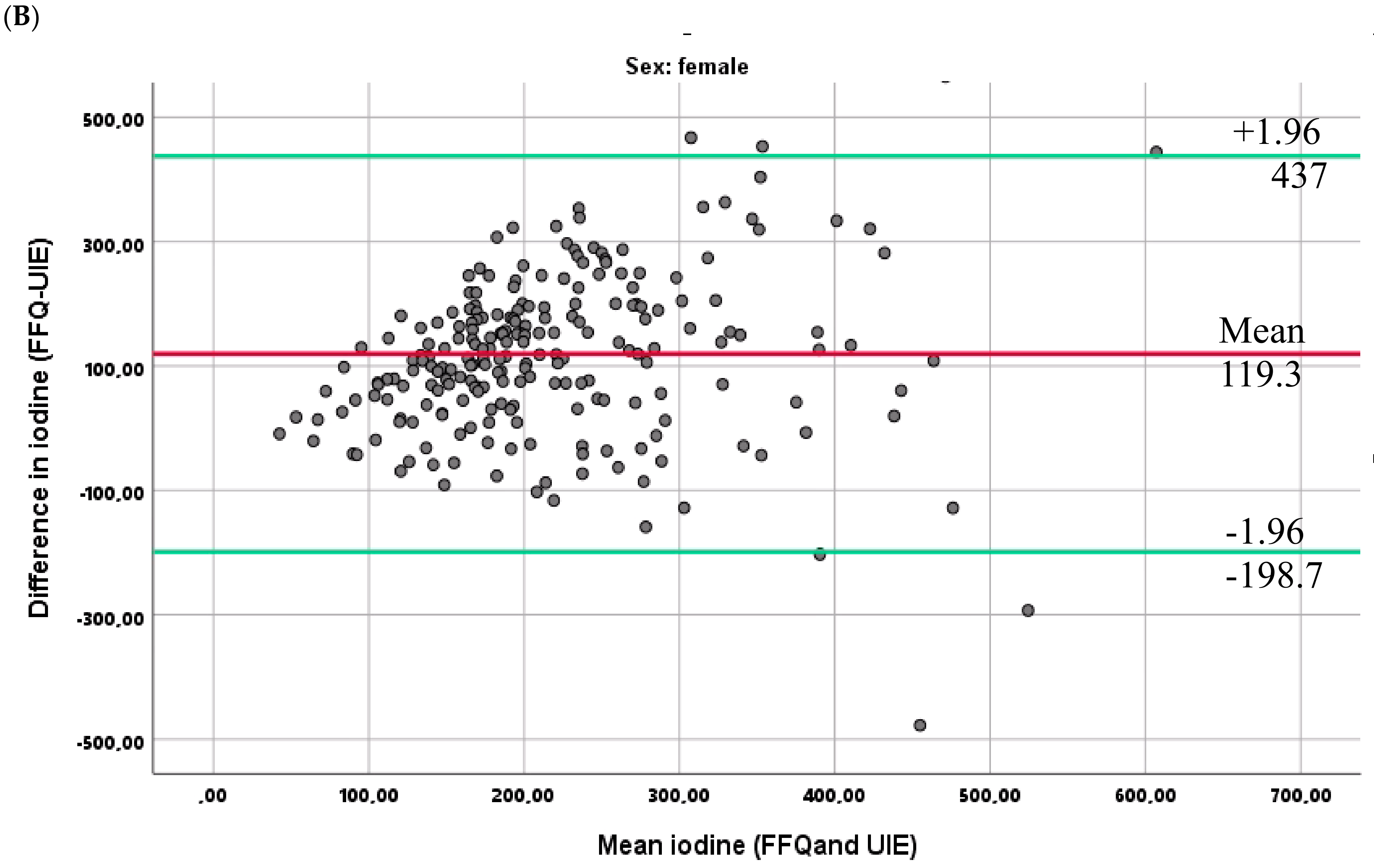
3.6. Relationship between the Two Methods for Estimating Iodine Intake
3.7. Sensitivity Analyses
4. Discussion
4.1. Variation in Iodine Intake by Gender, Age, and Education
4.2. Iodine Intake as Compared with Recommendations
4.3. Study Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EAR | Estimated Average Requirements |
| FFQ | Food frequency questionnaire |
| IDD | Iodine deficiency disorders |
| RDI | Recommended daily intake |
| UIC | Urinary iodine concentration |
| UIE | Urinary iodine excretion |
| TSH | Thyroid stimulating hormone |
References
- World Health Organization (WHO); United Nations Children’s Fund (UNICEF); International Council for Control of Iodine Deficiency Disorders (ICCIDD). Assessment of the Iodine Deficiency Disorders and Monitoring Their Elimination, 3rd ed.; World Health Organitzation: Geneva, Switzerland, 2007. [Google Scholar]
- Hetzel, B.S. Iodine deficiency disorders (IDD) and their eradication. Lancet 1983, 2, 1126–1129. [Google Scholar] [CrossRef]
- Aburto, N.J.; Abudou, M.; Candeias, V.; Wu, T. Effect and Safety of Salt Iodization to Prevent Iodine Deficiency Disorders: A Systematic Review with Meta-Analyses; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Andersson, M.; Karumbunathan, V.; Zimmermann, M.B. Global iodine status in 2011 and trends over the past decade. J. Nutr. 2012, 142, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Nystrom, H.F.; Brantsaeter, A.L.; Erlund, I.; Gunnarsdottir, I.; Hulthen, L.; Laurberg, P.; Mattisson, I.; Rasmussen, L.B.; Virtanen, S.; Meltzer, H.M. Iodine status in the Nordic countries-past and present. Food Nutr. Res. 2016, 60, 31969. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.H. Iodine status in europe in 2014. Eur. Thyroid J. 2014, 3, 3–6. [Google Scholar] [CrossRef]
- Mian, C.; Vitaliano, P.; Pozza, D.; Barollo, S.; Pitton, M.; Callegari, G.; di Gianantonio, E.; Casaro, A.; Nacamulli, D.; Busnardo, B.; et al. Iodine status in pregnancy: Role of dietary habits and geographical origin. Clin. Endocrinol. 2009, 70, 776–780. [Google Scholar] [CrossRef]
- Alvarez-Pedrerol, M.; Ribas-Fito, N.; Garcia-Esteban, R.; Rodriguez, A.; Soriano, D.; Guxens, M.; Mendez, M.; Sunyer, J. Iodine sources and iodine levels in pregnant women from an area without known iodine deficiency. Clin. Endocrinol. 2010, 72, 81–86. [Google Scholar] [CrossRef]
- Brantsaeter, A.L.; Knutsen, H.K.; Johansen, N.C.; Nyheim, K.A.; Erlund, I.; Meltzer, H.M.; Henjum, S. Inadequate Iodine Intake in Population Groups Defined by Age, Life Stage and Vegetarian Dietary Practice in a Norwegian Convenience Sample. Nutrients 2018, 10, 230. [Google Scholar] [CrossRef]
- Frey, H.; Tangen, T.; Lovik, J.; Thorsen, R.K.; Sand, T.; Rosenlund, B.; Kornstad, L. Endemic goiter no longer exists in the community of Modum. Tidsskr. Nor. Laegeforen. 1981, 101, 1184–1186. [Google Scholar]
- Dahl, L.; Meltzer, H.M. The Iodine Content of Foods and Diets: Norwegian Perspectives. In Comprehensive Handbook of Iodine; Preedy, V.R., Burrow, G.N., Watson, R.R., Eds.; Academic Press: London, UK, 2009; pp. 345–352. [Google Scholar]
- Totland, T.H.; Melnæs, B.K.; Lundberg-Hallén, N.; Helland-Kigen, K.M.; Lund-Blix, N.A.; Myhre, J.B.; Johansen, A.M.W.; Løken, E.B.; Andersen, L.F. Norkost 3 En Landsomfattende Kostholdsundersøkelse Blant Menn og Kvinner i Norge i Alderen 18-70 år, 2010–11; Helsedirektoratet: Oslo, Norway, 2012. [Google Scholar]
- Norwegian Directorate of Health. Utviklingen i Norsk Kosthold 2015; IS-2382; Helsedirektoratet: Oslo, Norway, 2015. Available online: https://wwwhelsedirektoratetno/rapporter/utviklingen-i-norsk-kosthold (accessed on 5 September 2020). (In Norwegian)
- Troan, G.; Dahl, L.; Meltzer, H.M.; Abel, M.H.; Indahl, U.G.; Haug, A.; Prestløkken, E. A model to secure a stable iodine concentration in milk. Food Nutr. Res. 2015, 59, 29829. [Google Scholar] [CrossRef]
- Brantsaeter, A.L.; Abel, M.H.; Haugen, M.; Meltzer, H.M. Risk of suboptimal iodine intake in pregnant Norwegian women. Nutrients 2013, 5, 424–440. [Google Scholar] [CrossRef]
- Henjum, S.; Lilleengen, A.M.; Aakre, I.; Dudareva, A.; Gjengedal, E.L.F.; Meltzer, H.M.; Brantsæter, A.L. Suboptimal Iodine Concentration in Breastmilk and Inadequate Iodine Intake among Lactating Women in Norway. Nutrients 2017, 9, 643. [Google Scholar] [CrossRef] [PubMed]
- Garnweidner-Holme, L.; Aakre, I.; Lilleengen, A.M.; Brantsaeter, A.L.; Henjum, S. Knowledge about Iodine in Pregnant and Lactating Women in the Oslo Area, Norway. Nutrients 2017, 9, 493. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.M.; Torheim, L.E.; Brantsæter, A.L.; Madar, A.; Abel, M.H.; Dahl, L. Risiko for Jodmangel i Norge—Identifisering av et Akutt Behov for Tiltak; Nasjonalt råd for Ernæring: Oslo, Norway, 2016; Available online: http://wwwernaeringsradetno/wp-content/uploads/2016/06/IS-0591_RisikoForJodmangeliNorgepdf (accessed on 2 July 2020). (In Norwegian)
- World Health Organization (WHO). Urinary Iodine Concentrations for Determining Iodine Status in Populations; (WHO/NMH/NHD/EPG/131); World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Zimmermann, M.B.; Andersson, M. Assessment of iodine nutrition in populations: Past, present, and future. Nutr. Rev. 2012, 70, 553–570. [Google Scholar] [CrossRef]
- Ministers NCo. Nordic Nutrition Recommendations 2012, 5th ed.; Nordic Council of Ministers: Copenhagen, Denmark, 2012.
- Tromsøundersøkelsen. Om Tromsøundersøkelsen 2017. Available online: https://uitno/forskning/forskningsgrupper/sub?p_document_id=367276&sub_id=377965 (accessed on 15 August 2020).
- Meyer, H.E.; Johansson, L.; Eggen, A.E.; Johansen, H.; Holvik, K. Sodium and Potassium Intake Assessed by Spot and 24-h Urine in the Population-Based Tromso Study 2015–2016. Nutrients 2019, 11, 1619. [Google Scholar] [CrossRef]
- Medin, A.C.; Carlsen, M.H.; Hambly, C.; Speakman, J.R.; Strohmaier, S.; Andersen, L.F. The validity of a web-based FFQ assessed by doubly labelled water and multiple 24-h recalls. Br. J. Nutr. 2017, 118, 1106–1117. [Google Scholar] [CrossRef]
- Matportalen. Old tables—The Norwegian Food Compostion Table 2015. Matportalen no. 2015. Available online: https://wwwmatportalenno/verktoy/the_norwegian_food_composition_table/old_tables (accessed on 15 August 2020).
- Lundblad, M.W.; Andersen, L.F.; Jacobsen, B.K.; Carlsen, M.H.; Hjartaker, A.; Grimsgaard, S.; Hopstock, L.A. Energy and nutrient intakes in relation to National Nutrition Recommendations in a Norwegian population-based sample: The Tromso Study 2015-16. Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef]
- Johansson, L.; Solvoll, K.N. Landsomfattende Kostholdsundersøkelse Blant Menn og Kvinner i Alderen 16-79 år (National Dietary Survey Among Males and Females, 16–79 Years); National Nutrition and Physical Education Council: Oslo, Norway, 1999. [Google Scholar]
- Haldimann, M.; Bochud, M.; Burnier, M.; Paccaud, F.; Dudler, V. Prevalence of iodine inadequacy in Switzerland assessed by the estimated average requirement cut-point method in relation to the impact of iodized salt. Public Health Nutr. 2015, 18, 1333–1342. [Google Scholar] [CrossRef]
- Carlsen, M.H.; Andersen, L.F.; Dahl, L.; Norberg, N.; Hjartaker, A. New Iodine Food Composition Database and Updated Calculations of Iodine Intake among Norwegians. Nutrients 2018, 10, 930. [Google Scholar] [CrossRef]
- Rasmussen, L.B.; Ovesen, L.; Bulow, I.; Jorgensen, T.; Knudsen, N.; Laurberg, P.; Pertild, H. Dietary iodine intake and urinary iodine excretion in a Danish population: Effect of geography, supplements and food choice. Br. J. Nutr. 2002, 87, 61–69. [Google Scholar] [CrossRef]
- Madar, A.A.; Meltzer, H.M.; Heen, E.; Meyer, H.E. Iodine Status among Somali Immigrants in Norway. Nutrients 2018, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, R.; Asakura, K.; Uechi, K.; Masayasu, S.; Sasaki, S. Iodine Excretion in 24-hour Urine Collection and Its Dietary Determinants in Healthy Japanese Adults. J. Epidemiol. 2016, 26, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Censi, S.; Manso, J.; Barollo, S.; Mondin, A.; Bertazza, L.; De Marchi, M.; Mian, C. Changing Dietary Habits in Veneto Region over Two Decades: Still a Long Road to Go to Reach an Iodine-Sufficient Status. Nutrients 2020, 12, 2399. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Biro, G.; Hulshof, K.F.; Ovesen, L.; Amorim Cruz, J.A. Selection of methodology to assess food intake. Eur. J. Clin. Nutr. 2002, 56 (Suppl. 2), S25–S32. [Google Scholar] [CrossRef] [PubMed]
- Althubaiti, A. Information bias in health research: Definition, pitfalls, and adjustment methods. J. Multidiscip. Healthc. 2016, 9, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Guo, X.; Tang, J.; Guo, X.; Lu, Z.; Zhang, J.; Bi, Z. Iodine nutritional status in the adult population of Shandong Province (China) prior to salt reduction program. Eur. J. Nutr. 2016, 55, 1933–1941. [Google Scholar] [CrossRef] [PubMed]
- Bath, S.C.; Sleeth, M.L.; McKenna, M.; Walter, A.; Taylor, A.; Rayman, M.P. Iodine intake and status of UK women of childbearing age recruited at the University of Surrey in the winter. Br. J. Nutr. 2014, 112, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M.P.; Stablein, U.; Brubaker, L. Urinary habits among asymptomatic women. Am. J. Obstet. Gynecol. 2002, 187, 1384–1388. [Google Scholar] [CrossRef] [PubMed]
- Perucca, J.; Bouby, N.; Valeix, P.; Bankir, L. Sex difference in urine concentration across differing ages, sodium intake, and level of kidney disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R700–R705. [Google Scholar] [CrossRef]
- Dahl, L.; Opsahl, J.A.; Meltzer, H.M.; Julshamn, K. Iodine concentration in Norwegian milk and dairy products. Br. J. Nutr. 2003, 90, 679–685. [Google Scholar] [CrossRef]
| All | Women | Men | |
|---|---|---|---|
| Gender, n | 493 | 252 | 241 |
| Age, years | 56 (8.4) | 57 (8.4) | 55 (8.4) |
| Primary education (up to 10 yrs.), % | 20.4 (100) | 21.5 (54) | 19.2 (46) |
| Secondary education (up to 13 yrs.), % | 28.3 (139) | 27.1 (68) | 29.6 (71) |
| Tertiary education (university), % | 51.3 (252) | 51.4 (129) | 51.2 (123) |
| Body mass index, kg/m2 | 27.2 (4.3) | 27.5 (3.7) | 26.8 (4.7) |
| Total Population | Women (n = 252) | Men (n = 241) | |||||
|---|---|---|---|---|---|---|---|
| All (40–69 Years (n = 493) | All | 40–54 Years (n = 112) | 55–69 Years | All | 40–54 Years | 55–69 Years | |
| (n = 252) | (n = 140) | (n = 241) | (n = 90) | (n = 151) | |||
| 24-h urine volume (L) | |||||||
| Mean (SD) | 1.74 (0.59) | 1.77 (0.60) | 1.74 (0.62) | 1.79 (0.58) | 1.67 (0.57) | 1.64 (0.61) | 1.70 (0.55) |
| Median (25th, 75th) * | 1.69 (1.31, 2.20) | 1.80 (1.29, 2.30) | 1.73 (1.24, 2.35) | 1.80 (1.36, 2.32) | 1.62 (1.27, 2.10) | 1.53 (1.13, 2.21) | 1.65 (1.32, 2.01) |
| 24-h UIC (µg/L) | |||||||
| Median (25th, 75th) | 88 (50, 125) | 63 (50, 100) | 63 (38, 100) | 75 (50, 113) | 100 (63, 150) | 88 (63, 125) | 100 (63, 153) |
| 24-h UIE (μg/day) # | |||||||
| Mean (SD) | 199 (545) | 182 (724) | 222 (1079) | 150 (116) | 214 (247) | 150 (96) | 253 (297) |
| Median (25th, 75th) * | 132 (87, 200) | 111 (78, 170) | 102 (79, 149) | 117 (75, 184) | 145 (108, 234) | 132 (95, 176) | 168 (113, 265) |
| 24-h iodine intake (μg/day) † | |||||||
| Mean (SD) | 241.6 (670) | 222.5 (890) | 271.6 (1327) | 183.2 (141) | 261.6 (302) | 182.9 (117) | 308.5 (364) |
| Median (25th, 75th) * | 158.9 (106, 245) | 133.2 (93, 205) | 122.8 (95, 182) | 141.1 (91, 223) | 174.4 (129, 284) | 159.3 (114, 216) | 202.9 (135, 321) |
| Subgroup | n | Mean (SD) | Median (P25, P75) * |
|---|---|---|---|
| All (40–69 years) | 450 | 314.3 (157.1) | 281 (212.5, 378.5) |
| Women | 234 | 288.1 (142.9) | 263 (200, 355.3) |
| 40–54 years | 102 | 271.6 (151.9) | 244 (186.5, 328.3) |
| 55–69 years | 132 | 300.8 (142.9) | 278.5 (216.3, 371.3) |
| Men | 216 | 342.7 (166.7) | 317.5 (228.3, 407.5) |
| 40–54 years | 80 | 304.6 (133) | 295 (215.8, 367.8) |
| 55–69 years | 136 | 365.2 (189.6) | 329 (246.3, 447.3) |
| N | Dairy (g) (Min–Max) | Fish (g) (Min–Max) | Eggs (g) (Min–Max) | |
|---|---|---|---|---|
| All (40–69 yrs) | 450 | 496.6 (0–3061) | 119.3 (0–606) | 27.1 (0–234) |
| Women | 234 | 422.6 (0–1957) | 108.2 (0–606) | 28.3 (0–234) |
| 40–54 (yrs) | 102 | 412.8 (0–1957) | 92.1 (0–606) | 32.9 (0–234) |
| 55–69 (yrs) | 132 | 430.1 (30–1657) | 120.6 (0–375) | 24.7 (0–233) |
| Men | 216 | 576.0 (16–3016) | 131.2 (0–489) | 25.8 (0–186) |
| 40–54 (yrs) | 80 | 596.7 (16–3061) | 107.9 (0–360) | 28.0 (0–101) |
| 55–69 (yrs) | 136 | 563.8 (26–1919) | 144.9 (10–489) | 24.4 (0–186) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madar, A.A.; Heen, E.; Hopstock, L.A.; Carlsen, M.H.; Meyer, H.E. Iodine Intake in Norwegian Women and Men: The Population-Based Tromsø Study 2015–2016. Nutrients 2020, 12, 3246. https://doi.org/10.3390/nu12113246
Madar AA, Heen E, Hopstock LA, Carlsen MH, Meyer HE. Iodine Intake in Norwegian Women and Men: The Population-Based Tromsø Study 2015–2016. Nutrients. 2020; 12(11):3246. https://doi.org/10.3390/nu12113246
Chicago/Turabian StyleMadar, Ahmed A, Espen Heen, Laila A Hopstock, Monica H Carlsen, and Haakon E Meyer. 2020. "Iodine Intake in Norwegian Women and Men: The Population-Based Tromsø Study 2015–2016" Nutrients 12, no. 11: 3246. https://doi.org/10.3390/nu12113246
APA StyleMadar, A. A., Heen, E., Hopstock, L. A., Carlsen, M. H., & Meyer, H. E. (2020). Iodine Intake in Norwegian Women and Men: The Population-Based Tromsø Study 2015–2016. Nutrients, 12(11), 3246. https://doi.org/10.3390/nu12113246





