The Role of Nutritional Support in Cured/Chronic Patients
Abstract
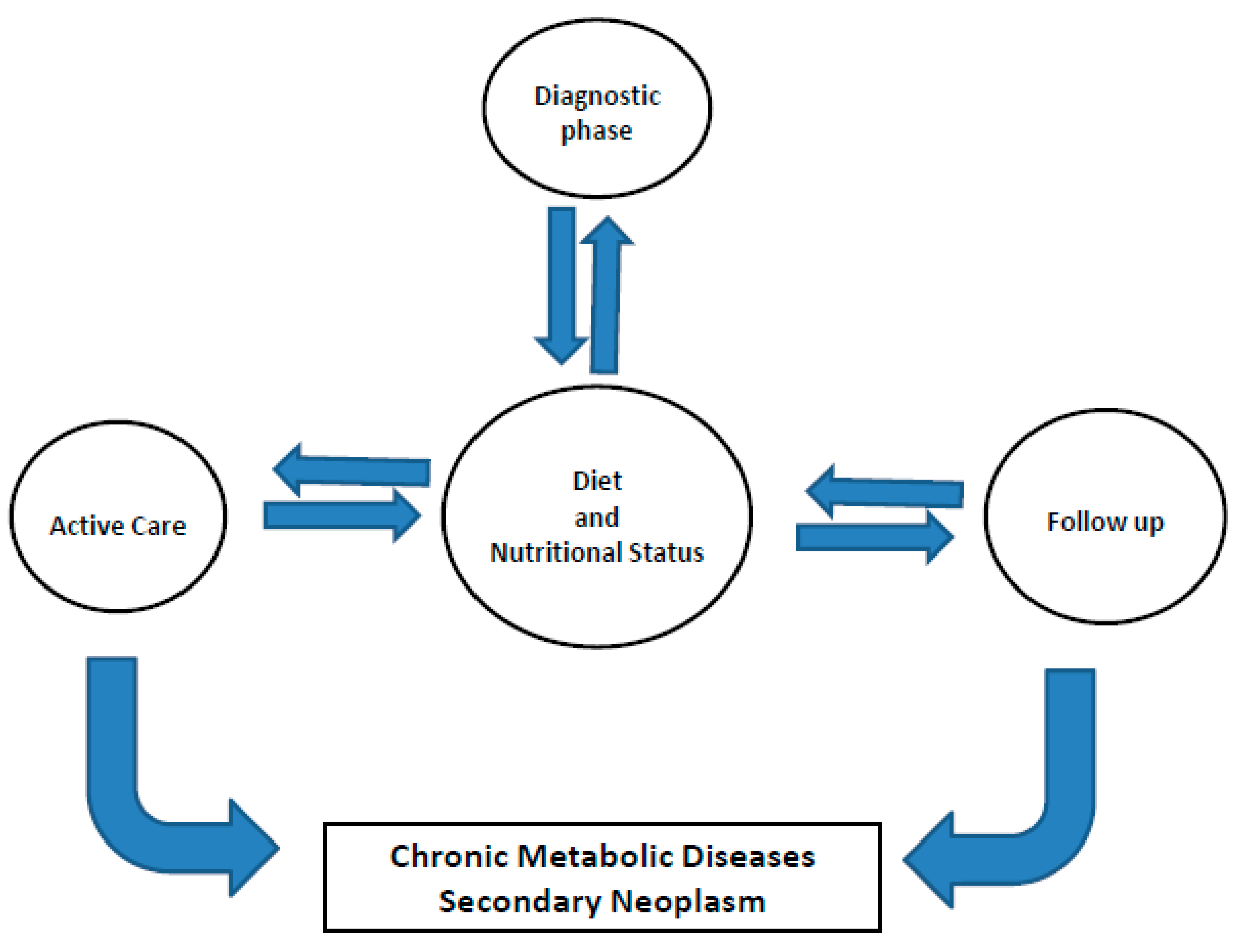
1. Cured and Long-Term Cancer Patients
2. General Dietary Information in This Setting
3. Hot Topics: Fibers, Protein and Carbohydrates
4. Dietary Supplements: Vitamins, Minerals and other Nutrients
5. Diet and Nutritional Therapy
6. Maintaining Body Weight
6.1. Weight Loss
6.2. Weight Gain
7. Constipation
8. Diet and Fatigue
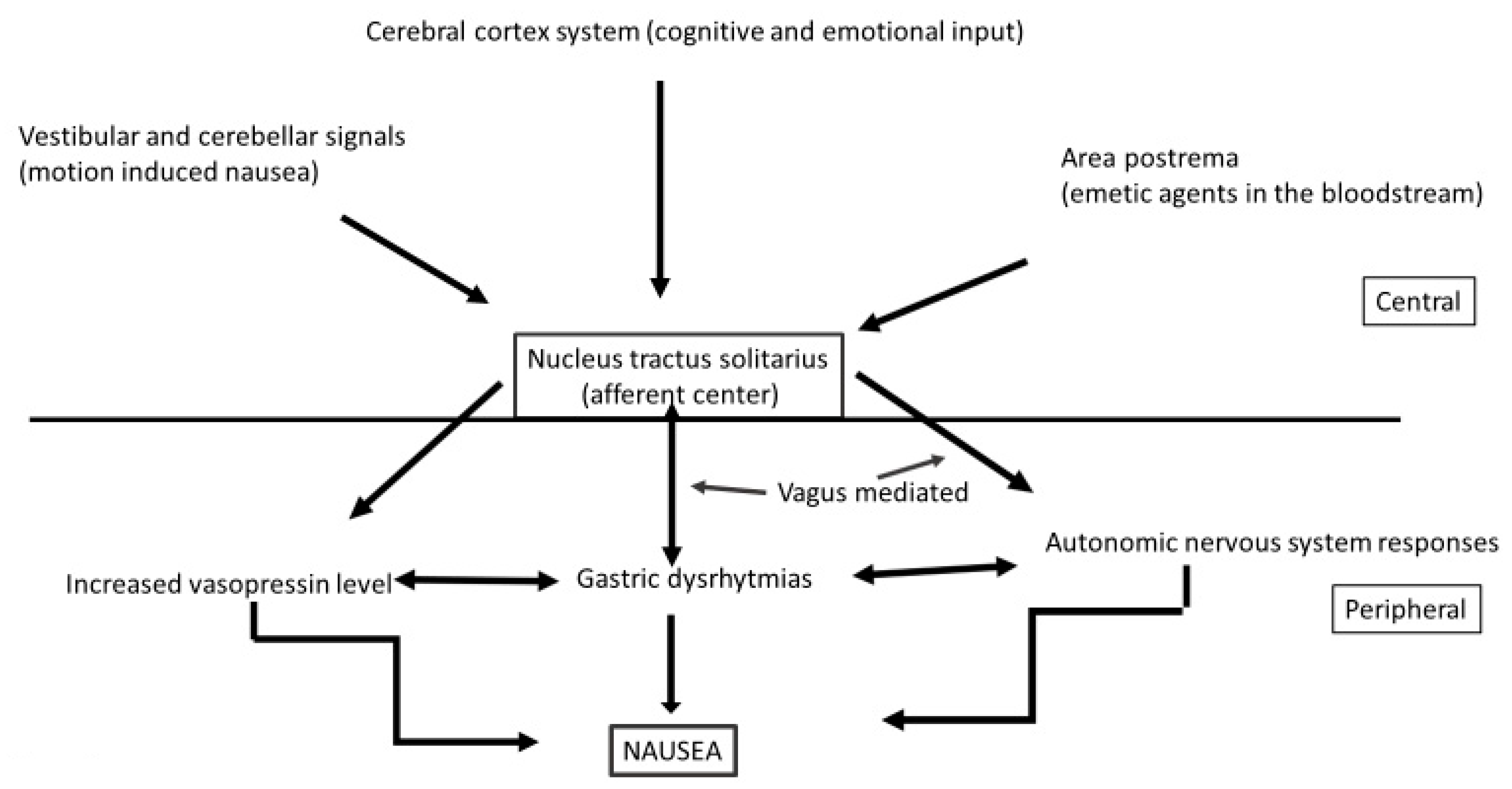
9. Diet and Nausea
10. Change in Lifestyle
Author Contributions
Funding
Conflicts of Interest
References
- Astrow, A.B. Cancer Survivorship and Beyond. JAMA 2012, 308, 1639–1640. Available online: https://europepmc.org/article/med/23093162 (accessed on 1 October 2012). [CrossRef]
- Bell, K.; Ristovski-Slijepcevic, S.; McGrath, S.E.; Webb, A.; Walker-Bone, K. Cancer Survivorship: Why Labels Matter. J. Clin. Oncol. 2013, 31, 409–411. Available online: https://ascopubs.org/doi/full/10.1200/JCO.2012.43.5891 (accessed on 1 February 2013). [CrossRef]
- AIOM AIRTUM I Numeri del Cancro in Italia 2017. Il Pensiero Scientifico Editore. Available online: http://media.aiom.it/userfiles/files/doc/AIOM-Fondazione/2017_numeri_del_cancro_ed_pazienti.pdf (accessed on 1 February 2013).
- Baade, P.D.; Youlden, D.R.; Chambers, S.K. When do I know I am Cured? Using Conditional Estimates to Provide Better Information about Cancer Survival Prospects. Med. J. Aust. 2011, 194, 73–77. [Google Scholar]
- Ellison, L.F.; Bryant, H.; Lockwood, G.; Shack, L. Conditional survival analyses across cancer sites. Heal. Rep. 2011, 22, 21–25. [Google Scholar]
- Janssen–Heijnen, M.L.; Gondos, A.; Bray, F.; Hakulinen, T.; Brewster, D.H.; Brenner, H.; Coebergh, J.W.W. Clinical relevance of conditional survival of cancer pa-tients in Europe: Age-Specific Analyses of 13 Cancers. J. Clin. Oncol. 2010, 28, 2520–2528. [Google Scholar] [CrossRef] [PubMed]
- Småstuen, M.; Aagnes, B.; Johannesen, T.B.; Møller, B.; Bray, F. Long-term Cancer Survival: Patterns and Trends in Norway 1965–2007; Cancer Registry of Norway: Oslo, Norway, 2008. [Google Scholar]
- Andrae, B.; Andersson, T.M.-L.; Lambert, P.C.; Kemetli, L.; Silfverdal, L.; Strander, B.; Ryd, W.; Dillner, J.; Törnberg, S.; Sparén, P. Screening and cervical cancer cure: Population based cohort study. BMJ 2012, 344, e900. [Google Scholar] [CrossRef] [PubMed]
- AIRTUM Working Group Italian Cancer Figures, report 2011. Survival of Cancer Patients in Italy. Epidemiol. Prev. 2011, 35 (Suppl. S3), 1–200.
- Tralongo, P.; Maso, L.D.; Surbone, A.; Santoro, A.; Tirelli, U.; Sacchini, V.; Pinto, C.; Crispino, S.; Ferraù, F.; Mandoliti, G.; et al. Use of the Word “Cured” for Cancer Patients—Implications for Patients and Physicians: The Siracusa charter. Curr. Oncol. 2014, 22, e38–e40. [Google Scholar] [CrossRef][Green Version]
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity. And Cancer: Impact and Future Directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef]
- Daar, A.S.; Singer, P.A.; Persad, D.L.; Pramming, S.K.; Matthews, D.R.; Beaglehole, R.; Bernstein, A.; Borysiewicz, L.K.; Colagiuri, S.; Ganguly, N.; et al. Grand Hallenges in Chronic Non-Communicable Diseases. Nat. Cell Biol. 2007, 450, 494–496. [Google Scholar] [CrossRef]
- Demark-Wahnefried, W.; Aziz, N.M.; Rowland, J.H.; Pinto, B.M. Riding the Crest of the Teachable Moment: Promoting Long-Term Health After the Diagnosis of Cancer. J. Clin. Oncol. 2005, 23, 5814–5830. [Google Scholar] [CrossRef] [PubMed]
- Ganz, P.A. A Teachable Moment for Oncologists: Cancer Survivors, 10 Million Strong and Growing! J. Clin. Oncol. 2005, 23, 5458–5460. [Google Scholar] [CrossRef]
- Nord, C.; Mykletun, A.; Thorsen, L.; Bjøro, T.; Fosså, S.D. Self-reported health and use of health care services in long-term cancer survivors. Int. J. Cancer 2004, 114, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Demark-Wahnefried, W.; Rogers, L.Q.; Alfano, C.M.; Thomson, C.A.; Courneya, K.S.; Meyerhardt, J.A.; Stout, N.L.; Kvale, E.; Ganzer, H.; Ligibel, J.A. Practical Clinical Interventions for Diet, Physical Activity, and Weight Control in Cancer Survivors. CA A Cancer J. Clin. 2015, 65, 167–189. [Google Scholar] [CrossRef]
- Burden, S.; Jones, D.J.; Sremanakova, J.; Sowerbutts, A.M.; Lal, S.; Pilling, M. Dietary interventions for adult cancer survivors. Cochrane. Database. Syst. Rev. 2019. [Google Scholar] [CrossRef]
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Byers, T. Nutrition and Physical Activ-ity Guidelines for Cancer Survivors. CA Cancer J. Clin. 2012, 62, 242–274. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Blackburn, G.L.; Thomson, C.A.; Nixon, D.W.; Shapiro, A.; Hoy, M.K.; Goodman, M.T.; Giuliano, A.E.; Karanja, N.; McAndrew, P.; et al. Dietary Fat Reduction and Breast Cancer Outcome: Interim Efficacy Results From the Women’s Intervention Nutrition Study. J. Natl. Cancer Inst. 2006, 98, 1767–1776. [Google Scholar] [CrossRef]
- Pierce, J.P.; Natarajan, L.; Caan, B.J.; Parker, B.A.; Greenberg, E.R.; Flatt, S.W.; Rock, C.L.; Kealey, S.; Al-Delaimy, W.K.; Bardwell, W.A.; et al. Influence of a Diet Very High in Vegetables, Fruit, and Fiber and Low in Fat on Prognosis Following Treatment for Breast Cancer. JAMA 2007, 298, 289–298. [Google Scholar] [CrossRef]
- Tohill, B.C.; Seymour, J.; Serdula, M.; Kettel-Khan, L.; Rolls, B.J. What epidemiologic studies tell us about the relationship between fruit and vegetable consumption and body weight. Nutr. Rev. 2004, 62, 365–374. [Google Scholar] [CrossRef]
- Kroenke, C.; Fung, T.T.; Hu, F.B.; Holmes, M.D. Dietary Patterns and Survival After Breast Cancer Diagnosis. J. Clin. Oncol. 2005, 23, 9295–9303. [Google Scholar] [CrossRef]
- Meyerhardt, J.A.; Niedzwiecki, D.; Hollis, D.; Saltz, L.B.; Hu, F.B.; Mayer, R.J.; Nelson, H.; Whittom, R.; Hantel, A.; Thomas, J.; et al. Association of Dietary Patterns With Cancer Recurrence and Survival in Patients With Stage III Colon Cancer. JAMA 2007, 298, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L. Diet and Breast Cancer: Can Dietary Factors Influence Survival? J. Mammary Gland. Biol. Neplasia 2003, 8, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.A.; Alberts, D.S. Diet and Survival after Ovarian Cancer: Where Are We and What’s Next? J. Am. Diet. Assoc. 2010, 110, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Holick, C.N.; Leitzmann, M.F.; Rimm, E.B.; Willett, W.C.; Stampfer, M.J.; Giovannucci, E.L. Diet After Diagnosis and the Risk of Prostate Cancer Progression, Recurrence, and death USA. Cancer Causes Control 2006, 17, 199–208. [Google Scholar] [CrossRef]
- Velicer, C.M.; Ulrich, C.M. Vitamin and Mineral Supplement Use Among US Adults after Cancer Diagnosis: A Systematic Review. J. Clin. Oncol. 2008, 26, 665–673. [Google Scholar] [CrossRef]
- Davies, A.A.; Smith, G.D.; Harbord, R.; Bekkering, G.E.; Sterne, J.A.C.; Beynon, R.; Thomas, S.J. Nutritional Interventions and Outcome in Patients With Cancer or Preinvasive Lesions: Systematic Review. J. Natl. Cancer Inst. 2006, 98, 961–973. [Google Scholar] [CrossRef]
- Pocobelli, G.; Peters, U.; Kristal, A.R.; White, E. Use of Supplements of Multivitamins, Vitamin C, and Vitamin E in Relation to Mortality. Am. J. Epidemiol. 2009, 170, 472–483. [Google Scholar] [CrossRef]
- Saquib, J.; Rock, C.L.; Natarajan, L.; Saquib, N.; Newman, V.A.; Patterson, R.E.; Pierce, J.P. Dietary Intake, Supplement Use, and Survival Among Women Diagnosed with Early-Stage Breast Cancer. Nutr. Cancer. 2011, 63, 327–333. [Google Scholar] [CrossRef]
- Kwan, M.L.; Greenlee, H.; Lee, V.S.; Castillo, A.; Gunderson, E.P.; Habel, L.A.; Caan, B.J. Multivitamin use and Breast Cancer Outcomes in Women with Early-Stage Breast Cancer: The Life after Cancer Epidemiology Study. Breast Cancer Res Treat. 2011, 130, 195–205. [Google Scholar] [CrossRef]
- Stacey, F.G.; James, E.L.; Chapman, K.; Courneya, K.S.; Lubans, D.R. A systematic review and me-ta-analysis of social cognitive theory-based physical activity and/or nutrition behaviour change interven-tions for cancer survivors. J. Cancer Surviv. 2015, 9, 305–338. [Google Scholar] [CrossRef]
- Irwin, M.L.; McTiernan, A.; Baumgartner, R.; Baumgartner, K.B.; Bernstein, L.; Gilliland, F.D.; Ballard-Barbash, R. Changes in Body Fat and Weight After a Breast Cancer Diagnosis:Influence of Demographic, Prognostic and Lifestyle Factors. J. Clin. Oncol. 2005, 23, 774–782. [Google Scholar] [CrossRef]
- Doyle, C.; Kushi, L.H.; Byers, T.; Courneya, K.S.; Demark-Wahnefried, W.; Grant, B.; Andrews, K.S. Nutrition and physical activity during and after cancer treatment. An American Cancer Society guide for informed choices. CA Cancer J. Clin. 2006, 56, 323–343. [Google Scholar] [CrossRef] [PubMed]
- Hopkinson, J.B.; Wright, D.N.M.; Foster, C. Management of Weight Loss and Anorexia. Ann. Oncol. 2008, 19, vii289–vii293. [Google Scholar] [CrossRef]
- Barrera, S.; Demark-Wahnefried, W. Nutrition During and After Cancer Therapy. Oncology 2009, 23, 15–21. [Google Scholar] [PubMed]
- Protani, M.; Coory, M.; Martin, J.H. Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Res. Treat. 2010, 123, 627–635. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, Obesity, and Mortality from Can-cer, in a Prospective Study Cohort of US Adults. NEJM 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.F.; Liu, S.; John, E.M.; Must, A.; Demark-Wahnefried, W. Diet quality of cancer survivors and noncancer individuals: Results from a national survey. Cancer 2015, 121, 4212–4221. [Google Scholar] [CrossRef] [PubMed]
- Schwedhelm, C.; Boeing, H.; Hoffmann, G.; Aleksandrova, K.; Schwingshackl, L. Effect of diet on mortality and cancer recurrence among cancer survivors: A systematic review and meta-analysis of cohort studies. Nutr. Rev. 2016, 74, 737–748. [Google Scholar] [CrossRef]
- Ligibel, J.A. Lifestyle Factors in Cancer Survivorship. J. Clin. Oncol. 2012, 30, 3697–3704. [Google Scholar] [CrossRef]
- Troeschel, A.N.; Hartman, T.J.; Jacobs, E.J.; Stevens, V.L.; Gansler, T.; Flanders, W.D.; McCullough, L.E.; Wang, Y. Postdiagnosis Body Mass Index, Weight Change, and Mortality From Prostate Cancer, Cardiovascular Disease, and All Causes Among Survivors of Nonmetastatic Prostate Cancer. J. Clin. Oncol. 2020, 38, 2018–2027. [Google Scholar] [CrossRef]
- Fallon, M. Constipation in cancer patients: Prevalence, pathogenesis, and cost-related issues. Eur. J. Pain 1999, 3, 3–7. [Google Scholar] [CrossRef]
- Yang, J.; Wang, H.P.; Zhou, L.; Xu, C.F. Effect of Dietary Fiber on Constipation: A Meta Analysis. World J. Gastroenterol. 2012, 18, 7378–7383. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3544045/ (accessed on 28 December 2013). [CrossRef] [PubMed]
- Respini, D.; Jacobsen, P.B.; Thors, C.; Tralongo, P.; Balducci, L. The prevalence and correlates of fatigue in old-er cancer patients. Crit. Rev. Oncol. Hematol. 2003, 47, 273–279. [Google Scholar] [CrossRef]
- Bower, J.E.; Ganz, P.A.; Aziz, N.; Fahey, J.L. Fatigue and Proinflammatory Cytokine Activity in Breast Cancer Survivors. Psychosom. Med. 2002, 64, 604–611. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4600148/ (accessed on 3 August 2015). [CrossRef] [PubMed]
- Gernier, F.; Joly, F.; Klein, D. Cancer-related fatigue among long-term survivors of breast, cervical, and colo-rectal cancer: A French registry-based controlled study. Support Care Cancer 2020. [Google Scholar] [CrossRef]
- Zick, S.M.; Colacino, J.; Cornellier, M.; Khabir, T.; Surnow, K.; Djuric, Z. Fatigue reduction diet in breast cancer survivors: A pilot ran-domized clinical trial. Breast Cancer Res. Treat. 2017, 161, 299–310. [Google Scholar] [CrossRef]
- Guest, D.D.; Evans, E.M.; Rogers, L.Q. Diet components associated with perceived fatigue in breast cancer survivors. Eur. J. Cancer Care 2013, 22, 51–59. [Google Scholar] [CrossRef]
- Baguley, B.J.; Bolam, K.A.; Wright, O.R.L.; Skinner, T.L. The Effect of Nutrition Therapy and Exercise on Cancer-Related Fatigue and Quality of Life in Men with Prostate Cancer: A Systematic Review. Nutrients 2017, 9, 1003. [Google Scholar] [CrossRef]
- Navari, R.M.; Aapro, M. Antiemetic Prophylaxis for Chemotherapy–Induced Nausea and Vomiting. N. Engl. J. Med. 2016, 374, 1356–1367. [Google Scholar]
- European Medicine Agency. European Medicines Agency Recommends Changes to the Use of Metoclopramide. 2013. Available online: https://www.ema.europa.eu/en/news/european-medicines-agency-recommends-changes-use-metoclopramide (accessed on 26 July 2013).
- Lete, I.; Allué, J. The Effectiveness of Ginger in the Prevention of Nausea and Vomiting During Pregnancy and Chemotherapy. Integr. Med. Insights 2016, 31, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Dundee, J.W.; Chestnutt, W.N.; Ghaly, R.G.; Lynas, A.G. Traditional Chinese acupuncture: A Potentially Useful Antiemetic? BMJ 1986, 293, 583–584. Available online: http://www.cjmb.org/uploads/pdf/pdf_CJMB_354.pdf (accessed on 1 January 2020). [CrossRef] [PubMed]
- Amand, P.; Kunnumakkara, E.; Sunderam, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Aggarwal, B.B. Cancer is a preventable disease that requires major life-style changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- Seyfried, T.N.; Flores, R.E.; Poff, A.M.; D’Agostino, D.P. Cancer as a metabolic disease: Implications for Novel Therapeutics. Carcinogenesis 2014, 35, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Pinto, B.M.; Trunzo, J.J. Health Behaviors During and After a Cancer Diagnosis. Cancer 2005, 104, 2614–2623. Available online: https://acsjournals.onlinelibrary.wiley.com/doi/full/10.1002/cncr.21248 (accessed on 24 October 2005). [CrossRef] [PubMed]
- Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Demark-Wahnefried, W.; Galvão, D.A.; Pinto, B.M.; Irwin, M.L.; Wolin, K.Y.; Segal, R.J.; Lucia, A.; et al. American College of Sports Medicine Roundtable on Exercise Guidelines for Cancer Survivors. Med. Sci. Sports Exerc. 2010, 42, 1409–1426. Available online: https://pubmed.ncbi.nlm.nih.gov/20559064/ (accessed on 1 July 2010). [CrossRef]
- European Society for Medical Oncology. Available online: www.esmo.org (accessed on 1 September 2019).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosti, G.; Romano, F.; Secondino, S.; Caccialanza, R.; Lobascio, F.; Carminati, O.; Pedrazzoli, P.; Tralongo, P. The Role of Nutritional Support in Cured/Chronic Patients. Nutrients 2020, 12, 3167. https://doi.org/10.3390/nu12103167
Rosti G, Romano F, Secondino S, Caccialanza R, Lobascio F, Carminati O, Pedrazzoli P, Tralongo P. The Role of Nutritional Support in Cured/Chronic Patients. Nutrients. 2020; 12(10):3167. https://doi.org/10.3390/nu12103167
Chicago/Turabian StyleRosti, Giovanni, Fabrizio Romano, Simona Secondino, Riccardo Caccialanza, Federica Lobascio, Ornella Carminati, Paolo Pedrazzoli, and Paolo Tralongo. 2020. "The Role of Nutritional Support in Cured/Chronic Patients" Nutrients 12, no. 10: 3167. https://doi.org/10.3390/nu12103167
APA StyleRosti, G., Romano, F., Secondino, S., Caccialanza, R., Lobascio, F., Carminati, O., Pedrazzoli, P., & Tralongo, P. (2020). The Role of Nutritional Support in Cured/Chronic Patients. Nutrients, 12(10), 3167. https://doi.org/10.3390/nu12103167






