Association between Altered Blood Parameters and Gut Microbiota after Synbiotic Intake in Healthy, Elderly Korean Women
Abstract
1. Introduction
2. Materials and Methods
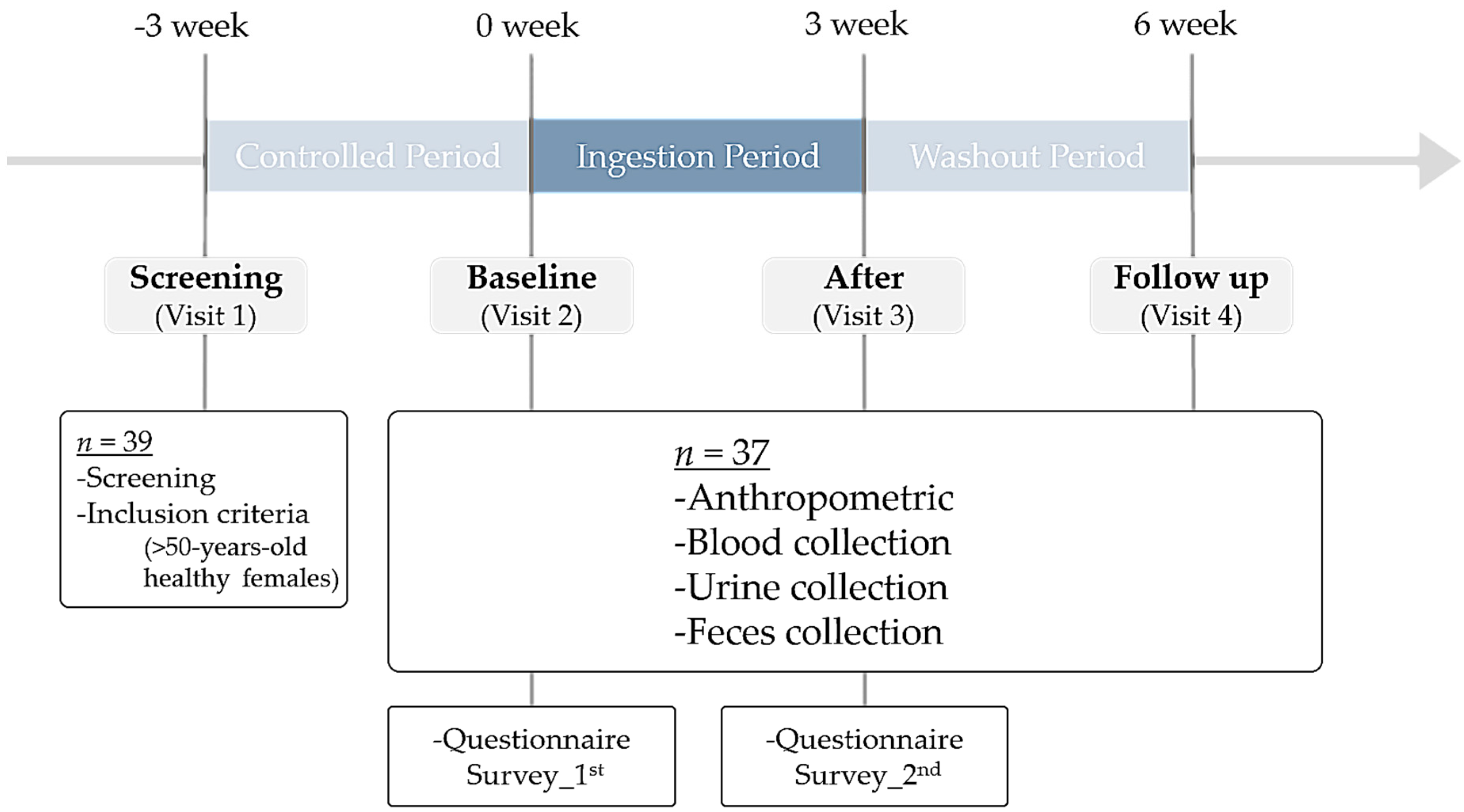
2.1. Participants and Study Design
2.2. Contents of the Synbiotic Drink
2.3. Sample Collection
2.4. Anthropometric Information
2.5. Blood Sample Collection
2.6. Urine Sample Collection
2.7. Fecal Sample Collection
2.8. Microbial Analysis
2.8.1. DNA Extraction
2.8.2. 16S rRNA Amplicon Library Preparation
2.8.3. Analysis of 16S rRNA Amplicon Sequence
2.9. Statistical Analysis
3. Results
3.1. Study Design and Characteristics of Participants
3.2. Blood Parameters and Anthropometric Measurements
3.3. Fecal Assessment
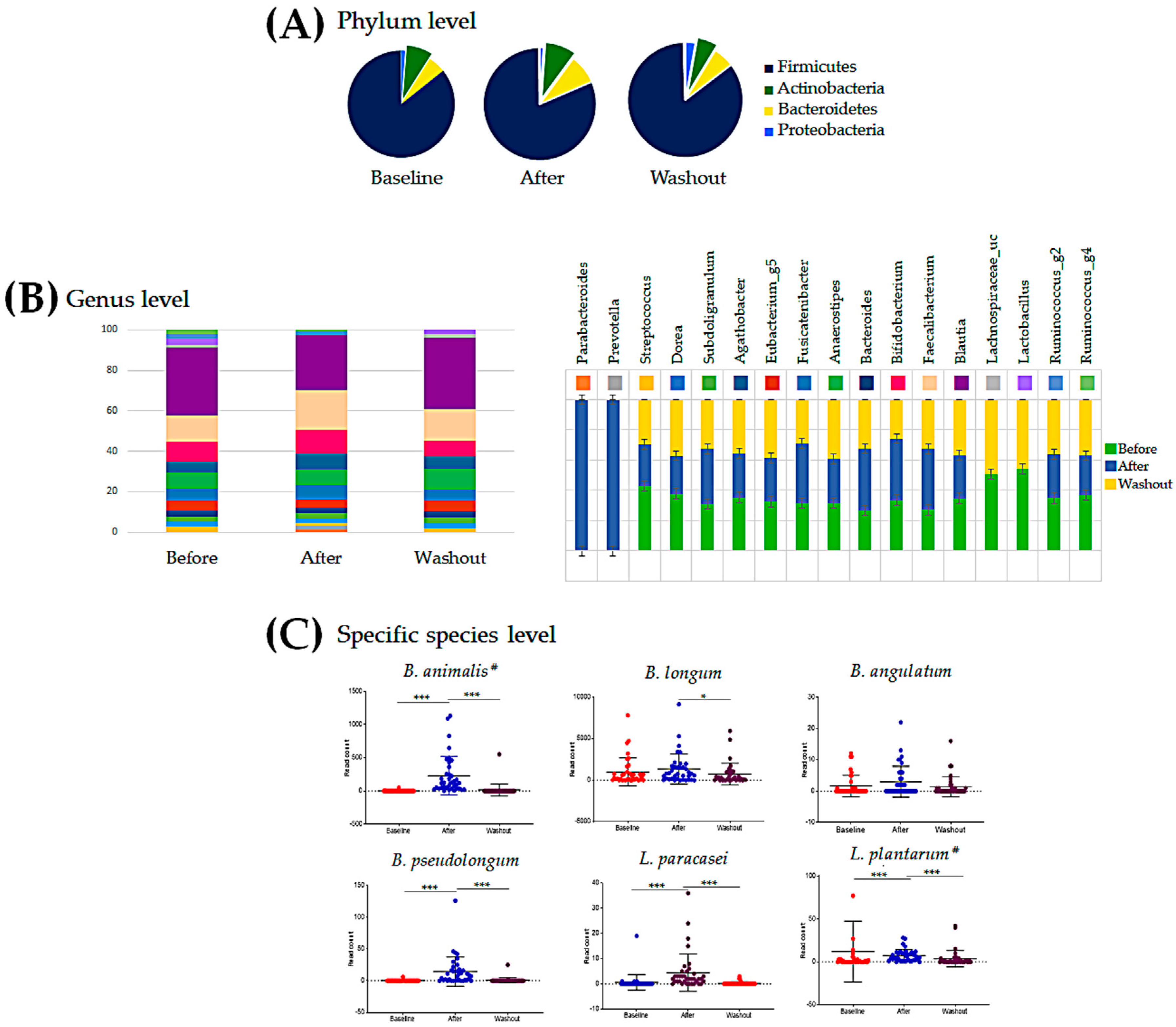
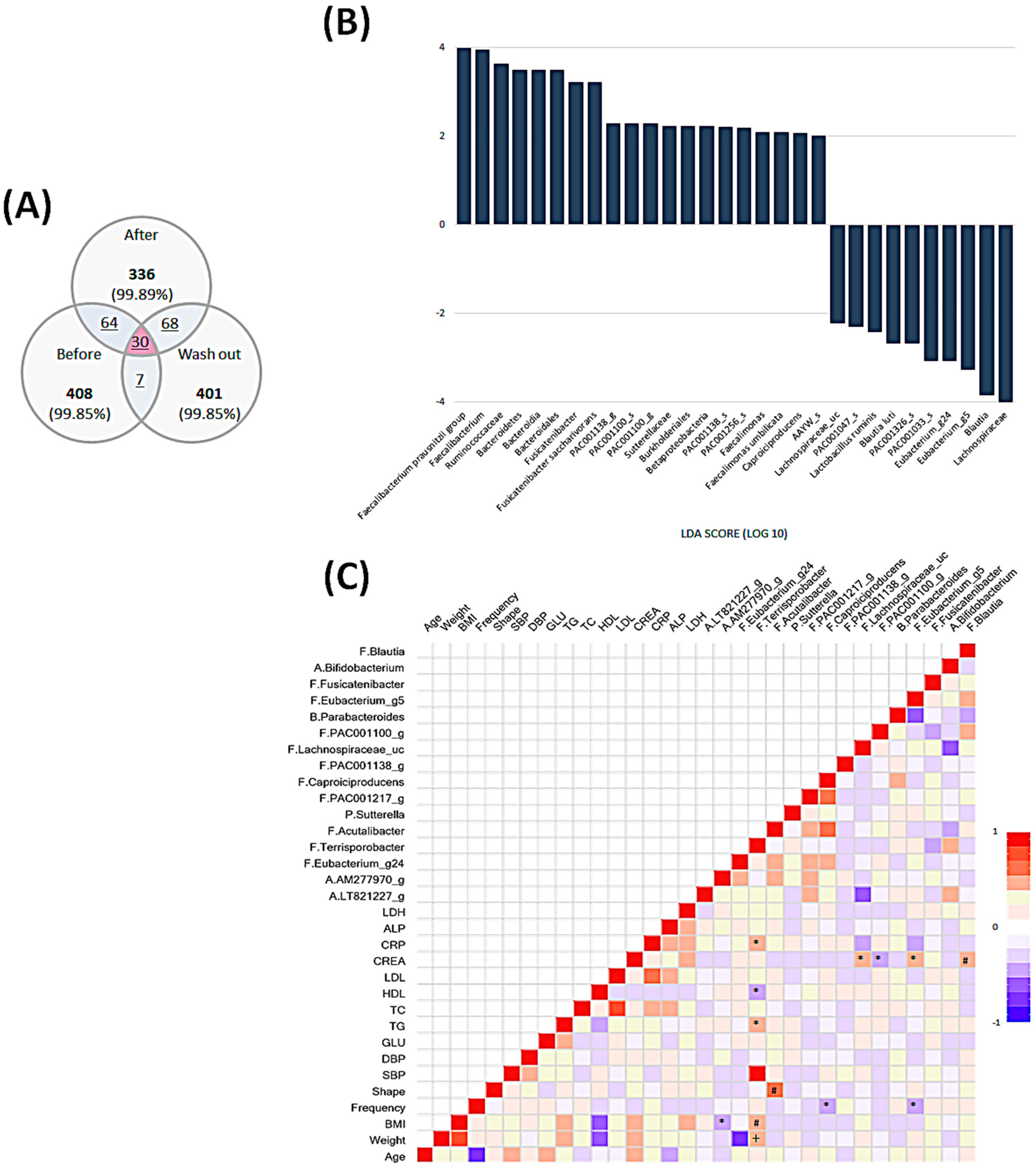
3.4. Intestinal Microbial Community Analysis
3.5. Temporal Changes in the Intestinal Microbiota
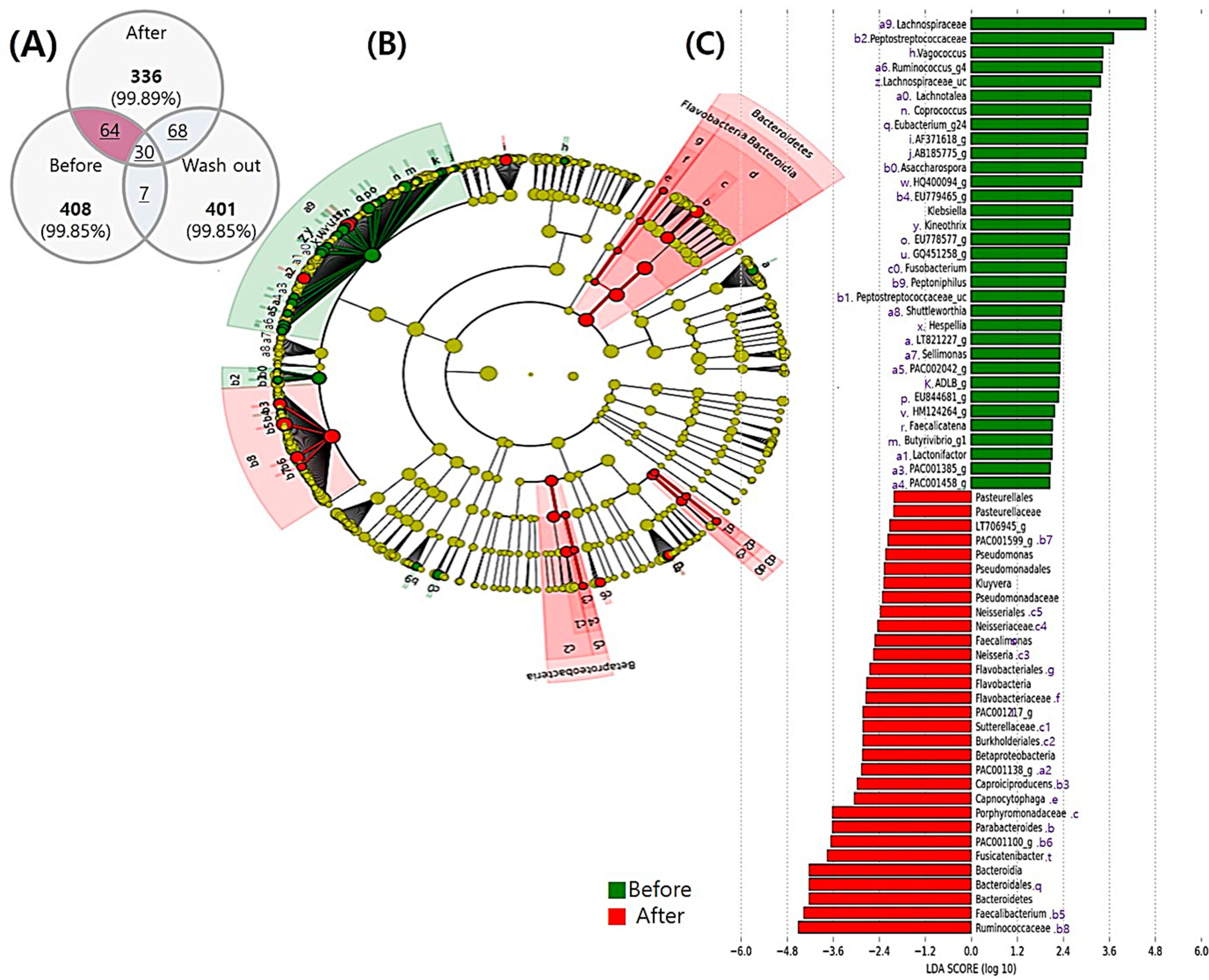
3.5.1. Comparison before and after the Intervention
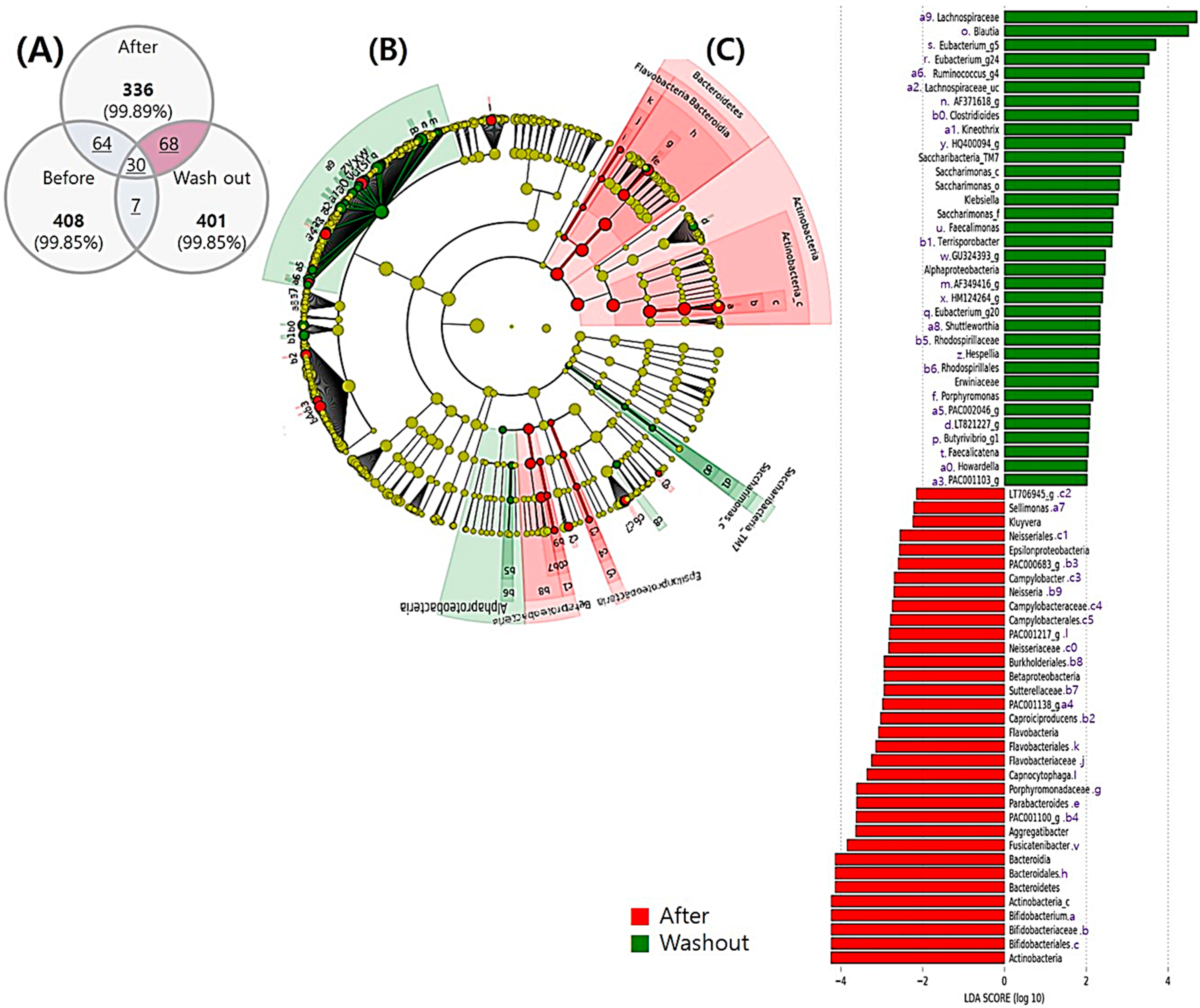
3.5.2. Comparison between the end of Intervention and Washout Period
3.6. Comprehensive Comparison of Each Time Point and Associations among All Parameters and Microbiota Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Korea National Statistical Office. Future Population Estimation. Future Life Expectancy/Nationwide. Korea: Population Trend Division, Statistics Korea. Available online: http://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1BPA201&vw_cd=MT_ZTITLE&list_id=A41_10&seqNo=&lang_mode=ko&language=kor&obj_var_id=&itm_id=&conn_path=MT_ZTITLE (accessed on 28 March 2019).
- Korea National Statistical Office. Life Table, National Approval Statistics No. 101035. Expected Life Expectancy (0-Year-Old Life Expectancy) and Life Expectancy (Excluding Health Period). Korea: Population Trend Division, Statistics Korea. Available online: http://www.index.go.kr/potal/stts/idxMain/selectPoSttsIdxSearch.do?idx_cd=2758&stts_cd=275801&freq=Y (accessed on 5 December 2017).
- Wang, K.; Yu, X.H.; Li, Y.; Guo, Y.; Ge, L.; Pu, F.F.; Ma, X.Y.; Cui, W.J.; Marrota, F.; He, F.; et al. Bifidobacterium bifidum TMC3115 Can Characteristically Influence Glucose and Lipid Profile and Intestinal Microbiota in the Middle-Aged and Elderly. Probiotics Antimicrob. Proteins 2019, 11, 1182–1194. [Google Scholar] [CrossRef] [PubMed]
- Ohkusa, T.; Koido, S.; Nishikawa, Y.; Sato, N. Gut Microbiota and Chronic Constipation: A Review and Update. Front. Med. 2019, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Saito, I. Epidemiological Evidence of Type 2 Diabetes Mellitus, Metabolic Syndrome, and Cardiovascular Disease in Japan. Circ. J. 2012, 76, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, P.; Parida, S.; Nanda, N.C.; Satpathy, R.; Pradhan, L.; Chandel, D.S.; Baccaglini, L.; Mohapatra, A.; Mohapatra, S.S.; Misra, P.R.; et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature 2017, 548, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.D.G.; McNaught, C.E.; Jain, P.K.; MacFie, J. Randomised clinical trial of synbiotic therapy in elective surgical patients. Gut 2004, 53, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Abney, K.N.; Susan, J.H. Probiotic supplementation and reducing inflammation in hemodialysis patients: A systematic review. J. Ren. Nutr. Metab. 2019, 5, 3. [Google Scholar] [CrossRef]
- Dore, M.P.; Bibbo, S.; Fresi, G.; Bassotti, G.; Pes, G.M. Side Effects Associated with Probiotic Use in Adult Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2019, 11, 2913. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.C.; Zhao, F.Y.; Liu, W.J.; Lv, R.R.; Khine, W.W.T.; Han, J.; Sun, Z.H.; Lee, Y.K.; Zhang, H.P. Probiotic-directed modulation of gut microbiota is basal microbiome dependent. Gut Microbes 2020, 12, 1736974. [Google Scholar] [CrossRef]
- Kristensen, N.B.; Bryrup, T.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Pedersen, O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: A systematic review of randomized controlled trials. Genome Med. 2016, 8, 52. [Google Scholar] [CrossRef]
- De Roos, N.M.; Schouten, G.; Katan, M.B. Yoghurt enriched with Lactobacillus acidophilus does not lower blood lipids in healthy men and women with normal to borderline high serum cholesterol levels. Eur. J. Clin. Nutr. 1999, 53, 277–280. [Google Scholar] [CrossRef][Green Version]
- Sergeev, I.N.; Aljutaily, T.; Walton, G.; Huarte, E. Effects of Synbiotic Supplement on Human Gut Microbiota, Body Composition and Weight Loss in Obesity. Nutrients 2020, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, C.; Taverniti, V.; Milani, C.; Fiore, W.; Laureati, M.; De Noni, I.; Stuknyte, M.; Chouaia, B.; Riso, P.; Guglielmetti, S. Modulation of Fecal Clostridiales Bacteria and Butyrate by Probiotic Intervention with Lactobacillus paracasei DG Varies among Healthy Adults. J. Nutr. 2014, 144, 1787–1796. [Google Scholar] [CrossRef] [PubMed]
- Neto, J.V.; de Melo, C.M.; Ribeiro, S.M.L. Effects of Three-Month Intake of Synbiotic on Inflammation and Body Composition in the Elderly: A Pilot Study. Nutrients 2013, 5, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Turroni, F.; Ventura, M.; Butto, L.F.; Duranti, S.; O’Toole, P.W.; Motherway, M.O.; van Sinderen, D. Molecular dialogue between the human gut microbiota and the host: Lactobacillus and Bifidobacterium perspective. Cell. Mol. Life Sci. 2014, 71, 183–203. [Google Scholar] [CrossRef]
- Collins, M.D.; Gibson, G.R. Probiotics, prebiotics, and synbiotics: Approaches for modulating the microbial ecology of the gut. Am. J. Clin. Nutr. 1999, 69, 1052S–1057S. [Google Scholar] [CrossRef]
- Neish, A.S. Microbes in gastrointestinal health and disease. Gastroenterology 2009, 136, 65–80. [Google Scholar] [CrossRef]
- Crittenden, R.; Laitila, A.; Forssell, P.; Matto, J.; Saarela, M.; Mattila-Sandholm, T.; Myllarinen, P. Adhesion of Bifidobacteria to granular starch and its implications in probiotic technologies. Appl. Environ. Microbiol. 2001, 67, 3469–3475. [Google Scholar] [CrossRef]
- Markowiak, P.; Slizewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Worthley, D.L.; Le Leu, R.K.; Whitehall, V.L.; Conlon, M.; Christophersen, C.; Belobrajdic, D.; Mallitt, K.A.; Hu, Y.; Irahara, N.; Ogino, S.; et al. A human, double-blind, placebo-controlled, crossover trial of prebiotic, probiotic, and synbiotic supplementation: Effects on luminal, inflammatory, epigenetic, and epithelial biomarkers of colorectal cancer. Am. J. Clin. Nutr. 2009, 90, 578–586. [Google Scholar] [CrossRef]
- Bartosch, S.; Woodmansey, E.J.; Paterson, J.C.; McMurdo, M.E.; Macfarlane, G.T. Microbiological effects of consuming a synbiotic containing Bifidobacterium bifidum, Bifidobacterium lactis, and oligofructose in elderly persons, determined by real-time polymerase chain reaction and counting of viable bacteria. Clin. Infect. Dis. 2005, 40, 28–37. [Google Scholar] [CrossRef]
- Macfarlane, S.; Cleary, S.; Bahrami, B.; Reynolds, N.; Macfarlane, G.T. Synbiotic consumption changes the metabolism and composition of the gut microbiota in older people and modifies inflammatory processes: A randomised, double-blind, placebo-controlled crossover study. Aliment. Pharmacol. Ther. 2013, 38, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.W.; Park, S.U.; Jang, Y.S.; Kim, Y.H.; Rhee, P.L.; Ko, S.H.; Joo, N.; Kim, S.I.; Kim, C.H.; Chang, D.K. Effect of composite yogurt enriched with acacia fiber and Bifidobacterium lactis. World J. Gastroenterol. 2012, 18, 4563–4569. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.C.; Kim, B.J.; Rhee, P.L.; Chang, D.K.; Son, H.J.; Kim, J.J.; Rhee, J.C.; Kim, S.I.; Han, Y.S.; Sim, K.H.; et al. Probiotic Fermented Milk Containing Dietary Fiber Has Additive Effects in IBS with Constipation Compared to Plain Probiotic Fermented Milk. Gut Liver 2011, 5, 22–28. [Google Scholar] [CrossRef][Green Version]
- Shinde, T.; Perera, A.P.; Vemuri, R.; Gondalia, S.V.; Karpe, A.V.; Beale, D.J.; Shastri, S.; Southam, B.; Eri, R.; Stanley, R. Synbiotic Supplementation Containing Whole Plant Sugar Cane Fibre and Probiotic Spores Potentiates Protective Synergistic Effects in Mouse Model of IBD. Nutrients 2019, 11, 818. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, M.; Ouwehand, A.C.; Forssten, S.D.; Nikkila, J.; Tiihonen, K.; Rautonen, N.; Lahtinen, S.J. Gut microbiota of healthy elderly NSAID users is selectively modified with the administration of Lactobacillus acidophilus NCFM and lactitol. Age 2012, 34, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Bedani, R.; Saad, S.M.I.; Sivieri, K. Potential benefits of probiotics, prebiotics, and synbiotics on the intestinal microbiota of the elderly. In Probiotics, Prebiotics, Synbiotics; Elsevier: Amsterdam, The Netherlands, 2016; pp. 525–538. [Google Scholar] [CrossRef]
- Jy, K.; Ey, C. Changes in Korean Adult Females’ Intestinal Microbiota Resulting from Kimchi Intake. J. Nutr. Food Sci. 2016, 6, 486. [Google Scholar] [CrossRef]
- Blake, M.R.; Raker, J.M.; Whelan, K. Validity, and reliability of the Bristol Stool Form Scale in healthy adults and patients with diarrhea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2016, 44, 693–703. [Google Scholar] [CrossRef]
- Shima, T.; Amamoto, R.; Kaga, C.; Kado, Y.; Sasai, T.; Watanabe, O.; Shiinoki, J.; Iwazaki, K.; Shigemura, H.; Tsuji, H. Association of life habits and fermented milk intake with stool frequency, defecatory symptoms and intestinal microbiota in healthy Japanese adults. Benef. Microbes 2019, 10, 841–854. [Google Scholar] [CrossRef]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics—A review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Vuljanic, D.; Dojder, A.; Spoljaric, V.; Saracevic, A.; Dukic, L.; Krleza, J.L.; Tanaskovic, J.V.; Maradin, I.; Grzunov, A.; Vogrinc, Z.; et al. Analytical verification of 12 most commonly used urine dipsticks in Croatia: Comparability, repeatability, and accuracy. Biochem. Med. 2019, 29, 123–132. [Google Scholar] [CrossRef]
- Lim, M.Y.; Song, E.J.; Kim, S.H.; Lee, J.; Nam, Y.D. Comparison of DNA extraction methods for human gut microbial community profiling. Syst. Appl. Microbiol. 2018, 41, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Dassi, E.; Ballarini, A.; Covello, G.; Quattrone, A.; Jousson, O.; De Sanctis, V.; Bertorelli, R.; Denti, M.A.; Segata, N. Enhanced microbial diversity in the saliva microbiome induced by short-term probiotic intake revealed by 16S rRNA sequencing on the IonTorrent PGM platform. J. Biotechnol. 2014, 190, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Ogata, K.; Takeshita, T.; Shibata, Y.; Matsumi, R.; Kageyama, S.; Asakawa, M.; Yamashita, Y. Effect of coffee on the compositional shift of oral indigenous microbiota cultured in vitro. J. Oral Sci. 2019, 61, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Adamiak, J.; Otlewska, A.; Tafer, H.; Lopandic, K.; Gutarowska, B.; Sterflinger, K.; Pinar, G. First evaluation of the microbiome of built cultural heritage by using the Ion Torrent next generation sequencing platform. Int. Biodeterior. Biodegrad. 2018, 131, 11–18. [Google Scholar] [CrossRef]
- Malapelle, U.; Vigliar, E.; Sgariglia, R.; Bellevicine, C.; Colarossi, L.; Vitale, D.; Pallante, P.; Troncone, G. Ion Torrent next-generation sequencing for routine identification of clinically relevant mutations in colorectal cancer patients. J. Clin. Pathol. 2015, 68, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Dave, V.; Street, K.; Francis, S.; Bradman, A.; Riley, L.; Eskenazi, B.; Holland, N. Bacterial microbiome of breast milk and child saliva from low-income Mexican-American women and children. Pediatric Res. 2016, 79, 846–854. [Google Scholar] [CrossRef]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Cech, M.; Chilton, J.; Clements, D.; Coraor, N.; Gruning, B.A.; et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef]
- Robles-Vera, I.; Callejo, M.; Ramos, R.; Duarte, J.; Perez-Vizcaino, F. Impact of Vitamin D Deficit on the Rat Gut Microbiome. Nutrients 2019, 11, 2564. [Google Scholar] [CrossRef]
- Minamida, K.; Nishimura, M.; Miwa, K.; Nishihira, J. Effects of dietary fiber with Bacillus coagulans lilac-01 on bowel movement and fecal properties of healthy volunteers with a tendency for constipation. Biosci. Biotechnol. Biochem. 2015, 79, 300–306. [Google Scholar] [CrossRef]
- Cancello, R.; Turroni, S. Effect of short-term dietary intervention and probiotic mix supplementation on the gut microbiota of elderly obese women. Nutrients 2019, 11, 3011. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Flint, H.J. Probiotics and prebiotics and health in ageing populations. Maturitas 2013, 75, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M.; Gaya, P.; Rodriguez, E.; Langa, S.; Peiroten, A.; Medina, M.; Arques, J.L. Probiotic Bacteria for Healthier Aging: Immunomodulation and Metabolism of Phytoestrogens. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.; Tang, J.; Gillings, R.; Perfecto, A.; Dutton, J.; Speakman, J.; Fraser, W.D.; Nicoletti, C.; Berendsen, A.A.M.; de Groot, L.; et al. Changing from a Western to a Mediterranean-style diet does not affect iron or selenium status: Results of the New Dietary Strategies Addressing the Specific Needs of the Elderly Population for Healthy Aging in Europe (NU-AGE) 1-year randomized clinical trial in elderly Europeans. Am. J. Clin. Nutr. 2020, 111, 98–109. [Google Scholar]
- Salazar, N.; Valdes-Varela, L.; Gonzalez, S.; Gueimonde, M.; de Los Reyes-Gavilan, C.G. Nutrition and the gut microbiome in the elderly. Gut Microbes 2017, 8, 82–97. [Google Scholar] [CrossRef]
- Gill, H.S.; Rutherfurd, K.J.; Cross, M.L.; Gopal, P.K. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. Am. J. Clin. Nutr. 2001, 74, 833–839. [Google Scholar] [CrossRef]
- Salazar, N.; Arboleya, S.; Valdes, L.; Stanton, C.; Ross, P.; Ruiz, L.; Gueimonde, M.; de los Reyes-Gavilan, C.G. The human intestinal microbiome at extreme ages of life. Dietary intervention as a way to counteract alterations. Front. Genet. 2014, 5, 406. [Google Scholar] [CrossRef]
- Kim, S.; Jazwinski, S.M. The Gut Microbiota and Healthy Aging: A Mini-Review. Gerontology 2018, 64, 513–520. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Claesson, M.J. Gut microbiota: Changes throughout the lifespan from infancy to elderly. Int. Dairy J. 2010, 20, 281–291. [Google Scholar] [CrossRef]
- Silvi, S.; Verdenelli, M.C.; Orpianesi, C.; Cresci, A. EU project Crownalife: Functional foods, gut microflora and healthy ageing—Isolation and identification of Lactobacillus and Bifidobacterium strains from fecal samples of elderly subjects for a possible probiotic use in functional foods. J. Food Eng. 2003, 56, 195–200. [Google Scholar] [CrossRef]
- Bouhnik, Y.; Achour, L.; Paineau, D.; Riottot, M.; Attar, A.; Bornet, F. Four-week short chain fructo-oligosaccharides ingestion leads to increasing fecal Bifidobacteria and cholesterol excretion in healthy elderly volunteers. Nutr. J. 2007, 6, 42. [Google Scholar] [CrossRef]
- Lahtinen, S.J.; Tammela, L.; Korpela, J.; Parhiala, R.; Ahokoski, H.; Mykkanen, H.; Salminen, S.J. Probiotics modulate the Bifidobacterium microbiota of elderly nursing home residents. Age 2009, 31, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Beltran, D.; Luna, M.C.; Romo-Vaquero, M.; Garcia-Villalba, R.; Mira, A.; Espin, J.C.; Tomas-Barberan, F.A. Isolation of Human Intestinal Bacteria Capable of Producing the Bioactive Metabolite Isourolithin A from Ellagic Acid. Front. Microbiol. 2017, 8, 1521. [Google Scholar] [CrossRef] [PubMed]
- Romo-Vaquero, M.R.-V.; Cortés-Martín, A.; Loria-Kohen, V.; De Molina, A.R.; García-Mantrana, I.; Collado, M.C.; Espín, J.C.; Selma, M.V. Deciphering the Human Gut Microbiome of Urolithin Metabotypes: Association with Enterotypes and Potential Cardiometabolic Health Implications. Mol. Nutr. Food Res. 2018, 63. [Google Scholar] [CrossRef]
- Cortés-Martín, A.; Romo-Vaquero, M.; García-Mantrana, I.; Rodríguez-Varela, A.; Collado, M.C.; Espín, J.C.; Selma, M.V. Urolithin Metabotypes can Anticipate the Different Restoration of the Gut Microbiota and Anthropometric Profiles during the First Year Postpartum. Nutrients 2019, 11, 2079. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Barberan, F.A.; Garcia-Villalba, R.; Gonzalez-Sarrias, A.; Selma, M.V.; Espin, J.C. Ellagic Acid Metabolism by Human Gut Microbiota: Consistent Observation of Three Urolithin Phenotypes in Intervention Trials, Independent of Food Source, Age, and Health Status. J. Agric. Food Chem. 2014, 62, 6535–6538. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.Y.; Wang, J.; Yu, H.; Wang, J.; Zhu, W.Y. Changes in Ileal Microbial Composition and Microbial Metabolism by an Early-Life Galacto-Oligosaccharides Intervention in a Neonatal Porcine Model. Nutrients 2019, 11, 1753. [Google Scholar] [CrossRef]
- Kameyama, K.; Itoii, K. Intestinal Colonization by a Lachnospiraceae Bacterium Contributes to the Development of Diabetes in Obese Mice. Microbes Environ. 2014, 29, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Z.; Thomsen, M.; Vitetta, L. Interaction of gut microbiota with dysregulation of bile acids in the pathogenesis of nonalcoholic fatty liver disease and potential therapeutic implications of probiotics. J. Cell. Biochem. 2019, 120, 2713–2720. [Google Scholar] [CrossRef]
- Guirong, Y.E.; Minjie, Z.; Lixin, Y.U.; Junsheng, Y.E.; Lin, Y.; Lisha, S. Gut microbiota in renal transplant recipients, patients with chronic kidney disease and healthy subjects. J. South. Med Univ. 2018, 38, 1401–1408. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Wang, F.Q.; Zhang, P.; Jiang, H.L.; Cheng, S.L. Gut Bacterial Translocation Contributes to Microinflammation in Experimental Uremia. Dig. Dis. Sci. 2012, 57, 2856–2862. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Montemurno, E.; Piccolo, M.; Vannini, L.; Lauriero, G.; Maranzano, V.; Gozzi, G.; Serrazanetti, D.; Dalfino, G.; Gobbetti, M.; et al. Microbiota and Metabolome Associated with Immunoglobulin A Nephropathy (IgAN). PLoS ONE 2014, 9, e99006. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.M.; Nguyen, T.H.; Andersen, G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, K.; Poussa, T.; Isolauri, E.; Nutrition, A.M.I.; Intestinal Microbiota, G. Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: A randomised controlled trial. Br. J. Nutr. 2009, 101, 1679–1687. [Google Scholar] [CrossRef]
- Ranganathan, N.; Friedman, E.A.; Tam, P.; Rao, V.; Ranganathan, P.; Dheer, R. Probiotic dietary supplementation in patients with stage 3 and 4 chronic kidney disease: A 6-month pilot scale trial in Canada. Curr. Med Res. Opin. 2009, 25, 1919–1930. [Google Scholar] [CrossRef]
- Firouzi, S.; Mohd-Yusof, B.N.; Majid, H.A.; Ismail, A.; Kamaruddin, N.A. Effect of microbial cell preparation on renal profile and liver function among type 2 diabetics: A randomized controlled trial. BMC Complem. Altern. Med. 2015, 15, 1–10. [Google Scholar] [CrossRef]
- Cirit, M.; Toprak, O.; Yesil, M.; Bayata, S.; Postaci, N.; Pupim, L.; Esi, E. Angiotensin-converting enzyme inhibitors as a risk factor for contrast-induced nephropathy. Nephron Clin. Pract. 2006, 104, C20–C27. [Google Scholar] [CrossRef]
- Kalliomaki, M.; Salminen, S.; Poussa, T.; Isolauri, E. Probiotics during the first 7 years of life: A cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2007, 119, 1019–1021. [Google Scholar] [CrossRef]
- Sugiura, S.H.; Hardy, R.W.; Roberts, R.J. The pathology of phosphorus deficiency in fish—A review. J. Fish Dis. 2004, 27, 255–265. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, H.J.; Lee, H.S.; Jang, Y.A.; Kim, C.I. Analytical dietary fiber database for the National Health and Nutrition Survey in Korea. J. Food Compos. Anal. 2008, 21, S35–S42. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, M.S.; Lee, M.S.; Park, Y.S.; Lee, H.J.; Kang, S.-A.; Lee, H.S.; Lee, K.-E.; Yang, H.J.; Kim, M.J.; et al. Korean diet: Characteristics and historical background. J. Ethn. Foods 2016, 3, 26–31. [Google Scholar] [CrossRef]
- Shin, J.H.; Jung, S.; Kim, S.A.; Kang, M.S.; Kim, M.S.; Joung, H.; Hwang, G.S.; Shin, D.M. Differential Effects of Typical Korean Versus American-Style Diets on Gut Microbial Composition and Metabolic Profile in Healthy Overweight Koreans: A Randomized Crossover Trial. Nutrients 2019, 11, 2450. [Google Scholar] [CrossRef] [PubMed]
| Variables | N | Mean (SD) or Frequency (%) | ||
|---|---|---|---|---|
| Age (Years) | ||||
| 50–59 | 11 | 57.9 (1.2) | ||
| 60–69 | 15 | 63.3 (3.2) | ||
| 70–79 | 11 | 73.5 (3.0) | ||
| Total | 37 | 65 (6.7) | ||
| Menopausal transition | Menstruating | 0 | 0 | |
| Post-Menopausal | 37 | 100% | ||
| Smoking | No (Non-smoking) | 37 | 100% | |
| Yes (Smoking) | 0 | 0 | ||
| Ex-smoker | 0 | 0 | ||
| Alcohol | No | 29 | 78% | |
| Yes | 8 | 22% | ||
| Alcohol frequency of consumption | Alcohol (Yes) | Daily | 5 | |
| Weekly | 1 | |||
| Occasionally | 2 | |||
| Variables | Baseline | After | Washout | p-Value | ||
|---|---|---|---|---|---|---|
| Baseline vs. After | After vs. Washout | |||||
| Anthropometric measures | ||||||
| Weight (kg) | 55.27 (7.33) | 55.56 (7.29) | 55.80 (7.44) | 0.221 | 0.08 | |
| BMI (kg/m2) | 23.02 (2.92) | 23.16 (3.00) | 23.22 (3.00) | 0.253 | 0.744 | |
| Blood parameters | ||||||
| Systolic blood pressure (mmHg) | 115.92 (13.84) | 116.30 (11.51) | 117.73 (15.50) | 0.800 | 0.508 | |
| Diastolic blood pressure (mmHg) | 73.54 (7.77) | 74.22 (6.61) | 74.76 (7.42) | 0.606 | 0.611 | |
| FBG (mg/dL) | 94.21 (16.40) | 88.95 (15.26) | 88.89 (14.21) | 0.005 | 0.974 | |
| Triglyceride (mg/dL) | 155.22 (126.26) | 150.95 (69.63) | 159.11 (83.25) | 0.474 | 0.391 | |
| Cholesterol (mg/dL) | 206.73 (42.54) | 205.38 (45.20) | 200.95 (41.40) | 0.733 | 0.370 | |
| HDL cholesterol (mg/dL) | 54.30 (10.42) | 54.43 (12.08) | 53.68 (11.78) | 0.897 | 0.916 | |
| LDL cholesterol (mg/dL) | 121.05 (35.74) | 120.76 (43.38) | 115.54 (35.65) | 0.930 | 0.294 | |
| Creatinine (mg/dL) | 0.92 (0.28) | 0.78 (0.42) | 0. 56 (0.10) | 0.025 | 0.768 | |
| CRP (mg/dL) | 0.15 (0.23) | 0.12 (0.13) | 0.15 (0.17) | 0.317 | 0.228 | |
| ALP (U/L) | 75.32 (17.48) | 73.24 (16.25) | 73.68 (15.41) | 0.047 | 0.181 | |
| LDH (U/L) | 182.43 (27.13) | 181.81 (27.50) | 167.62 (28.12) | 0.239 | 0.000 | |
| Fecal characteristics 2 | Shape | 4.3 (0.9) | 4.0 (0.8) | 4.4 (1.1) | 0.110 | 0.083 |
| Frequency | 6.16 (1.25) | 6.35 (1.22) | 0.346 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.H.; You, H.S.; Kang, H.-G.; Kang, S.S.; Hyun, S.H. Association between Altered Blood Parameters and Gut Microbiota after Synbiotic Intake in Healthy, Elderly Korean Women. Nutrients 2020, 12, 3112. https://doi.org/10.3390/nu12103112
Lee SH, You HS, Kang H-G, Kang SS, Hyun SH. Association between Altered Blood Parameters and Gut Microbiota after Synbiotic Intake in Healthy, Elderly Korean Women. Nutrients. 2020; 12(10):3112. https://doi.org/10.3390/nu12103112
Chicago/Turabian StyleLee, Song Hee, Hee Sang You, Hee-Gyoo Kang, Sang Sun Kang, and Sung Hee Hyun. 2020. "Association between Altered Blood Parameters and Gut Microbiota after Synbiotic Intake in Healthy, Elderly Korean Women" Nutrients 12, no. 10: 3112. https://doi.org/10.3390/nu12103112
APA StyleLee, S. H., You, H. S., Kang, H.-G., Kang, S. S., & Hyun, S. H. (2020). Association between Altered Blood Parameters and Gut Microbiota after Synbiotic Intake in Healthy, Elderly Korean Women. Nutrients, 12(10), 3112. https://doi.org/10.3390/nu12103112







