Abstract
Background: Rare plants that contain corrinoid compounds mostly comprise cobalamin analogues, which may compete with cobalamin (vitamin B12 (B12)) metabolism. We examined the presence of B12 in a cultivated strain of an aquatic plant: Wolffia globosa (Mankai), and predicted functional pathways using gut-bioreactor, and the effects of long-term Mankai consumption as a partial meat substitute, on serum B12 concentrations. Methods: We used microbiological assay, liquid-chromatography/electrospray-ionization-tandem-mass-spectrometry (LC-MS/MS), and anoxic bioreactors for the B12 experiments. We explored the effect of a green Mediterranean/low-meat diet, containing 100 g of frozen Mankai shake/day, on serum B12 levels during the 18-month DIRECT-PLUS (ID:NCT03020186) weight-loss trial, compared with control and Mediterranean diet groups. Results: The B12 content of Mankai was consistent at different seasons (p = 0.76). Several cobalamin congeners (Hydroxocobalamin(OH-B12); 5-deoxyadenosylcobalamin(Ado-B12); methylcobalamin(Me-B12); cyanocobalamin(CN-B12)) were identified in Mankai extracts, whereas no pseudo B12 was detected. A higher abundance of 16S-rRNA gene amplicon sequences associated with a genome containing a KEGG ortholog involved in microbial B12 metabolism were observed, compared with control bioreactors that lacked Mankai. Following the DIRECT-PLUS intervention (n = 294 participants; retention-rate = 89%; baseline B12 = 420.5 ± 187.8 pg/mL), serum B12 increased by 5.2% in control, 9.9% in Mediterranean, and 15.4% in Mankai-containing green Mediterranean/low-meat diets (p = 0.025 between extreme groups). Conclusions: Mankai plant contains bioactive B12 compounds and could serve as a B12 plant-based food source.
1. Introduction
Cobalamin is an essential nutrient for humans. It has the largest molecular mass (1355.4 g/mol) and the most complex structure of all vitamins [1]. The term “vitamin B12” is the name usually used for cyanocobalamin (CN-B12), which is the most chemically stable form of cobalamin. In this study, vitamin B12 will be used to refer to all corrinoids exhibiting the qualitative biological activity of CN-B12 [2], including the following three natural forms: Hydroxocobalamin (OH-B12), 5-deoxyadenosylcobalamin (Ado-B12), and methylcobalamin (Me-B12). CN-B12 is the form used in most dietary supplements and is readily converted to the coenzyme forms, Me-B12 and Ado-B12 in the body [1]. Me-B12 functions as a cofactor for the methionine synthase reaction involved in the conversion of homocysteine to methionine through a transfer of a methyl group from methyltetrahydrofolate; Ado-B12 functions as a cofactor for methylmalonyl-CoA mutase in which methylmalonyl-CoA, a product of amino acid and odd-chain fatty acid catabolism, is converted to succinyl-CoA [1]. At the cellular level, these enzymes play an important role in several crucial functions, such as DNA synthesis, methylation, and mitochondrial metabolism [3,4].
De novo synthesis of vitamin B12 appears to be restricted to some bacteria and archaea [2,5]. The vitamin is therefore found solely in foods fermented by B12-producing bacteria, or in those derived from the tissues of animals that have ingested B12-containing foods or which have obtained it from B12-producing microbiota of their commensal microflora [2]. Hence, animal-derived foods (meat, milk, eggs, and shellfish) are considered to be the exclusive dietary source of B12 vitamin in humans [5,6]. However, a preference for diets that limit intake of animal products has arisen during the past decade, largely from the belief that lower animal-source protein diets reduce risk factors for cardiometabolic diseases, such as hypertension, dyslipidemia, hyperglycemia, type 2 diabetes, and cardiovascular diseases [7,8,9,10]. On the other hand, since vitamin B12 is not measurably present in plant-based foods, individuals adhering to a vegan diet without vitamin B12 supplementation are at risk of developing vitamin B12 deficiency with potentially serious and sometimes irreversible consequences [3,11]. Indeed, various types of edible algae have been reported to contain vitamin B12 [4,12]. However, recent data indicate that pseudo B12 forms, such as OH-pseudoB12, Ado-pseudoB12, Me-pseudoB12, and CN-pseudoB12, which are considered inactive in humans, and might compete with B12, are the predominant corrinoids present in the algae [4,12].
Wolffia globosa ‘Mankai’ is an aquatic plant of the duckweed family recently identified for its nutritional value [13,14]. It has a unique nutritional composition profile, which includes about 45% protein of its dry weight, with all nine essential amino acids in a ratio equivalent to that of egg protein [15], a source of omega-3 fatty acids [16]; dietary fiber; polyphenols; iron; and several other micronutrients that tend to have low abundance in animal-based foods diets (e.g., vitamin A as beta-carotene, riboflavin, vitamin B6, and folate). One cup of Mankai shake, which is equivalent to ~20 g of dry matter, provides the following proportions of recommended intakes: 18% whole bioavailable protein [15], 75% bioavailable iron [17], 60% folic acid, and 21% vitamin B12. In our previous bioavailability study, we found, unexpectedly, that the serum vitamin B12 concentrations increase and attain higher levels than the increase observed following other protein source meals [15].
To exclude the sporadic presence of B12 and to evaluate the stability levels in Mankai biomass, various Mankai samples, grown under different conditions, ranging from lab scale under artificial light to commercial scale under sunlight, were examined for their B12 content by two different methods. In the DIRECT PLUS weight-loss trial, among 294 participants with abdominal obesity and normal B12 levels, we explored the effect of an 18-month intake of Mankai, consumed as an evening green shake, as a partial protein plant-based substitute, on vitamin B12 serum levels. Besides, we examined changes in the gut microbiome when directly exposed to Mankai using anoxic bioreactors, to simulate the human colon environment/microbiota. We hypothesized that Mankai might serve as a consistent vitamin B12 source, despite the reduction in red meat intake.
2. Materials and Methods
2.1. Mankai Laboratory Analyses
2.1.1. Plant Sources
Vitamin B12 Detection in Plants Cultivated Under Greenhouse Conditions
Cultivated plant samples: Mankai biomass is grown in closed controlled highly monitored aquatic greenhouses using a proprietary precision agriculture cultivation system. We sampled the plant for B12 analysis at different seasons during the years 2014 to 2019. Plant biomass was sieve harvested, washed with tap water for 2 min, and dried in a food dehydrator (Excalibur, Sacramento, USA) at 65 °C for 16 h. Each dried plant sample was stored in a vacuum-sealed aluminum bag at 4 °C, until analysis was performed.
Vitamin B12 Detection in Axenic Culture
Generating axenic culture: Plant sterilization was achieved by submerging and agitating plants in predetermined concentrations of sodium hypochlorite for 1–3 min. Treated fronds were transferred to a 12-well plate containing sterile Hoagland solution (MgSO4·7H2O 0.246 g/L, Ca(NO3)2·4H2O 542 mg/L, KH2PO4 68 mg/L, KNO3 250 mg/L, FeNa·EDTA 37 mg/L, H3BO3 1.5 mg/L, MnCl2·4H2O 9.1 mg/L, ZnSO4·7H2O 0.11 mg/L, Na2MoO4·2H2O 0.045 mg/L, CuSO4·5H2O 0.045 mg/L, and 1% Sucrose (All purchased from Fisher Scientific, Leicestershire, UK). The Hoagland formulation does not contain cobalt compounds. Furthermore, ICP-MS analysis performed by an accredited laboratory was applied to this 10× concentrated Hoagland solution and revealed no cobalt traces (<0.01 ppm). The plate was covered with aluminum foil and kept at 25 °C for 24 h. After the foil was removed, the plants were allowed to recover for an additional 6 days under a 24-h light regime at 120 µE. Bleached mother fronds with green daughter fronds were transferred to a new sterile well to establish a sterile Mankai culture. Three sterile cultures, derived from three independent treatments, were continuously grown in vitamin B12-free Hoagland medium that was replaced once a week. Culture sterility was verified by incubation of whole and crushed fronds on PCA (plate count agar, Neogen, Michigan, USA) at 30 °C for at least 5 days. Vitamin B12 analysis was performed on 5-month-old independent plant cultures that were intensively washed with running tap water for two minutes and dried in a food dehydrator as described above.
2.1.2. Vitamin B12 Analyses
Bioassay Method
Total vitamin B12 in the plant samples was measured by the AOAC 952.20 microbiological analytical method, utilizing the B12-requiring bacterium Lactobacillus delbrueckii subsp. lactis ATCC7830, which is the established vitamin B12 determination method for foods [18]. The analysis was performed by Eurofins Laboratories, Inc. (Des Moines, IA, USA) and by Bactochem Ltd. (Nes Ziona, Israel). Some tests were done by Hinoman Ltd., analyzing one gram of dried plant by the Vitafast B12 microbiological assay kit (R-Biopharm, AG, Darmstadt, Germany) according to the manufacturer’s instructions.
Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry (ESI LC-MS/MS) Assay
Extraction of Vitamin B12: The extraction of dried Mankai samples and two commercial Spirulina powders that served as a reference for pseudo vitamin B12 are described in Supplementary File S1.
Purification of vitamin B12 and LC-MS/MS: B12 extracts were evaporated to dryness under reduced pressure and then re-dissolved in 9 mL of double-distilled water. The obtained solutions were loaded onto an immunoaffinity column (EASI-EXTRACT vitamin B12 immunoaffinity column (AOAC 2014.02), R-Biopharm AG, Darmstadt, Germany) and purified according to the manufacturer’s protocol. The recovery efficiency of pseudo CN-B12 was considered to be similar to that of authentic CN-B12. Subsequently, 10-μL aliquots of extracts were analyzed in optimized conditions determined using individual B12 standards. The concentrations based on standard curves were calculated using TargetLynx (Waters, Milford, MA, USA). The LC-MS/MS assay was performed at the Life Sciences Core Facilities of Weizmann Institute of Science. Further extraction and purification methods, as well as retention times and Multiple Reaction Monitoring (MRM) parameters for the detection of corrinoids, are given in Supplementary File S1 and Table S1.
2.2. The DIRECT PLUS Dietary Intervention Trial
2.2.1. Study Design
The 18-month DIRECT-PLUS (dietary intervention randomized controlled trial polyphenols-unprocessed) trial (clinicaltrials.gov ID: NCT03020186) aimed to address the residual beneficial effect of a green Mediterranean diet, richer in green plants and lower in meat, compared with other healthy lifestyle strategies. The trial was initiated in May 2017 and was conducted in an isolated workplace (Nuclear Research Center Negev (NRCN), Dimona, Israel), where a monitored lunch was provided. This workplace includes a medical department where most of the medical measurements were taken and where lifestyle intervention sessions were held. Of the 378 volunteers, 294 met the inclusion criteria of age >30 years and characterized by abdominal obesity (waist circumference (WC): men > 102 cm, women > 88 cm) or dyslipidemia (TG > 150 mg/dL and high-density lipoprotein cholesterol (HDL-c) ≤ 40 mg/dL for men, ≤ 50 mg/dL for women). Exclusion criteria are detailed in Supplementary File S2.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Medical Ethics Board and Institutional Review Board at Soroka University Medical Centre, Be’er Sheva, Israel (0280-16-SOR). All participants did not receive any financial compensation.
2.2.2. Randomization and Intervention
Randomization and intervention were described elsewhere [17,19]. Briefly, participants were randomly assigned to one of three intervention groups, all combined with physical activity recommendation (along with a free gym membership):
Healthy dietary guidelines (HDG) group: In addition to the workout program, the participants received basic health-promoting guidelines for achieving a healthy diet.
Mediterranean (MED) group: In addition to the workout program, participants were instructed to adopt a calorie-restricted Mediterranean diet as described in our previous trials: DIRECT [20] and CENTRAL [21] trials, supplemented with 28 g/day of walnuts.
Green Mediterranean (green-MED) group: In addition to the Mediterranean intervention (including the provided walnuts), the green Mediterranean dieters were further guided to avoid red and processed meat, with the diet being richer in plants and polyphenols. The participants were guided to further consume the two following provided items: 3–4 cups/day of 100 g frozen cubes of Mankai (whole plant), replacing dinner and a potential source of protein, iron, and vitamin B12. The MED and green-MED diets were equally calorie restricted (1500–1800 kcal/day for men and 1200–1400 kcal/day for women). All the above (walnuts, green tea, and Mankai) were provided free of charge.
2.2.3. Outcomes
Blood samples were taken at 8:00 AM after a 12-h fast, at baseline and after 18 months of intervention. The samples were centrifuged and stored at −80 °C. Serum vitamin B12 was analyzed with a competitive Elektro Chemiluminescence-Immuno Assay “ECLIA” (Cobas 8000, Roche Diagnostics, Mannheim, Germany) using Intrinsic Factor as a binding protein. Serum folate was also measured by the ECLIA competitive approach and was used as a marker for green leaf consumption [22]. All biochemical analyses were performed at the laboratories of the University of Leipzig, Germany. Chemical and hematological parameters in freshly drawn blood samples were assessed at the workplace clinic at baseline and at the end of the intervention measurements (±1 month before/after initiating blood draws). Additional outcomes measures (i.e., anthropometric, electronic questionnaires) are presented in Supplementary File S3.
2.2.4. Statistical Analysis
The primary outcomes of the DIRECT PLUS study, as stated in clinicaltrials.gov, were 18-month changes in adiposity parameters (a flow diagram for the study is presented in Figure S1). In this analysis, we primarily aimed to assess serum vitamin B12 change during the study period. Continuous variables are presented as means ± standard deviations for normally distributed variables and medians for non-normally distributed variables, with the Kolmogorov–Smirnov test used to determine the variable’s distribution. Nominal variables are expressed as numbers and percentages. Differences between time points were tested using the paired sample T-test or Wilcoxon test. Differences between groups (i.e., intervention groups or tertiles) were tested using analysis of variance (ANOVA), Kruskal–Wallis test, or Chi-square test. Ln transformations were applied when necessary to achieve normal distribution. Kendal Tau correlation was used to examine p of trend. Multiple comparisons were addressed using the Tukey post hoc test (for ANOVA), and Bonferroni correction (for Kruskal–Wallis). For adjustments, we used general linear regression models, with the specific adjustments detailed with the results. Sample size calculations were detailed elsewhere [17]. Statistical analysis was performed using SPSS (version 25.0, IBM, Armonk, NY, USA). Statistical significance was set at 0.05 level, two-sided.
2.3. Anoxic Gut Microbiome Bioreactors Pilot Experiment
2.3.1. Microbiota Reactors (Human Fecal Mixture)
A mixture of human fecal samples obtained from 20 healthy male and female volunteers (age: 18–65 years) collected for a research study in 2017 (Krajmalnik-Brown Lab; IRB#STUDY00004850, Arizona State University) was used to inoculate anoxic bioreactors. After donation, fecal samples were kept at 4 °C and 1 g of sample was supplemented with 500 μL of 40% (v/v) anaerobic glycerol solution. The fecal mixtures, consisting of 20 homogenized fecal samples obtained from each donor, were stored in anaerobic freezer bags at −80 °C. Prior to use, 1 mL of fecal mixture was added to a serum bottle filled with 70 mL of anoxic Base medium (see below). The bottle, containing the starter culture, was placed in a shaking incubator for 24 h at 100 rpm and 37 °C. Headspace gas quantification was used to confirm microbial activity.
2.3.2. Media, Anoxic Bioreactor, Mankai Lysate, and Sampling
Two anoxic media were used to examine the potential effect of Mankai on human-derived gut microbiota. Both media were based on the protocol described by McDonald et al. [23], with the following modification to provide the same chemical oxygen demand (COD) amount (200 meq/L) to all treatments. The final media consisted of an anoxic micronutrient-containing solution and an anoxic macronutrient solution (Table S2). COD was measured to quantify the reducing equivalents in both solutions. To obtain a (a) base medium for the bioreactors that lacked Mankai and for the starter culture (see above), and (b) Mankai medium for the Mankai-supplemented bioreactors, micronutrient-containing solution, and macronutrient solution were combined, accordingly (Table S2). Before bioreactor inoculation (adding 1 mL of the starter culture (see above)), Mankai lysate was prepared by blending 5 g of frozen Mankai biomass (Wolffia globosa ‘Mankai’) with 400 mL of deionized (DI) water for 5 min and subsequently flushing with nitrogen for 5 min. After inoculation and before the first fill and draw, the bioreactors were incubated for 48 h in the dark at 37 °C and mixed continuously at 100 rpm. Full details regarding the media, anoxic bioreactor, Mankai lysate, and the sampling are provided in Supplementary File S4.
2.3.3. Chemical and Molecular Analysis
Total COD was determined by adding 400 µL of solution, medium, or lysate to a HACH COD vial (HACH, High Range 20–1500 mg COD/L) with 1600 μL of DI water followed by a 2-h incubation at 150 °C (HACH DRB200). The vials were then cooled and measured for COD concentration in mgCOD/L using a spectrophotometer (HACH DR2800 Laboratory Spectrophotometer). For microbiome composition analysis, we performed 16S rRNA gene amplicon sequencing using Illumina sequencing technology and found core differences as described [24,25]. Further detailing regarding the 16S rRNA amplicon sequences is presented in Supplementary File S5.
3. Results
3.1. Mankai Plant Analyses
3.1.1. Content and Stability of Vitamin B12 Levels during Different Seasons
Overall, Mankai contained 2.8 ± 0.5 µg B12/100 g dry weight (DW) and the concentration remained relatively stable during the seasons (Figure 1), regardless of the water temperature (17 °C–29 °C) or duration of light hours (10–14): autumn: 2.84 ± 0.5 µg/100 g DW, n = 5 (range: 2.34 µg/100 g to 3.62 µg/100 g DW); winter: 2.83 ± 0.6 µg/100 g DW, n = 5 (range: 1.96 µg/100 g to 3.44 µg/100 g DW); spring: 2.94 ± 0.6 µg/100 g DW, n = 4 (range: 2.19 µg /100 g to 3.52 µg/100 g DW); and summer: 2.6 ± 0.5 µg/100 g DW, n = 6 (range: 1.83 µg/100 g to 3.26 µg/100 g DW). (p = 0.76 between seasons).
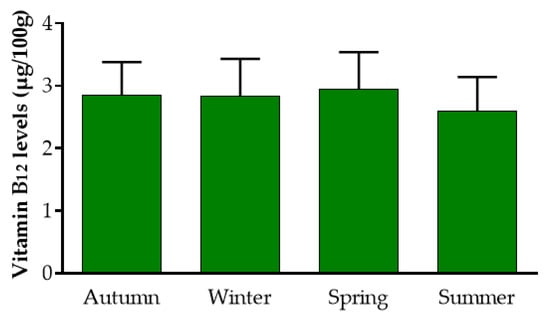
Figure 1.
Stability of vitamin B12 levels in Mankai™ along the year. “Autumn” refers to water temperatures of 22–24.5 °C and 10:20–10:50 h of light. “Winter” refers to water temperatures of 17–20 °C and 10–10:20 h of light. “Spring” refers to water temperatures of 21–24 °C and 11:30–13:30 h of light. “Summer” refers to water temperatures of 25–29 °C and 13:50–14:15 h of light. For each season, the weekly average water temperatures and daily light hours relate to the sampling date.
3.1.2. Inherent Presence of Vitamin B12 in Mankai Axenic Cultures
B12 concentrations in three independent axenic cultures, which were vegetatively propagated for at least 5 months post establishment, were 2.08, 2.34, and 1.6 µg/100 g DW.
3.1.3. Identification of Vitamin B12 Purified from Mankai
To verify that the corrinoid detected by the bioassay was indeed a bioactive form of cobalamin, we used LC-MS/MS. The presence of the active form was validated in all 10 tested samples: four plant samples representing three different seasons (spring, summer, and autumn) and 6 samples grown under indoor conditions. Representative data of a Mankai sample collected during mid-March 2019 from an outdoor basin are shown in Figure 2. Standard CN-B12 was eluted as a peak with a retention time of 2.11 min (Figure 2A) and the plant extract sample showed a corresponding peak with the same retention time (Figure 2B) for all MRM transitions. The intensity ratios between individual MRM signals were kept similar in both standard and plant samples (Figure S2).
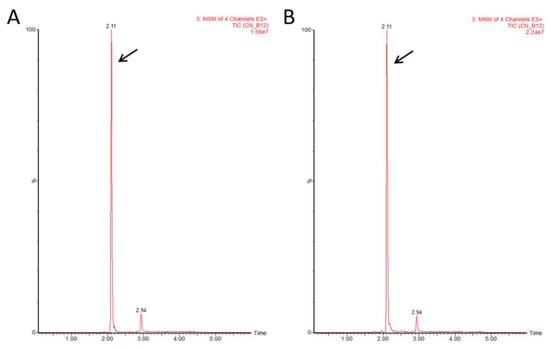
Figure 2.
Liquid chromatography/electrospray ionization tandem mass spectrometry (LC-MS/MS) chromatograms of CN-B12. (A) Retention time for CN-B12 standard (arrow). (B) Retention time for CN-B12 extracted from Mankai sample (arrow). ES, electrospray; MRM, multiple reaction monitoring; TIC, total ion current.
3.1.4. Quantification of Total Vitamin B12 Purified from Mankai
The extractions described above were performed in the presence of KCN, which converts the naturally occurring forms of cobalamin to the stable CN-B12 form. Since this conversion is not always complete [26], we analyzed all four vitamin B12 forms by LC-MS/MS, with the aim of determining the total vitamin B12 content of Mankai. Commercial OH-B12, CN-B12, Ado-B12, and Me-B12 standards were eluted as peaks with retention times of 1.87, 2.1, 2.25, and 2.31 min, respectively, and the plant extract samples showed corresponding peaks with the same retention times (Figure S3). The intensity ratios between individual MRM signals were kept similar in both standard and plant samples (data not shown). These results indicate that all three natural forms were present in Mankai and that incomplete conversion to CN-B12 had occurred. The identification of CN-B12, OH-B12, Ado-B12, and Me-B12 was further validated by four, three, two, and four MRMs, respectively. In order to calculate the total vitamin B12 in the plants, we measured the recovery rate of each form by analyzing the standards, with or without immunoaffinity column purification. Namely, the solutions containing the standard mix of four B12 forms in equal amounts were divided in two halves. One half was diluted with acetate buffer and passed through a EASI-EXTRACT vitamin B12 immunoaffinity column according to the manufacturer’s purification protocol. The obtained eluate was evaporated and re-dissolved to the same volume as the second half. Samples thus obtained were analyzed by LC-MS/MS. The results showed recovery rates of 55%, 37%, 16%, and 100% for CN-B12, OH-B12, Ado-B12, and Me-B12, respectively. The analysis was performed on three plant samples that were obtained from greenhouse cultivation basins during spring, summer, and autumn. The amount of each form was then measured in plant extracts and the total B12 level was calculated according to the recovery rates. The data showed that the average total authentic vitamin B12 concentrations in Mankai is 3.23 µg ± 0.5/100 g DW and stable during different seasons: spring 2.86 µg, summer 3.84 µg, and autumn 2.99 µg/100 g DW. These concentrations are in line with the results received by the bioassay method.
3.1.5. Authentic CN-B12 and Pseudo CN-B12 in Mankai
To further study Mankai as a vitamin B12 food source, we estimated the concentrations of pseudo B12 in the plant. To this end, we used LC-MS/MS to analyze samples of spirulina that are known to produce large amounts of pseudo B12 [27] and therefore can be used as a reference. This measurement was performed assuming similar ionization products for both CN-B12 and pseudo CN-B12, so the standard CN-B12 curve was used as a reference to quantify both compounds. Based on the different molecular masses of CN-B12 and pseudo CN-B12, the data revealed the presence of CN-B12 and pseudo CN-B12 in a ratio 1:3 in two different spirulina samples, whereas no pseudo CN-B12 was detected in the Mankai samples (Figure 3 and Figure S4).
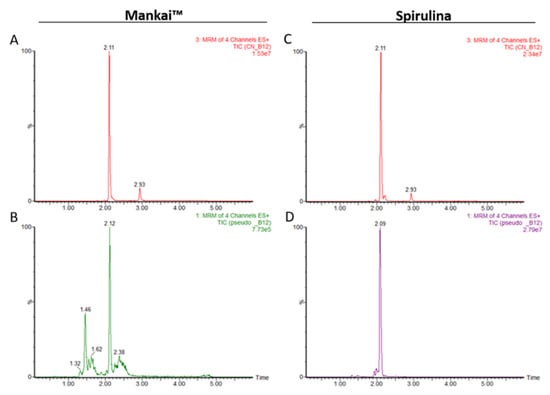
Figure 3.
A comparison of chromatograms of TIC for authentic CN-B12 and pseudo CN-B12 in Mankai and spirulina samples. (A–C): Active CN-B12; (B–D) Pseudo CN-B12 in Mankai™ (A,B) and spirulina (C,D) samples. In panel B, a peak at 2.12 min does not represent pseudo CN-B12 because pseudo CN-B12 should appear before the peak of CN-B12 [27,28] as is observed with a peak from a spirulina sample (at 2.09 min, panel D) and is present not just in one but in all 4 MRM transitions at measurable levels (Figure S4). ES, electrospray; MRM, multiple reaction monitoring; TIC, total ion current.
3.2. DIRECT PLUS Trial
3.2.1. Baseline Characteristics
The baseline characteristics are presented in Table 1. The mean vitamin B12 concentration was 420.4 ± 187.8 pg/mL (range: 150–1500 pg/mL), with a mean of 414.3 ± 182.5 pg/mL for men and 465.5 ± 220.9 pg/mL for women (p = 0.21 between sexes). Triglyceride levels were lower in the highest vitamin B12 tertile compared with the lowest tertile (p = 0.01). Details regarding baseline vitamin supplementation are presented in Supplementary File S6.

Table 1.
Baseline characteristics of the DIRECT PLUS participants across sex-specific vitamin B12 tertiles.
All chemical and hematological parameters (mean corpuscular volume (MCV), mean cell hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red blood cells (RBCs) hemoglobin and hematocrit; n = 290 for hemoglobin; n = 124 for other parameters) were similar and within the normal range across intervention groups (data not shown).
3.2.2. The Effect of the Intervention on Serum B12 Levels
The trial’s 18-month subject retention rate was 89.8%. Higher and similar weight reductions were observed, following a caloric deficit, in the two MED groups (MED: −2.9 ± 5.2%; Green-MED/low-meat: −3.9 ± 6.5%) compared with the HDG group (−0.6 ± 5.1%, p < 0.05 for both MEDs vs. HDG). Overall, the green-MED/low-meat diet group significantly increased intake of fish, Mankai, and green tea, and decreased red meat and poultry compared with the two other groups (p < 0.01 for all). Both MED groups increased egg and milk consumption compared with the HDG group [16]. Vitamin supplementation usage at the end of the intervention did not differ between the intervention groups (Supplementary File S6).
Differences in serum vitamin B12 concentrations between intervention groups are presented in Figure 4. After 18 months, the HDG group had a non-significant 1.245 ± 126.5 pg/mL (+5.2%) change in serum vitamin B12 levels (p = 0.93 vs. baseline), while MED had a significant increase in serum vitamin B12 levels (32.6 ± 76.2 pg/mL (+9.9%); p < 0.001 vs. baseline) as well in group Green-MED/low-meat (48.8 ± 124.9 pg/mL (+15.4%); p < 0.001 vs. baseline). P-of-trend was observed between the groups (p = 0.02), with a significant difference between the HDG and the green-MED/low-meat groups (p = 0.025). When further adjusted for age, sex, and baseline B12 concentrations, these significant differences remained (p = 0.01).
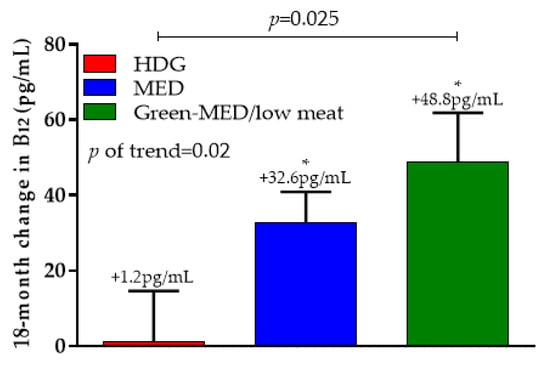
Figure 4.
The 18-month change in serum vitamin B12 across intervention groups. * Indicates within-group change (baseline vs. T18) at the 0.05 level. Data presented as means and SEM. HDG, healthy dietary guidelines; MED, Mediterranean.
3.2.3. Changes in chemical and hematological Parameters
After 18 months of intervention, among the sub-group of participants with available hematological and chemical measurements (n = 71 for hemoglobin; n = 41 for other hematological parameters), all groups demonstrated no significant changes in MCV, MCH, MCHC, RBC hemoglobin, or hematocrit, and also did not differ between the groups (p > 0.05 for all comparisons).
3.2.4. Dietary Vitamin B12 Sources
Next, we examined red meat (reported as increased, decreased, or no change in consumption) vs. Mankai frequency of intake tertiles, and change in serum folate (Green-MED/low-meat group only). Those who decreased red meat intake throughout the intervention showed a significantly increased serum folate associated with more frequent intake of Mankai (p of trend < 0.05; Figure S5a). Across all intervention groups, among those who decreased red meat consumption, increased serum folate was associated with increased serum vitamin B12 (p < 0.05) (Figure 5). The less red meat/increased serum folate group had a comparable increase of serum vitamin B12 to the mor -red meat/decreased serum folate group (86.0 ± 117.6 pg/mL vs. 77.9 ± 118.6 pg/mL, p = 0.88). In a similar analysis, replacing red meat with fish, we observed that among participants who increased fish intake throughout the intervention, an increase in vitamin B12 was observed, as well as serum folate (p of trend <0.01 for both). Significant increases in serum folate and vitamin B12 were observed for participants who both consumed more fish and Mankai, and demonstrated an increase in serum folate levels, as compared with other groups (Figure S5b,c).
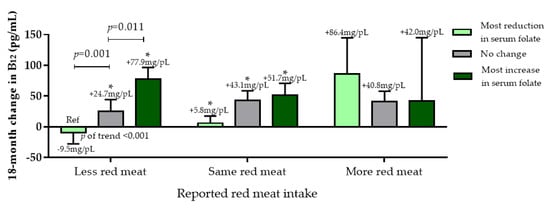
Figure 5.
Red meat consumption change at the end of the intervention (tertiles) vs. 18-month serum folate change (tertiles) vs. 18-month change in vitamin B12. * indicated within-group significance (baseline vs. T18) at the 0.05 level. Data presented as means and SEM.
No significant difference between extreme groups less red meat/most increase in serum folate and more red meat/most reduction in serum folate was observed.
3.3. Anoxic Bioreactors Pilot Experiment
Predicted Functional Pathways-Gut Bioreactor
Based on 16S rRNA gene amplicon sequences obtained from all bioreactors at the end of incubation (day 14), we predicted KEGG (Kyoto Encyclopedia of Genes and Genomes) present in the genomes of the bacteria identified, using Predicted functional profile analysis via PICRUSt [29]. This analysis, allowing us to predict KEGGS and the linear discriminant analysis effect size (LEFSE), showed that Mankai-supplemented bioreactors displayed a significantly higher relative abundance of 16S rRNA gene sequences associated with a genome containing a KEGG ortholog involved in vitamin B12 uptake (btuB; KEGG identifier K16092) than control bioreactors that lacked Mankai. Statistical analyses revealed a linear discriminant analysis (LDA) score of 2.19 (log10) and a relative btuB abundance of 0.034 ± 0.008 and 0.00 ± 0.001 in Mankai-supplemented reactors and reactors that lacked Mankai, respectively (p < 0.05 between reactors).
In total, 1180 of 5257 different 16S rRNA gene amplicon sequences were identified in the three replicated Mankai-supplemented bioreactors that contributed to the increased predicted abundance of microbes containing btuB. Six 16S rRNA gene amplicon sequences displayed a greater than 0.5% relative 16S rRNA gene amplicon abundance, and three of these sequences (closely related to Aeromonas hydrophila, Pelomonas aquatica, and Geobacter anodireducens) were present in all three replicated Mankai-supplemented bioreactors (Figure S3). In marked contrast, only nine different 16S rRNA gene amplicon sequences—associated with microbes potentially containing btuB—were identified in the control reactors that lacked Mankai, of which (a) five of these nine were present in all three replicates and (b) only one sequence (closely related to Escherichia coli) displayed a relative abundance greater than 0.5% (Table S3).
4. Discussion
In the current study, we examined, using different methodologies, the presence of vitamin B12 in a cultivated strain of Wolffia globosa (Mankai). We found that Mankai, cultured under closed-controlled greenhouse conditions, contains a substantial amount of the known bioactive forms of vitamin B12 and that its presence is stable throughout the year. In inoculated gut microbiome anoxic bioreactors, a significantly higher relative abundance of 16S rRNA gene sequences associated with a genome containing the KEGG ortholog involved in vitamin B12 uptake was observed, compared with control bioreactors that lacked Mankai. In our human studies, results suggest that long-term consumption of this plant, as part of a whole flexitarian diet, may increase rather than impair vitamin B12 levels, without additional red meat intake. To our knowledge, this is the first reported study on the B12 content and bioavailability in duckweed and specifically in Wolffia globosa.
Although some evidence for the presence of vitamin B12 in Actinorhizal plants has been reported [28], it is generally recognized that vitamin B12 is absent from plant-derived food sources [1,2,5,30]. Plants neither require nor synthesize vitamin B12 because they contain no cobalamin-dependent enzymes and instead encode a B12-independent form of methionine synthase [31]. To carefully examine our hypothesis regarding the presence of vitamin B12 in Mankai, we analyzed, over a period of 5 years, samples that were obtained from intensively grown plant cultures. Repeated microbiological assay analyses revealed the presence of stable levels of vitamin B12 in Mankai. Furthermore, to exclude B12 presence due to absorption from an external source, we tested vitamin B12 in axenic Mankai cultures, generated by propagating a green daughter frond that emerged from a bleached mother frond, for several months under sterile conditions. We speculated that in Mankai plants grown under these conditions, the level of any absorbed vitamin B12 from an external source, such as occasional bacteria or microalgal contamination, would be expected to decline and probably become undetectable in the axenic culture as the plants propagated for successive generations in the sterile culture and as the biomass increased by several orders. However, B12 analysis performed on cultures that were propagated for at least 5 months, under sterile conditions in a B12-free medium, revealed similar levels of the vitamin. Since the results described above were obtained by the microbiological assay method, the reliability of which was recently put in question because lactic bacterium, L. delbrueckii, was found to be able to utilize other corrinoids as well [1], we decided to further study the B12 nature in the Mankai plant tissue.
The LC-MS/MS method is a reliable method to analyze and identify vitamin B12 and its congeneric forms. We analyzed the four major forms of the vitamin: OH-B12, Ado-B12, Me-B12, and CN-B12 in all Mankai samples. The results revealed the presence of all four B12 forms in the Mankai samples. It is well known that in animal cells, Me-B12 serves as a cofactor for methionine synthase, while Ado-B12 is a cofactor of methylmalonyl-CoA mutase. However, plants contain no cobalamin-dependent enzymes [31] and therefore, while one can assume that these metabolites do not play a biological role in Mankai plants, it remains possible that the coenzyme forms of B12 are produced in endophytic bacteria, which are the presumed source of the B12. As the analysis was performed using the KCN extraction method, we were unable to assess the original content of each of the three natural B12 forms in Mankai. However, we were able to determine the total level of B12 in Mankai, and importantly, these results were comparable to the microbiological assay method. Moreover, we further investigated the presence of pseudo B12 due to reports on the identification of large quantities of this compound in non-animal food sources, such as algae [27]. Since pseudo CN-B12 is not commercially available, we used spirulina extracts as a reference source of pseudo CN-B12 and compared it with Mankai extracts. Under the LC-MS/MS conditions used in this study, no pseudo CN-B12 forms bearing identity with the pseudo CN-B12 seen in the spirulina extract were detected in any of the Mankai samples. Therefore, the bioassay analysis is a reliable method to measure vitamin B12 levels in Mankai.
Although the affinity of the gastric intrinsic factor binding protein for authentic B12 is 500 times greater than for pseudo B12 [32,33], according to Herbert and Drivas [34], non-cobalamin vitamin B12 analogues, produced by algae and other organisms, may interfere with vitamin B12 metabolism. A recent study by Bito et al. demonstrated that pseudo B12 can inhibit transcobalamin II-mediated absorption in mammalian cultured COS-7 cells [35].
Functional microbial composition analysis based on genome prediction and sequence matching of microbes in reactors that were inoculated with human fecal samples indicated that Mankai-supplemented reactors displayed a significantly enhanced relative abundance of 16S rRNA gene sequences of microorganisms that have the gene required to produce the vitamin B12 transporter BtuB. BtuB, located in the outer membrane of Gram-negative bacteria, is essential for the active uptake of cobalamin across the outer membrane [36]. We could infer that the increased abundance in gut microorganisms that produce the vitamin B12 transporter is due to the increased abundance of thisvitamin B12 in the Mankai reactors. Vitamin B12 is an essential cofactor in several microbial anaerobic processes (e.g., propionate fermentation, butyrate fermentation via 3-methylaspartate, methanogenesis), suggesting that this vitamin has the potential to stimulate fermentation and, thus, the production of short-chain fatty acids [37,38,39,40,41], which provide many benefits to the host [42].
The origin of the vitamin B12 in Mankai was not determined in this study, but we speculate that it is derived from an endophyte bacterial source. The fact that we did find B12 in the axenic cultures does not negate this hypothesis as axenic duckweed cultures, although often termed in the literature as “sterile” cultures, may still contain a plant tissue that carries microbes, in its internal core, as described by Gilbert et al. [12]. One may reasonably assume that a single or several such endophytic bacteria are responsible for the production of B12 found in Mankai.
Collectively, these results indicate that the presence of B12 in Mankai is not an occasional event nor a result of uptake from the surrounding medium but is stably and consistently produced within or in close association with the plant. Further studies should be conducted to identify the vitamin B12-producing bacteria and characterize their interaction with the plant. These studies may lead to novel strategies for B12 enrichment in Mankai and would contribute to its nutritional value as a potential vitamin B12 food source, particularly for individuals who prefer a vegetarian lifestyle or who eschew any animal products in their diet.
The recommended dietary allowance of vitamin B12 for adults is set at 2.4 μg/day [43]. The vitamin B12 content in Mankai, according to our repeated analyses, is about 0.5 μg/20 g DW (equivalent to 100 g of frozen Mankai, as given to our participants as a green dinner shake), thus making it a desirable plant substitute. Although advised to completely reduce red meat intake, we observed a significant increase in vitamin B12 levels among participants who were under a semi-vegetarian weight loss diet, compared with participants who, although advised to adopt a healthy lifestyle, did not significantly change their routine red meat intake. It has to be noted that a significant trend in vitamin B12 increase was observed between the intervention groups, even though the green-MED dieters were instructed to avoid red/processed meat and their diet was further fortified with Mankai shake and green tea. In addition, participants who reduced red meat had an increase in serum folate (a marker for green leafy vegetable consumption [22]), and in this study for Mankai consumption [17] had an increase in vitamin B12 comparable to participants who increased red meat and had a decrease in serum folate levels. Reducing red meat consumption, especially processed meat products, has been a focus of attention in recent years, due to increasing evidence of the association between meat consumption and health risks [44]. However, reducing red meat, as vegan or some vegetarian eating patterns suggest, might put one at risk of vitamin B12 deficiency, which could result in megaloblastic anemia and neurological damage [45,46]. Vegetarians and vegans in particular are at risk of developing vitamin B12 deficiency and infants born to mothers who follow such diets run a risk of neurodevelopmental abnormalities and feeding difficulties [47]. Therefore, the identification of a natural alternative vitamin B12 source would be of major interest to nutrition professionals.
Natural sources of authentic vitamin B12 include red meat and fish but also dairy and eggs [31,46]. However, it is well known that growing cattle for food requires a lot of land, water, and energy, and generates considerable waste [48,49]. In the search for a sustainable vitamin B12 source, it has been reported that some plant foods (e.g., mushrooms and edible Algae) are rich in corrinoids, but those foods either lack the bioactive form of vitamin B12, must be consumed in impractical amounts, or because of controversial data are a questionable source of bioavailable B12 [50,51,52]. Alternatively, insects have been proposed as a promising source of food for vitamin B12. Mealworms, grasshoppers, crickets, and cockroaches were studied regarding their content of bioactive vitamin B12 but exhibited marked variations in their vitamin B12 content [53]. Moreover, esthetic, religious, and psychological barriers may further limit their use as a source of vitamin B12 replacement.
The limitations of this study include the inability to assess the origin of the vitamin B12 in the plant, as well as the bioavailability and specific digestibility pathway of vitamin B12 directly among our human participants. The bioassay method, based on the B12-requiring bacteria Lactobacillus Delbrueckii, cannot determine whether Mankai contains cobalamin or inactive corrinoids or both [1]. However, the fact that the LC-MS/MS method, which is a direct physico-chemical assay for B12, revealed comparable levels to the bioassay method indicates that, in the case of Mankai, the bioassay results reflect solely the concentrations of authentic B12 forms and not analogues. We were also not able to isolate Mankai as a sole source of vitamin B12 from other dietary components rich in vitamin B12 in the long-term human trial. In order to overcome this limitation, we presented additional analyses from the electronic questionnaires of other B12 sources. We did not measure homocysteine or methylmalonic acid, which might better reflect metabolic deficiencies of vitamin B12 or serum folate [54], thus we cannot evaluate the effect of the intervention in cases with low B12 and high levels of these serum/plasma markers indicative of biochemical B12 deficiency. Furthermore, our participants had baseline serum B12 levels within the normal range, so, although we could observe significant increases, we could not demonstrate efficacy for correction of B12 deficiency status and further studies should be carried out to examine this question. We also cannot point out the exact mechanism that explains the substantial B12 content in the Mankai plant, nor the way in which this may be controlled in the plant tissue. The data we showed for our bioreactors are from a small pilot study, and we consider them preliminary. Thus, an open question remains concerning the possibility that the Mankai plant may modify the microbiota in the intestinal tract with possible effects on the bioavailability of B12 normally present in bile [5]. Strengths of the data that we report here include the comprehensive multi-assessment of several aspects of B12, including laboratory, gut-related, and a long-term human randomized controlled trial, with monitored lunch and daily supply of Mankai to the participants.
5. Conclusions
The Mankai plant contains bioactive B12 compounds and could potentially serve as a plant-based food source of vitamin B12. Results from this study could provide additional insight regarding a much-needed alternative healthy and sustainable B12 source.
Supplementary Materials
The following are available: https://www.mdpi.com/2072-6643/12/10/3067/s1. File S1: Further details on the extraction and purification of Vitamin B12, File S2: Exclusion criteria DIRECT PLUS trial, File S3: Further outcome measurements of DIRECT PLUS trial, File S4: Full details regarding the media, anoxic bioreactor, Mankai lysate and the sampling, File S5: Further details regarding the 16S rRNA amplicon sequences, File S6: Supplementation usage, DIRECT PLUS trial, Table S1: LC and MS parameters for detection of corrinoids, Table S2: Composition of micronutrient-containing solution and macronutrient solution that were used to prepare Base medium and Mankai medium, Table S3: Relative 16S rRNA gene amplicon abundance and taxonomy of phylotypes that (a) are predicted to contain btuB in their genome and (b) displayed a greater than 0.5% relative abundance in either the Mankai-supplemented or control reactor, Figure S1: The DIRECT PLUS trial flow diagram, Figure S2: Comparison of chromatograms of different MRMs for CN-B12 standard 0.1 µg/mL (A-D) and plant sample (E-H), Figure S3: Liquid chromatography/electrospray ionization−tandem mass spectrometry chromatograms of bioactive B12 compounds, Figure S4: Comparison of chromatograms of different MRMs for Pseudo CN-B12 in Mankai (A-D) and Spirulina (E-H) samples, Figure S5: Further nutritional analysis.
Author Contributions
Conceptualization, I.S. (Ilan Sela), A.Y.M., R.K.-B., L.Z., G.T., A.K., E.R., H.Z., M.L., I.S. (Iris Shai); Formal analysis, I.S. (Ilan Sela), A.Y.M., A.B., R.K.-B., L.Z., D.C., B.D., S.A., U.C., B.I., M.L., I.S. (Iris Shai); Investigation, A.Y.M., G.T., A.K., E.R., H.Z., I.S. (Iris Shai); Resources, I.S. (Iris Shai), R.K.-B.; Supervision, I.S. (Iris Shai), R.G.; Writing—Original draft, I.S. (Ilan Sela), A.Y.M., M.L., I.S. (Iris Sha); Writing—Review and editing, I.S. (Ilan Sela), A.Y.M., A.B., R.K.-B., L.Z., G.T., A.K., E.R., H.Z., U.C., R.G., I.S. (Iris Shai). All authors have read and agreed to the published version of the manuscript.
Funding
DIRECT-PLUS was supported by the Deutsche Forschungsgemeinschaft (DFG—German Research Foundation)—project no. 209933838, grant SFB1052; the Deutsche Forschungsgemeinschaft, Obesity Mechanisms; Israel Ministry of Health grant 87472511 (to I Shai); Israel Ministry of Science and Technology grant 3-13604 (to I Shai); and the California Walnuts Commission (to I Shai). Gut microbiome study was supported by Arizona-BGU collaborative grant (PIs: Rosa Krajmalnik-Brown, Iris Shai). Mankai plant B12 analysis was funded by Hinoman Ltd. and Weitzman Institute. None of the funding providers were involved in any stage of the design, conduct, or analysis of the study and they had no access to the study results before publication.
Acknowledgments
We thank the DIRECT PLUS participants for their valuable contribution. We thank the California Walnut Commission, Wissotzky Tea Company, and Hinoman, Ltd. for kindly supplying food items for this study. We thank Dov Brikner, Efrat Pupkin, Eyal Goshen, Avi Ben Shabat, Evyatar Cohen and Benjamin Sarusi from the Nuclear Research Center Negev, Liz Shabtai and Yulia Kovshan from Ben-Gurion University of the Negev, Monica Colt from Hinoman Ltd. and Janet King from UC Davis for their valuable contributions to this study.
Conflicts of Interest
Sela I., Arinos S. and Lapidot M. are employees of Hinoman Ltd.; Shai I. advises to the Hinoman, Ltd. nutritional committee. All other authors declare no conflict of interest.
References
- Watanabe, F.; Bito, T. Determination of cobalamin and related compounds in foods. J. AOAC Int. 2018, 101, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Burgess, C.M.; Smid, E.J.; van Sinderen, D. Bacterial vitamin B2, B11 and B12 overproduction: An overview. Int. J. Food Microbiol. 2009, 133, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Green, R. Vitamin B12 deficiency from the perspective of a practicing hematologist. Blood J. Am. Soc. Hematol. 2017, 129, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, F.; Takenaka, S.; Kittaka-Katsura, H.; Ebara, S.; Miyamoto, E. Characterization and bioavailability of vitamin B12-compounds from edible algae. J. Nutr. Sci. Vitaminol. 2002, 48, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Miller, J.W. Vitamin B12. In Handbook of Vitamins; Zempleni, J., Suttie, J.W., Gregory, J.F., III, Stover, P.J., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 447–489. [Google Scholar]
- Rizzo, G.; Laganà, A.S.; Rapisarda, A.M.C.; Ferrera, L.; Grazia, G.M.; Buscema, M.; Rossetti, P.; Nigro, A.; Muscia, V.; Valenti, G.; et al. Vitamin B12 among vegetarians: Status, assessment and supplementation. Nutrients 2016, 8, 767. [Google Scholar] [CrossRef] [PubMed]
- Crowe, F.L.; Appleby, P.N.; Travis, R.C.; Key, T.J. Risk of hospitalization or death from ischemic heart disease among British vegetarians and nonvegetarians: Results from the EPIC-Oxford cohort study. Am. J. Clin. Nutr. 2013, 97, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Barnard, N.D.; Levin, S.M.; Watanabe, M. Vegetarian diets and glycemic control in diabetes: A systematic review and meta-analysis. Cardiovasc. Diagn. Ther. 2014, 4, 373. [Google Scholar]
- Wang, F.; Zheng, J.; Yang, B.; Jiang, J.; Fu, Y.; Li, D. Effects of vegetarian diets on blood lipids: A systematic review and meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2015, 4, e002408. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Nishimura, K.; Barnard, N.D.; Miyamoto, Y. Blood pressure and vegetarian diets. In Vegetarian and Plant-Based Diets in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2017; pp. 395–413. [Google Scholar]
- Sukumar, N.; Saravanan, P. Investigating vitamin B12 deficiency. BMJ 2019, 365, l1865. [Google Scholar] [CrossRef]
- Gilbert, S.; Xu, J.; Acosta, K.; Poulev, A.; Lebeis, S.; Lam, E. Bacterial production of indole related compounds reveals their role in association between duckweeds and endophytes. Front. Chem. 2018, 6, 265. [Google Scholar] [CrossRef]
- Appenroth, K.J.; Sree, K.S.; Bog, M.; Ecker, J.; Boehm, V.; Lorkowski, S.; Sommer, K.; Vetter, W.; Tolzin-Banasch, K.; Kirmse, R.; et al. Nutritional value of the duckweed species of the genus Wolffia (Lemnaceae) as human food. Front. Chem. 2018, 6, 483. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, Y.; Shibui, Y.; Takumi, A.; Seki, T.; Shimada, T.; Hashimoto, M.; Inoue, N.; Kobayashi, H.; Narita, T. Genotoxicity and repeated-dose toxicity evaluation of dried Wolffia globosa Mankai. Toxicol. Rep. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.; Zelicha, H.; Tsaban, G.; Yaskolka Meir, A.; Rinott, E.; Kovsan, J.; Novack, L.; Thiery, J.; Ceglarek, U.; Burkhardt, R.; et al. Protein bioavailability of Wolffia globosa duckweed, a novel aquatic plant–A randomized controlled trial. Clin. Nutr. 2019, 38. [Google Scholar] [CrossRef]
- Yan, Y.; Candreva, J.; Shi, H.; Ernst, E.; Martienssen, R.; Schwender, J.; Shanklin, J. Survey of the total fatty acid and triacylglycerol composition and content of 30 duckweed species and cloning of a Δ6-desaturase responsible for the production of γ-linolenic and stearidonic acids in Lemna gibba. BMC Plant Biol. 2013, 13, 201. [Google Scholar] [CrossRef]
- Yaskolka Meir, A.; Tsaban, G.; Zelicha, H.; Rinott, E.; Kaplan, A.; Youngster, I.; Rudich, A.; Shelef, I.; Tirosh, A.; Brikner, D.; et al. A Green-mediterranean diet, supplemented with mankai duckweed, preserves iron-homeostasis in humans and is efficient in reversal of anemia in rats. J. Nutr. 2019, 149. [Google Scholar] [CrossRef]
- Ball, G.F.M. Microbiological methods for the determination of the B-group vitamins. In Water-Soluble Vitamin Assays in Human Nutrition; Springer: Berlin, Germany, 1994; pp. 317–364. [Google Scholar]
- Rinott, E.; Youngster, I.; Meir, A.Y.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Fava, F.; Scholz, M.U.; et al. Effects of diet-modulated autologous fecal microbiota transplantation on weight regain. Gastroenterology 2020. [Google Scholar] [CrossRef]
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H.; et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N. Engl. J. Med. 2008, 359, 229–241. [Google Scholar] [CrossRef]
- Gepner, Y.; Shelef, I.; Schwarzfuchs, D.; Zelicha, H.; Tene, L.; Meir, A.Y.; Tsaban, G.; Cohen, N.; Bril, N.; Rein, M.; et al. Effect of distinct lifestyle interventions on mobilization of fat storage pools: Central magnetic resonance imaging randomized controlled trial. Circulation 2018, 137, 1143–1157. [Google Scholar] [CrossRef]
- Moll, R.; Davis, B. Iron, vitamin B12 and folate. Medicine 2017, 45, 198–203. [Google Scholar] [CrossRef]
- McDonald, J.A.K.; Schroeter, K.; Fuentes, S.; Heikamp-deJong, I.; Khursigara, C.M.; de Vos, W.M.; Allen-Vercoe, E. Evaluation of microbial community reproducibility, stability and composition in a human distal gut chemostat model. J. Microbiol. Methods 2013, 95, 167–174. [Google Scholar] [CrossRef]
- Gutierrez, D.; Weinstock, A.; Antharam, V.C.; Gu, H.; Jasbi, P.; Shi, X.; Dirks, B.; Krajmalnik-Brown, R.; Maldonado, J.; Guinan, J.; et al. Antibiotic-induced gut metabolome and microbiome alterations increase the susceptibility to Candida albicans colonization in the gastrointestinal tract. FEMS Microbiol. Ecol. 2020, 96, fiz187. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, Z.E.; di Baise, J.K.; Dautel, S.E.; Isern, N.G.; Kim, Y.M.; Hoyt, D.W.; Schepmoes, A.A.; Brewer, H.M.; Weitz, K.K.; Metz, T.O.; et al. Temporospatial shifts in the human gut microbiome and metabolome after gastric bypass surgery. NPJ Biofilms Microbiomes 2020, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jägerstad, M.; Arkbåge, K. Cobalamins properties and determination. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Trugo, L.C., Finglas, P.M., Eds.; Academic: Cambridge, MA, USA, 2003; p. 1419. [Google Scholar]
- Watanabe, F.; Katsura, H.; Takenaka, S.; Fujita, T.; Abe, K.; Tamura, Y.; Nakatsuka, T.; Nakano, Y. Pseudovitamin B12 is the predominant cobamide of an algal health food, spirulina tablets. J. Agric. Food Chem. 1999, 47, 4736–4741. [Google Scholar] [CrossRef] [PubMed]
- Chamlagain, B.; Edelmann, M.; Kariluoto, S.; Ollilainen, V.; Piironen, V. Ultra-high performance liquid chromatographic and mass spectrometric analysis of active vitamin B12 in cells of Propionibacterium and fermented cereal matrices. Food Chem. 2015, 166, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Thurber, R.L.V.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, F.; Yabuta, Y.; Bito, T.; Teng, F. Vitamin B12-containing plant food sources for vegetarians. Nutrients 2014, 6, 1861–1873. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, F. Vitamin B12 sources and bioavailability. Exp. Biol. Med. 2007, 232, 1266–1274. [Google Scholar] [CrossRef]
- Fedosov, S.N.; Fedosova, N.U.; Kräutler, B.; Nexø, E.; Petersen, T.E. Mechanisms of discrimination between cobalamins and their natural analogues during their binding to the specific B12-transporting proteins. Biochemistry 2007, 46, 6446–6458. [Google Scholar] [CrossRef]
- Stupperich, E.; Nexø, E. Effect of the cobalt-N coordination on the cobamide recognition by the human vitamin B12 binding proteins intrinsic factor, transcobalamin and haptocorrin. Eur. J. Biochem. 1991, 199, 299–303. [Google Scholar] [CrossRef]
- Herbert, V.; Drivas, G. Spirulina and vitamin B12. JAMA 1982, 248, 3096–3097. [Google Scholar] [CrossRef]
- Bito, T.; Bito, M.; Hirooka, T.; Okamoto, N.; Harada, N.; Yamaji, R.; Nakano, Y.; Inui, H.; Watanabe, F. Biological activity of pseudovitamin B12 on cobalamin-dependent methylmalonyl-CoA mutase and methionine synthase in mammalian cultured COS-7 Cells. Molecules 2020, 25, 3268. [Google Scholar] [CrossRef] [PubMed]
- Schauer, K.; Rodionov, D.A.; de Reuse, H. New substrates for TonB-dependent transport: Do we only see the ‘tip of the iceberg’? Trends Biochem. Sci. 2008, 33, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xiang, S.; Ye, K.; Zheng, Y.; Feng, X.; Zhu, X.; Chen, J.; Chen, Y. Cobalamin (vitamin B12) induced a shift in microbial composition and metabolic activity in an in vitro colon simulation. Front. Microbiol. 2018, 9, 2780. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Takahashi-Iñiguez, T.; García-Hernandez, E.; Arreguín-Espinosa, R.; Flores, M.E. Role of vitamin B12 on methylmalonyl-CoA mutase activity. J. Zhejiang Univ. Sci. B 2012, 13, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Ragsdale, S.W. The many faces of vitamin B12: Catalysis by cobalamin-dependent enzymes. Annu. Rev. Biochem. 2003, 72, 209–247. [Google Scholar] [CrossRef]
- Buckel, W. Unusual enzymes involved in five pathways of glutamate fermentation. Appl. Microbiol. Biotechnol. 2001, 57, 263–273. [Google Scholar] [CrossRef]
- Van Treuren, W.; Dodd, D. Microbial contribution to the human Metabolome: Implications for health and disease. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 345–369. [Google Scholar] [CrossRef]
- Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes: Recommended Intakes for Individuals; National Academy of Sciences: Washington, DC, USA, 2004.
- Micha, R.; Penalvo, J.L.; Cudhea, F.; Imamura, F.; Rehm, C.D.; Mozaffarian, D. association between dietary factors and mortality from heart disease, stroke, and Type 2 diabetes in the united states. JAMA 2017, 317, 912–924. [Google Scholar] [CrossRef]
- Obeid, R.; Heil, S.G.; Verhoeven, M.M.A.; van den Heuvel, E.G.H.M.; de Groot, L.C.; Eussen, S.J.P.M. Vitamin B12 intake from animal foods, biomarkers, and health aspects. Front. Nutr. 2019, 6, 93. [Google Scholar] [CrossRef]
- Green, R.; Allen, L.H.; Bjørke-Monsen, A.L.; Brito, A.; Guéant, J.L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.H.; et al. Vitamin B 12 deficiency. Nat. Rev. Dis. Prim. 2017, 3, 17040. [Google Scholar] [CrossRef]
- Oussalah, A.; Levy, J.; Berthezène, C.; Alpers, D.H.; Guéant, J.L. Health outcomes associated with vegetarian diets: An umbrella review of systematic reviews and meta-analyses. Clin. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lebacq, T.; Baret, P.V.; Stilmant, D. Sustainability indicators for livestock farming. A review. Agron. Sustain. Dev. 2013, 33, 311–327. [Google Scholar] [CrossRef]
- Van Zanten, H.H.E.; Herrero, M.; van Hal, O.; Röös, E.; Muller, A.; Garnett, T.; Gerber, P.J.; Schader, C.; Boer, I.J.M. De Defining a land boundary for sustainable livestock consumption. Glob. Chang. Biol. 2018, 24, 4185–4194. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, F.; Yabuta, Y.; Tanioka, Y.; Bito, T. Biologically active vitamin B12 compounds in foods for preventing deficiency among vegetarians and elderly subjects. J. Agric. Food Chem. 2013, 61, 6769–6775. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H. Serum vitamin B12 levels in young vegans who eat brown rice. J. Nutr. Sci. Vitaminol. 1995, 41, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Dagnelie, P.C.; van Staveren, W.A.; van den Berg, H. Vitamin B-12 from algae appears not to be bioavailable. Am. J. Clin. Nutr. 1991, 53, 695–697. [Google Scholar] [CrossRef]
- Schmidt, A.; Call, L.M.; Macheiner, L.; Mayer, H.K. Determination of vitamin B12 in four edible insect species by immunoaffinity and ultra-high performance liquid chromatography. Food Chem. 2019, 281, 124–129. [Google Scholar] [CrossRef]
- Klee, G.G. Cobalamin and folate evaluation: Measurement of methylmalonic acid and homocysteine vs vitamin B12 and folate. Clin. Chem. 2000, 46, 1277–1283. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).