Cognitive Outcomes and Relationships with Phenylalanine in Phenylketonuria: A Comparison between Italian and English Adult Samples
Abstract
:1. Introduction
2. Method
2.1. Participants
2.2. Ethical Approval
2.3. Metabolic Measures
2.4. Cognitive Assessment
2.4.1. Visual Attention
2.4.2. Visuo-Motor Coordination
2.4.3. Complex Executive Functions
2.4.4. Short-term Memory/Working Memory
2.4.5. Sustained Attention
2.4.6. Verbal Memory and Learning
2.4.7. Visual Memory and Learning
3. Data Analyses
4. Results
4.1. Demographics
4.2. Cognitive Performance
4.2.1. AwPKU vs. Controls
4.2.2. Italian AwPKU vs. English AwPKU
4.3. Cognitive Outcomes in Relation to Metabolic Control
5. Discussion
5.1. Metabolic Control
5.2. Cognitive Impairments
- -
- -
- Executive functions in terms of flexibility and planning (TMT B and B–A and verbal fluency), but no impairment in the WCST (see also Moyle et al. [49] for impairments in fluency, but no impairments in the TMT B; see also Brumm et al. [25]; Smith et al. [50], for impairments in the WCST; see Ris et al. [51], and Channon et al. [52], for impairments in a test similar to the WCST).
- -
- -
6. Conclusions
Limitations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hardelid, P.; Cortina-Borja, M.; Munro, A.; Jones, H.; Cleary, M.; Champion, M.P.; Foo, Y.; Scriver, C.R.; Dezateux, C. The Birth Prevalence of PKU in Populations of European, South Asian and Sub-Saharan African Ancestry Living in South East England. Ann. Hum. Genet. 2007, 72, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A.; Prevor, M.B.; Callender, G.; Druin, D.P. Prefrontal Cortex Cognitive Deficits in Children Treated Early and Continuously for PKU. Monogr. Soc. Res. Child. Dev. 1997, 62, 1–208. [Google Scholar] [CrossRef]
- Douglas, T.D.; Ramakrishnan, U.; Kable, J.A.; Singh, R.H. Longitudinal quality of life analysis in a phenylketonuria cohort provided sapropterin dihydrochloride. Heal. Qual. Life Outcomes 2013, 11, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The American College of Medical Genetics and Genomics Therapeutic Committee; Vockley, J.; Andersson, H.C.; Antshel, K.M.; Braverman, N.E.; Burton, B.K.; Frazier, D.M.; Mitchell, J.J.; Smith, W.E.; Thompson, B.H.; et al. Phenylalanine hydroxylase deficiency: Diagnosis and management guideline. Genet. Med. 2013, 16, 188–200. [Google Scholar] [CrossRef] [Green Version]
- Romani, C.; Palermo, L.; Macdonald, A.; Limback, E.; Hall, S.K.; Geberhiwot, T. The impact of phenylalanine levels on cognitive outcomes in adults with phenylketonuria: Effects across tasks and developmental stages. Neuropsychology 2017, 31, 242–254. [Google Scholar] [CrossRef]
- Romani, C.; Manti, F.; Nardecchia, F.; Valentini, F.; Fallarino, N.; Carducci, C.; De Leo, S.; Macdonald, A.; Palermo, L.; Leuzzi, V. Adult cognitive outcomes in phenylketonuria: Explaining causes of variability beyond average Phe levels. Orphanet J. Rare Dis. 2019, 14, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Blau, N.; Bélanger-Quintana, A.; Demirkol, M.; Feillet, F.; Giovannini, M.; Macdonald, A.; Trefz, F.K.; Van Spronsen, F. Management of phenylketonuria in Europe: Survey results from 19 countries. Mol. Genet. Metab. 2010, 99, 109–115. [Google Scholar] [CrossRef]
- Walter, J.; White, F.; Hall, S.; Macdonald, A.; Rylance, G.; Boneh, A.; Francis, D.; Shortland, G.; Schmidt, M.; Vail, A. How practical are recommendations for dietary control in phenylketonuria? Lancet 2002, 360, 55–57. [Google Scholar] [CrossRef]
- Weglage, J.; Fromm, J.; Van Teeffelen-Heithoff, A.; Möller, H.E.; Koletzko, B.; Marquardt, T.; Rutsch, F.; Feldmann, R. Neurocognitive functioning in adults with phenylketonuria: Results of a long term study. Mol. Genet. Metab. 2013, 110, S44–S48. [Google Scholar] [CrossRef]
- Moyle, J.; Fox, A.M.; Bynevelt, M.; Arthur, M.; Burnett, J.R. A neuropsychological profile of off-diet adults with phenylketonuria. J. Clin. Exp. Neuropsychol. 2007, 29, 436–441. [Google Scholar] [CrossRef]
- Albrecht, J.; Garbade, S.F.; Burgard, P. Neuropsychological speed tests and blood phenylalanine levels in patients with phenylketonuria: A meta-analysis. Neurosci. Biobehav. Rev. 2009, 33, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Christ, S.E.; Huijbregts, S.C.; De Sonneville, L.M.; White, D.A. Executive function in early-treated phenylketonuria: Profile and underlying mechanisms. Mol. Genet. Metab. 2010, 99, S22–S32. [Google Scholar] [CrossRef] [PubMed]
- Deroche, K.; Welsh, M. Twenty-Five Years of Research on Neurocognitive Outcomes in Early-Treated Phenylketonuria: Intelligence and Executive Function. Dev. Neuropsychol. 2008, 33, 474–504. [Google Scholar] [CrossRef] [PubMed]
- Janzen, D.; Nguyen, M. Beyond executive function: Non-executive cognitive abilities in individuals with PKU. Mol. Genet. Metab. 2010, 99, S47–S51. [Google Scholar] [CrossRef] [PubMed]
- Palermo, L.; Geberhiwot, T.; Macdonald, A.; Limback, E.; Hall, S.K.; Romani, C. Cognitive outcomes in early-treated adults with phenylketonuria (PKU): A comprehensive picture across domains. Neuropsychology 2017, 31, 255–267. [Google Scholar] [CrossRef]
- Romani, C.; Macdonald, A.; De Felice, S.; Palermo, L. Speed of processing and executive functions in adults with phenylketonuria: Quick in finding the word, but not the ladybird. Cogn. Neuropsychol. 2017, 35, 171–198. [Google Scholar] [CrossRef]
- Koch, R.; Moseley, K. Phenylketonuria: Newborn identification through to adulthood. In Amino Acids in Human Nutrition and Health; CABI Publishing: Wallingford, UK, 2011; pp. 406–417. [Google Scholar]
- Rohr, F.J.; Wessel, A.; Brown, M.; Charette, K.; Levy, H.L. Adherence to tetrahydrobiopterin therapy in patients with phenylketonuria. Mol. Genet. Metab. 2015, 114, 25–28. [Google Scholar] [CrossRef]
- Thomas, J.; Levy, H.; Amato, S.; Vockley, J.; Zori, R.; Dimmock, D.; Harding, C.O.; Bilder, D.A.; Weng, H.H.; Olbertz, J.; et al. Pegvaliase for the treatment of phenylketonuria: Results of a long-term phase 3 clinical trial program (PRISM). Mol. Genet. Metab. 2018, 124, 27–38. [Google Scholar] [CrossRef]
- Harding, C.O.; Amato, R.S.; Stuy, M.; Longo, N.; Burton, B.K.; Posner, J.; Weng, H.H.; Meriläinen, M.; Gu, Z.; Jiang, J.; et al. Pegvaliase for the treatment of phenylketonuria: A pivotal, double-blind randomized discontinuation Phase 3 clinical trial. Mol. Genet. Metab. 2018, 124, 20–26. [Google Scholar] [CrossRef]
- Pascucci, T.; Rossi, L.; Colamartino, M.; Gabucci, C.; Carducci, C.; Valzania, A.; Sasso, V.; Bigini, N.; Pierigè, F.; Viscomi, M.T.; et al. A new therapy prevents intellectual disability in mouse with phenylketonuria. Mol. Genet. Metab. 2018, 124, 39–49. [Google Scholar] [CrossRef]
- Manti, F.; Nardecchia, F.; Paci, S.; Chiarotti, F.; Carducci, C.; Carducci, C.; Dalmazzone, S.; Cefalo, G.; Salvatici, E.; Banderali, G.; et al. Predictability and inconsistencies in the cognitive outcome of early treated PKU patients. J. Inherit. Metab. Dis. 2017, 40, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Van Spronsen, F.; Huijbregts, S.; Bosch, A.; Leuzzi, V. Cognitive, neurophysiological, neurological and psychosocial outcomes in early-treated PKU-patients: A start toward standardized outcome measurement across development. Mol. Genet. Metab. 2011, 104, S45–S51. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, D.; Van Wegberg, A.M.; Ahring, K.; Bik-Multanowski, M.; Blau, N.; Bulut, F.D.; Casas, K.; Didycz, B.; Djordjevic, M.; Federico, A.; et al. Can untreated PKU patients escape from intellectual disability? A systematic review. Orphanet J. Rare Dis. 2018, 13, 149. [Google Scholar] [CrossRef]
- Brumm, V.L.; Azen, C.; Moats, R.A.; Stern, A.M.; Broomand, C.; Nelson, M.D.; Koch, R. Neuropsychological outcome of subjects participating in the PKU Adult Collaborative Study: A preliminary review. J. Inherit. Metab. Dis. 2004, 27, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Nardecchia, F.; Manti, F.; Chiarotti, F.; Carducci, C.; Carducci, C.; Leuzzi, V. Neurocognitive and neuroimaging outcome of early treated young adult PKU patients: A longitudinal study. Mol. Genet. Metab. 2015, 115, 84–90. [Google Scholar] [CrossRef]
- Jahja, R.; Huijbregts, S.; De Sonneville, L.M.J.; Van Der Meere, J.J.; Legemaat, A.M.; Bosch, A.M.; Hollak, C.E.; Rubio-Gozalbo, M.E.; Brouwers, M.C.G.J.; Hofstede, F.C.; et al. Cognitive profile and mental health in adult phenylketonuria: A PKU-COBESO study. Neuropsychology 2017, 31, 437–447. [Google Scholar] [CrossRef]
- Christ, S.E.; Steiner, R.; Grange, D.K.; Abrams, R.A.; White, D.A. Inhibitory Control in Children With Phenylketonuria. Dev. Neuropsychol. 2006, 30, 845–864. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, R.; Denecke, J.; Grenzebach, M.; Weglage, J. Frontal lobe-dependent functions in treated phenylketonuria: Blood phenylalanine concentrations and long-term deficits in adolescents young adults. J. Inherit. Metab. Dis. 2005, 28, 445–455. [Google Scholar] [CrossRef]
- Channon, S.; Mockler, C.; Lee, P. Executive Functioning and Speed of Processing in Phenylketonuria. Neuropsychology 2005, 19, 679–686. [Google Scholar] [CrossRef]
- Janos, A.L.; Grange, D.K.; Steiner, R.D.; White, D.A. Processing speed and executive abilities in children with phenylketonuria. Neuropsychology 2012, 26, 735–743. [Google Scholar] [CrossRef] [Green Version]
- De Felice, S.; Romani, C.; Geberhiwot, T.; Macdonald, A.; Palermo, L. Language processing and executive functions in early treated adults with phenylketonuria (PKU). Cogn. Neuropsychol. 2018, 35, 148–170. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. Wechsler Adult Intelligence Scale, 3rd ed.; The Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Wechsler, D. Wechsler Abbreviated Scale of Intelligence (WASI); Harcourt Assessment: San Antonio, TX, USA, 1999. [Google Scholar]
- Schrimsher, G.W.; O’Bryant, S.E.; O’Jile, J.R.; Sutker, P.B. Comparison of Tetradic WAIS-III Short Forms in Predicting Full Scale IQ Scores in Neuropsychiatric Clinic Settings. J. Psychopathol. Behav. Assess. 2007, 30, 235–240. [Google Scholar] [CrossRef]
- Trites, R. Grooved Pegboard Test; Lafayette Instrument: Lafayette, IN, USA, 1977. [Google Scholar]
- AITB. Army Individual Test. Battery. Manual of Directions and Scoring; War Department, Adjutant General’s Office: Washington, DC, USA, 1944. [Google Scholar]
- Sánchez-Cubillo, I.; A Periañez, J.; Adrover-Roig, D.; Rodríguez-Sánchez, J.; Rios-Lago, M.; Tirapu, J.; Barcelo, F. Construct validity of the Trail Making Test: Role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J. Int. Neuropsychol. Soc. 2009, 15, 438–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kongs, S.K.; Thompson, L.L.; Iverson, G.L.; Heaton, R.K. WCST-64: Wisconsin Card Sorting Test.-64 Card Version, Professional Manual; Psychological Assessment Resources: Odessa, FL, USA, 2000. [Google Scholar]
- Novelli, G.; Papagno, C.; Capitani, E.; Laiacona, M.; Vallar, G.; Cappa, S.F. Tre test clinici di ricerca e produzione lessicale. Taratura su soggetti normali. Arch. Neurol. Psychiatry 1986, 47, 477–506. [Google Scholar]
- Benton, A.L.; deS, K.; Sivan, A.B. Multilingual Aphasia Examination; AJA Associates: Iowa City, IA, USA, 1994. [Google Scholar]
- Rosen, W.G. Verbal fluency in aging and dementia. J. Clin. Neuropsychol. 1980, 2, 135–146. [Google Scholar] [CrossRef]
- Costa, A.; Bagoj, E.; Monaco, M.; Zabberoni, S.; De Rosa, S.; Papantonio, A.M.; Mundi, C.; Caltagirone, C.; Carlesimo, G.A. Standardization and normative data obtained in the Italian population for a new verbal fluency instrument, the phonemic/semantic alternate fluency test. Neurol. Sci. 2013, 35, 365–372. [Google Scholar] [CrossRef]
- Corsi, P.M. Human Memory and the Medial Temporal Region of the Brain. Unpublished Doctoral Dissertation, McGill University, Montreal, QC, Canada, 1972. [Google Scholar]
- Sahakian, B.J.; Jones, G.; Levy, R.; Gray, J.; Warburton, D. The Effects of Nicotine on Attention, Information Processing, and Short-Term Memory in Patients with Dementia of the Alzheimer Type. Br. J. Psychiatry 1989, 154, 797–800. [Google Scholar] [CrossRef]
- Rey, A. L’Examen Clinique en Psychologie (Clinical Examination in Psychology); Presses Universitaires de France: Paris, France, 1964. [Google Scholar]
- Schmidt, E.; Rupp, A.; Burgard, P.; Pietz, J.; Weglage, J.; De Sonneville, L.M.J. Sustained attention in adult phenylketonuria: The influence of the concurrent phenylalanine-blood-level. J. Clin. Exp. Neuropsychol. 1994, 16, 681–688. [Google Scholar] [CrossRef]
- Sahakian, B.J.; Morris, R.G.; Evenden, J.L.; Heald, A.; Levy, R.; Philpot, M.; Robbins, T. A Comparative Study of Visuospatial Memory and Learning in Alzheimer-Type Dementia and Parkinson’s Disease. Brain 1988, 111, 695–718. [Google Scholar] [CrossRef]
- Moyle, J.J.; Fox, A.M.; Arthur, M.; Bynevelt, M.; Burnett, J.R. Meta-Analysis of Neuropsychological Symptoms of Adolescents and Adults with PKU. Neuropsychol. Rev. 2007, 17, 91–101. [Google Scholar] [CrossRef]
- Smith, M.L.; Klim, P.; Mallozzi, E.; Hanley, W.B. A test of the frontal-specificity hypothesis in the cognitive performance of adults with phenylketonuria. Dev. Neuropsychol. 1996, 12, 327–341. [Google Scholar] [CrossRef]
- Ris, M.; Williams, S.E.; Hunt, M.M.; Berry, H.K.; Leslie, N. Early-treated phenylketonuria: Adult neuropsychologic outcome. J. Pediatr. 1994, 124, 388–392. [Google Scholar] [CrossRef]
- Channon, S.; German, E.; Cassina, C.; Lee, P. Executive Functioning, Memory, and Learning in Phenylketonuria. Neuropsychology 2004, 18, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Bik-Multanowski, M.; Pietrzyk, J.J.; Mozrzymas, R. Routine use of CANTAB system for detection of neuropsychological deficits in patients with PKU. Mol. Genet. Metab. 2011, 102, 210–213. [Google Scholar] [CrossRef]
- Griffiths, P.; Paterson, L.; Harvie, A. Neuropsychological effects of subsequent exposure to phenylalanine in adolescents and young adults with eariy-treated phenylketonuria. J. Intellect. Disabil. Res. 1995, 39, 365–372. [Google Scholar] [CrossRef]
- Pietz, J.; Dunckelmann, R.; Rupp, A.; Rating, D.; Meinck, H.-M.; Schmidt, H.; Bremer, H.J. Neurological outcome in adult patients with early-treated phenylketonuria. Eur. J. Nucl. Med. Mol. Imaging 1998, 157, 824–830. [Google Scholar] [CrossRef]
- Hofman, D.L.; Champ, C.L.; Lawton, C.L.; Henderson, M.; Dye, L. A systematic review of cognitive functioning in early treated adults with phenylketonuria. Orphanet J. Rare Dis. 2018, 13, 150. [Google Scholar] [CrossRef]
- Cleary, M.; Trefz, F.; Muntau, A.C.; Feillet, F.; Van Spronsen, F.J.; Burlina, A.; Bélanger-Quintana, A.; Gizewska, M.; Gasteyger, C.; Bettiol, E.; et al. Fluctuations in phenylalanine concentrations in phenylketonuria: A review of possible relationships with outcomes. Mol. Genet. Metab. 2013, 110, 418–423. [Google Scholar] [CrossRef]
- Bartus, A.; Palasti, F.; Juhasz, E.; Kiss, E.; Simonova, E.; Sumanszki, C.; Reismann, P. The influence of blood phenylalanine levels on neurocognitive function in adult PKU patients. Metab. Brain Dis. 2018, 33, 1609–1615. [Google Scholar] [CrossRef]
- Vilaseca, M.A.; Campistol, J.; Cambra, F.J.; Lambruschini, N. Index of dietary control of PKU patients. Quím. Clín. 1995, 14, 271. [Google Scholar]
- Vilaseca, M.A.; Lambruschini, N.; Gómez-López, L.; Gutiérrez, A.; Fusté, E.; Gassió, R.; Artuch, R.; Campistol, J. Quality of dietary control in phenylketonuric patients and its relationship with general intelligence. Nutr. Hosp. 2010, 25, 60–66. [Google Scholar] [PubMed]
- Feldmann, R.; Osterloh, J.; Onon, S.; Fromm, J.; Rutsch, F.; Weglage, J. Neurocognitive functioning in adults with phenylketonuria: Report of a 10-year follow-up. Mol. Genet. Metab. 2019, 126, 246–249. [Google Scholar] [CrossRef] [PubMed]
| Italian AwPKU | English AwPKU | English vs. Italian | |||
|---|---|---|---|---|---|
| N = 19 | N = 19 | p Value | |||
| Mean | SD | Mean | SD | ||
| Age | 25.4 (range: 19–33) | 4.1 | 25.3 (range: 18–36) | 6.1 | n.s. |
| Education (in years) | 14 | 1.8 | 14.6 | 1.9 | n.s. |
| Gender (M/F) | 8//11 | 8//11 | |||
| Verbal IQ | 98.8 | 12.9 | 100.4 | 8.9 | n.s. |
| Performance IQ | 99.3 | 15 | 103.3 | 12.9 | n.s. |
| Full IQ | 98.9 | 14.5 | 102.1 | 10.4 | n.s. |
| Childhood (0–10 years) | |||||
| Phe Average Median | 499 | 149 | 386 | 168 | p = 0.04 |
| Phe Fluctuation | 227 | 63 | 198 | 50 | n.s. |
| Mean N observationsper participant | 208 | 79 | 259 | 156 | n.s. |
| Adolescence (11–16 years) | |||||
| Phe Average Median | 702 | 194 | 612 | 293 | n.s. |
| Phe Fluctuation | 170 | 51 | 165 | 34 | n.s. |
| Mean N obs. per participant | 77 | 54 | 98 | 74 | n.s. |
| Adulthood (17 years +) | |||||
| Phe Average Median | 970 | 239 | 733 | 344 | p = 0.02 |
| Phe Variation | 217 | 65 | 122 | 41 | p < 0.001 |
| Mean N obs. per participant | 58 | 49 | 62 | 58 | n.s. |
| Lifetime | |||||
| Phe Average Median | 695 | 198 | 516 | 233 | p = 0.02 |
| Phe Fluctuation (SD) | 208 | 46 | 171 | 33 | p < 0.01 |
| Mean N observations. per participant | 344 | 149 | 419 | 232 | n.s. |
| Current Phe | 1042 | 428 | 677 | 382 | p = 0.01 |
| Range | 454–2081 | 65–1465 | |||
| ITALIAN PARTICIPANTS (N = 19 In Each Group) | ENGLISH PARTICIPANTS (N = 19 In Each Group) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AwPKU | Controls | Diff. | AwPKU | Controls | Diff. | |||||
| Mean | SD | Mean | SD | p Value | Mean | SD | Mean | SD | p Value | |
| Age | 25.4 | 4.1 | 24.7 | 3.4 | n.s. | 25.3 | 6.1 | 24.4 | 5.35 | n.s. |
| Education (in years) | 14.0 | 1.8 | 13.9 | 1.7 | n.s. | 14.6 | 1.9 | 14.8 | 1.7 | n.s. |
| Gender (M/F) | 8//11 | 8//11 | 7//12 | 6//13 | ||||||
| VIQ | 98.8 | 13 | 108.5 | 13.1 | 0.03 | 100.4 | 8.9 | 109.6 | 9.6 | <0.001 # |
| PIQ | 99.3 | 15 | 107.1 | 9.6 | 0.07 | 103.3 | 12.9 | 110.2 | 12.6 | n.s. |
| FIQ | 98.9 | 15 | 110.1 | 12.0 | 0.01 | 102.1 | 10.4 | 111.1 | 10.3 | 0.01 |
| VISUAL ATTENTION | ||||||||||
| Simple Detection (RT—ms) | 325 | 46 | 315 | 44.6 | n.s. | 331 | 50.7 | 304.9 | 51.2 | 0.06 |
| Detention with Distractors | ||||||||||
| RT—ms | 483 | 104 | 430 | 58 | 0.02 | 433 | 56.4 | 392 | 54.2 | 0.03 |
| % errors | 0.8 | 0.6 | 0.5 | 1.1 | n.s. | 0.8 | 0.8 | 0.5 | 0.6 | n.s. |
| Feature Search | ||||||||||
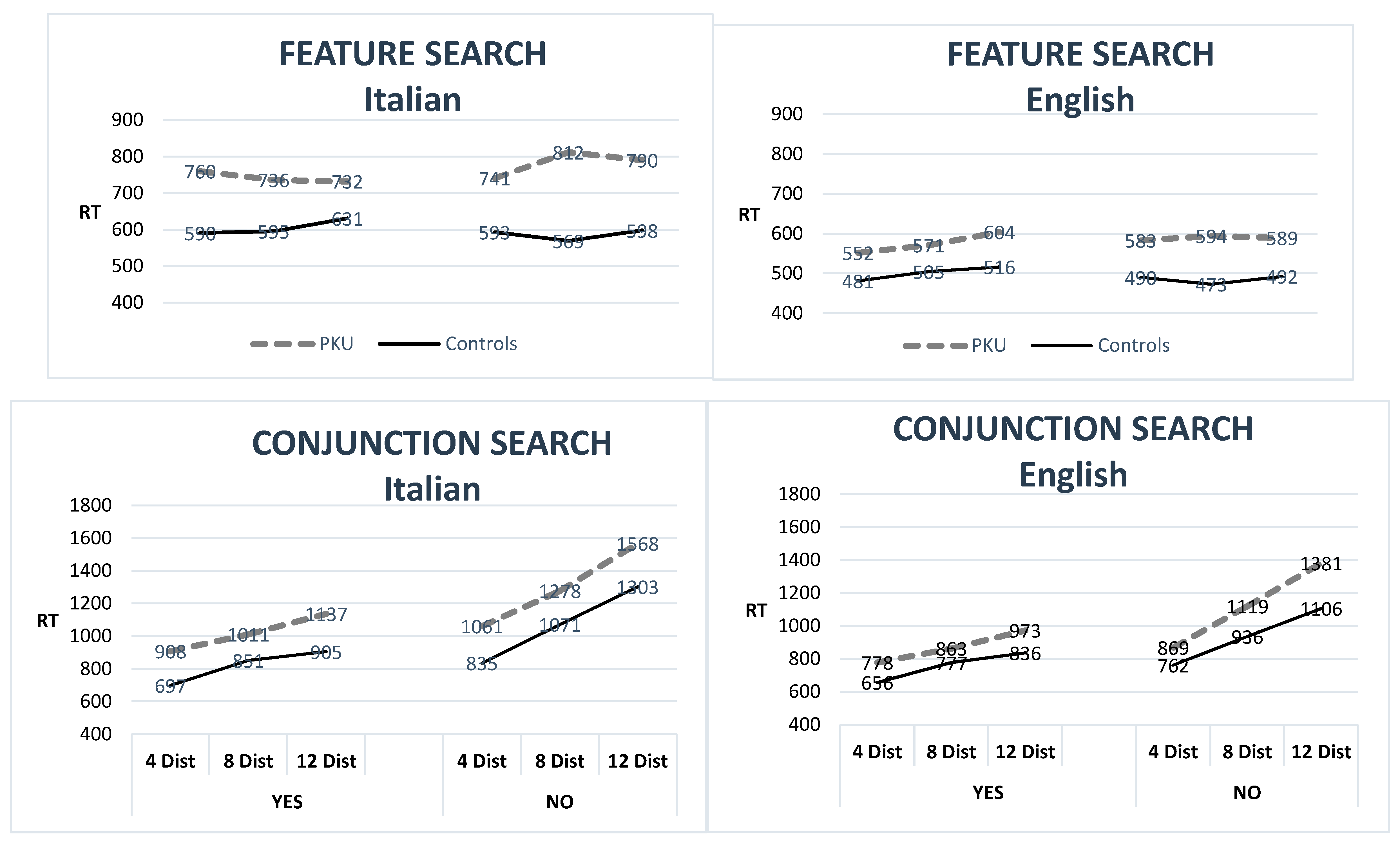
| RT—ms | 752 | 262 | 586 | 113 | 0.02 | 581 | 111 | 492.3 | 70.5 | 0.01 |
| % errors | 1.6 | 4.7 | 0.6 | 1.2 | n.s. | 1.9 | 2.6 | 2.3 | 2.3 | n.s. |
| Conjunction Search | ||||||||||
| RT—ms | 1152 | 237 | 938 | 203 | <0.001 # | 998 | 162 | 846 | 129.0 | <0.001 # |
| % errors | 3.2 | 4.7 | 2.8 | 4.7 | n.s. | 2 | 2.2 | 3.5 | 5.4 | n.s. |
| VISUO-MOTOR COORDINATION | ||||||||||
| Pegboard (Time–s) | 76.7 | 11 | 70.6 | 9.0 | 0.06 | 71.9 | 9.9 | 66.0 | 6.1 | 0.03 |
| Digit Symbol (%errors in 90 s) | 41.6 | 12 | 39.4 | 9.1 | <0.001 # | 37.1 | 11.6 | 27.9 | 10.3 | 0.01 |
| Trail-Making Test A (Time–s) | 32.4 | 15 | 29.9 | 12.1 | n.s. | 24.0 | 7.0 | 20.2 | 3.7 | 0.04 |
| EXECUTIVE FUNCTIONS | ||||||||||
| WCST | ||||||||||
| Total errors | 13.6 | 6.9 | 13.6 | 5.3 | n.s. | 13.8 | 8.6 | 11.9 | 5.5 | n.s. |
| Perseverative responses | 7.0 | 3.1 | 7.8 | 3.3 | n.s. | 7.9 | 5.1 | 7.5 | 5.3 | n.s. |
| N of Completed Categories | 4.0 | 1.1 | 4.1 | 1.0 | n.s. | 3.9 | 1.2 | 4.3 | 0.9 | n.s. |
| Trail-Making Test | ||||||||||
| B (sec) | 79.8 | 34 | 54.4 | 19.7 | 0.01 | 42 | 10.8 | 43.2 | 16.0 | n.s. |
| B–A (sec) | 47.5 | 29 | 24.4 | 13.1 | <0.001 # | 18 | 8.0 | 22.9 | 14.3 | n.s. |
| Verbal Fluency | ||||||||||
| Letter (correct answers) | 39.0 | 11 | 47.5 | 9.3 | 0.01 | 36.3 | 11.6 | 40.5 | 15.0 | n.s. |
| Semantic (correct answers) | 20.1 | 4.8 | 23.5 | 3.6 | 0.02 | 21.6 | 5.8 | 25.1 | 5.0 | 0.06 |
| Rey Auditory Verbal Learning | ||||||||||
| Retention after interference (% errors A6) | 17.2 | 13 | 8.4 | 7.6 | 0.02 | 19.6 | 18.3 | 15.1 | 16.9 | n.s. |
| SUSTAINED ATTENTION | ||||||||||
| RVP (% of errors) | 24.2 | 12 | 15.9 | 9.6 | 0.03 | 16.8 | 9.5 | 13.2 | 9.4 | n.s. |
| SHORT-TERM MEMORY | ||||||||||
| Digit span | 5.9 | 0.9 | 5.9 | 1.1 | n.s. | 6.2 | 0.8 | 6.5 | 0.9 | n.s. |
| Corsi Block span | 5.9 | 1.0 | 5.0 | 1.1 | 0.01 | 5.3 | 0.8 | 5.6 | 0.9 | n.s. |
| (opposite to expected) | ||||||||||
| MEMORY and LEARNING | ||||||||||
| Rey Auditory Verbal Learning | ||||||||||
| Trial A1–A5 (% errors) | 20.8 | 8.8 | 19.7 | 6.1 | n.s. | 24.5 | 11.4 | 21.7 | 9.3 | n.s. |
| Delayed Recall (% errors) | 10.2 | 11 | 4.9 | 7.0 | 0.08 | 18.6 | 18 | 14.7 | 14.8 | n.s. |
| Paired Associate Visual learning (% errors) | 2.3 | 2.2 | 1.2 | 1.3 | 0.07 | 2.5 | 3 | 1.8 | 1.8 | n.s. |
| Italian AwPK Z Score | English AwPKU Z Score | Diff. | |||
|---|---|---|---|---|---|
| DOMAIN/TASK | Mean | SD | Mean | SD | p |
| IQ | 1.0 | 1.0 | 0.9 | 1.0 | n.s. |
| VISUO-SPATIAL ATTENTION RTs | |||||
| Simple Detection—ms | 0.2 | 1.0 | 0.5 | 1.0 | n.s. |
| Detention with Distractors—ms | 1.3 | 2.1 | 0.8 | 1.0 | n.s. |
| Feature Search—ms | 1.5 | 2.3 | 1.3 | 1.6 | n.s. |
| Conjunction Search ms | 1.1 | 1.2 | 1.2 | 1.2 | n.s. |
| VISUAL ATTENTION accuracy | |||||
| Detention with Distractors—% errors | 0.2 | 0.6 | 0.5 | 1.3 | n.s. |
| Feature Search—% errors | 0.9 | 4.0 | −0.2 | 1.1 | n.s. |
| Conjunction Search—% errors | 0.1 | 1.0 | −0.3 | 0.4 | n.s. |
| VISUO-MOTOR COORDINATION | |||||
| Pegboard—s | 0.7 | 1.2 | 1.0 | 1.6 | n.s. |
| Digit Symbol—% errors in 90 s | 0.2 | 1.3 | 0.9 | 1.1 | n.s. |
| Trail-Making Test A—s | 0.2 | 1.2 | 1.0 | 1.9 | n.s. |
| EXECUTIVE FUNCTIONS | |||||
| WCST | |||||
| Total errors | 0.0 | 1.3 | 0.3 | 1.5 | n.s. |
| Perseverative responses | −0.3 | 0.8 | 0.1 | 1.0 | n.s. |
| N of Completed Categories | 0.1 | 1.0 | 0.3 | 1.3 | n.s. |
| Trail-Making Tests | |||||
| B (s) | 1.3 | 1.7 | −0.1 | 0.7 | <0.001# |
| B–A (s) | 1.8 | 2.2 | −0.3 | 0.6 | <0.001# |
| Verbal Fluency | |||||
| Letter (correct answers) | 0.9 | 1.1 | 0.3 | 0.8 | 0.05 |
| Semantic (correct answers) | 1.0 | 1.3 | 0.7 | 1.2 | n.s. |
| Rey Auditory Verbal Learning | |||||
| Retention after interference (% errors A6) | 1.1 | 1.7 | 0.3 | 1.0 | 0.07 |
| SUSTAINED ATTENTION | |||||
| RVP (% of errors) | 0.9 | 1.3 | 0.4 | 1.0 | n.s. |
| SHORT-TERM MEMORY | |||||
| Digit span | 0.0 | 0.8 | 0.3 | 0.9 | n.s. |
| Corsi Block span | −0.8 | 0.9 | 0.3 | 0.9 | <0.001# |
| LEARNING | |||||
| Rey Auditory Verbal Learning Test | |||||
| Trial A1–A5—% errors | 0.2 | 1.4 | 0.3 | 1.2 | n.s. |
| Delayed Recall—% errors | 0.8 | 1.5 | 0.3 | 1.2 | n.s. |
| Paired Associate Visual learning—% err | 0.9 | 1.7 | 0.4 | 1.7 | n.s. |
| OVERALL Z SCORE excluding IQ | |||||
| mean | 0.59 | 0.42 | n.s. | ||
| SD | 0.76 | 0.58 | |||
| VISUAL ATTENTION SPEED | VISUO-MOTOR | EF-MONITORING | SUST. | LEARNING AND MEMORY | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ITALIAN PKU | FSIQ | Simple | Detection | Feature | Conj. | Peg-Board | Digit | WCST | TMT | Digit | Corsi | Sem | ATTENT | Rey | Rey | Paired Ass. |
| PHE | RT | With distractors RT | Search RT | Search RT | Sec. | Symbol | Total err | b–a | Span | Span | Fluency | RVP % | Words a1–a5 | Words Delayed | Visual Learning | |
| 0–10 yrs | ||||||||||||||||
| Average | 0.38 | 0.43 | 0.42 | 0.15 | 0.30 | 0.05 | 0.28 | 0.19 | 0.55 * | 0.57 * | 0.37 | 0.03 | 0.43 | 0.17 | 0.08 | 0.30 |
| SD | 0.31 | 0.73 ** | 0.73 ** | 0.34 | 0.49 * | 0.02 | 0.13 | −0.10 | 0.55 * | 0.66 ** | 0.42 | 0.08 | 0.57 * | 0.34 | 0.25 | 0.36 |
| 11–16 yrs | ||||||||||||||||
| Average | 0.53 * | 0.08 | 0.30 | 0.41 | 0.35 | 0.18 | 0.38 | 0.28 | 0.44 | 0.32 | 0.29 | 0.41 | 0.63 ** | 0.14 | 0.08 | 0.49 * |
| SD | 0.02 | 0.29 | 0.32 | 0.37 | 0.20 | −0.25 | −0.10 | −0.08 | 0.20 | 0.16 | −0.04 | 0.38 | 0.37 | −0.18 | −0.29 | 0.30 |
| 17 yr to now | ||||||||||||||||
| Average | 0.58 ** | 0.03 | 0.37 | 0.38 | 0.41 | 0.32 | 0.60 ** | 0.59 ** | 0.56 * | 0.60 ** | 0.60 ** | 0.24 | 0.48 * | 0.11 | 0.09 | 0.30 |
| SD | 0.52 * | −0.06 | 0.06 | 0.16 | 0.22 | 0.29 | 0.43 | 0.46 * | 0.52 * | 0.38 | 0.28 | 0.21 | 0.33 | 0.05 | −0.11 | −0.20 |
| Current | 0.41 | −0.10 | 0.05 | 0.03 | 0.01 | 0.12 | 0.61 ** | 0.58 ** | 0.54 * | 0.60 ** | 0.37 | 0.00 | 0.30 | 0.21 | 0.16 | 0.11 |
| Lifetime | ||||||||||||||||
| Average | 0.55 * | 0.23 | 0.42 | 0.29 | 0.39 | 0.03 | 0.37 | 0.31 | 0.55 * | 0.58 ** | 0.33 | 0.23 | 0.58 * | 0.12 | 0.12 | 0.38 |
| SD | 0.47 * | 0.45 | 0.54 * | 0.40 | 0.45 | 0.05 | 0.24 | 0.16 | 0.60 * | 0.55 * | 0.35 | 0.28 | 0.58 * | 0.13 | 0.00 | 0.26 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romani, C.; Manti, F.; Nardecchia, F.; Valentini, F.; Fallarino, N.; Carducci, C.; De Leo, S.; MacDonald, A.; Palermo, L.; Leuzzi, V. Cognitive Outcomes and Relationships with Phenylalanine in Phenylketonuria: A Comparison between Italian and English Adult Samples. Nutrients 2020, 12, 3033. https://doi.org/10.3390/nu12103033
Romani C, Manti F, Nardecchia F, Valentini F, Fallarino N, Carducci C, De Leo S, MacDonald A, Palermo L, Leuzzi V. Cognitive Outcomes and Relationships with Phenylalanine in Phenylketonuria: A Comparison between Italian and English Adult Samples. Nutrients. 2020; 12(10):3033. https://doi.org/10.3390/nu12103033
Chicago/Turabian StyleRomani, Cristina, Filippo Manti, Francesca Nardecchia, Federica Valentini, Nicoletta Fallarino, Claudia Carducci, Sabrina De Leo, Anita MacDonald, Liana Palermo, and Vincenzo Leuzzi. 2020. "Cognitive Outcomes and Relationships with Phenylalanine in Phenylketonuria: A Comparison between Italian and English Adult Samples" Nutrients 12, no. 10: 3033. https://doi.org/10.3390/nu12103033
APA StyleRomani, C., Manti, F., Nardecchia, F., Valentini, F., Fallarino, N., Carducci, C., De Leo, S., MacDonald, A., Palermo, L., & Leuzzi, V. (2020). Cognitive Outcomes and Relationships with Phenylalanine in Phenylketonuria: A Comparison between Italian and English Adult Samples. Nutrients, 12(10), 3033. https://doi.org/10.3390/nu12103033








