Importance of Dietary Phosphorus for Bone Metabolism and Healthy Aging
Abstract
1. Introduction
2. Phosphate Absorption from the Diet in the Gut
2.1. Paracellular Phosphate Absorption Pathway
2.2. Transcellular Absorption Pathway/Transporter-Mediated Phosphate Absorption
3. Endocrine Regulation of Phosphate Homeostasis
3.1. Clinical Chemistry of Phosphate
3.2. Regulation of Phosphate Absorption in the Gut
3.2.1. Calcitriol
3.2.2. Phosphorus Depletion
3.2.3. Estrogen
3.2.4. Glucorticoids
3.2.5. Epidermal Growth Factor
4. Regulation of Systemic Phosphate Homeostasis
4.1. PTH
4.2. FGF23
4.3. Calcitriol
5. Disorders of Phosphate Homeostasis
5.1. Phosphorus Content in the Western Diet
5.2. Influence of Dietary Components and Drugs on the Bioaccessibility of Phosphate
5.3. Influence of Dietary Components, Drugs, and Disorders on the Bioavailability of Phosphate
5.4. Genetic Disorders of Intestinal Phosphate Absorption
5.5. Other Disorders of Phosphate Homeostasis
6. Metabolic Phosphate Sensing
6.1. Extracellular Phosphate Sensing
6.2. Intracellular Phosphate Sensing
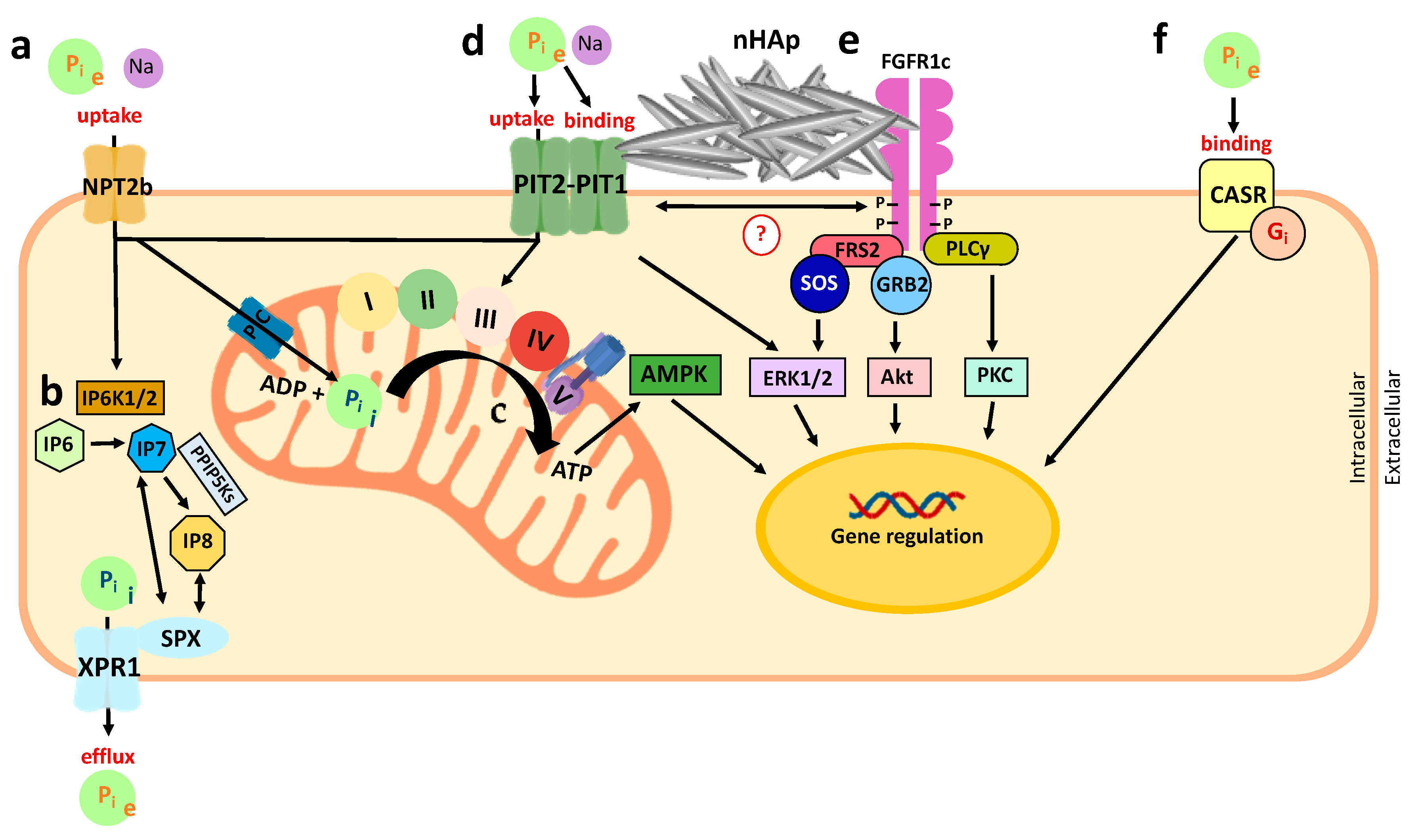
7. Importance of Dietary Phosphorus for Bone Health
7.1. General Importance of Phosphate for Bone Health
7.2. Role of Phosphate in Chondrocytes
7.3. Role of Phosphate in Osteoblasts and Osteocytes
7.4. Role of Phosphate in Osteoclasts and Bone Resorption
8. Importance of Dietary Phosphorus for Teeth (or Dental Health)
Role of Phosphate in the Tooth
9. Importance of Dietary Phosphorus for Cardiovascular Health
9.1. Role of Phosphate in Cardiac Muscle Function
9.2. Role of Phosphate in Vascular Health
9.3. Role of Phosphate in Erythrocyte Function
10. Importance of Dietary Phosphorus for Skeletal Muscle Health
Role of Phosphate in Skeletal Muscle
11. Importance of Dietary Phosphorus for Healthy Aging
11.1. High Dietary Phosphate Reduces Longevity in Lower Species
11.2. High Dietary Phosphorus Reduces Longevity in Higher Species and Humans
12. Conclusions
Key Points
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Glossary
| Key Term/Abbreviation | Definition |
| (I, II, III, IV, V) | Complexes I-V of the mitochondrial respiratory chain |
| 1,25-dihydroxyvitamin D | 1,25(OH)2D |
| 2′-PP | 2′-Phosphophloretin |
| 2,3-BPG | 2,3-Bisphosphoglycerate |
| AD | Autosomal dominant |
| AKT | Protein kinase B |
| Alp | Alkaline phosphatase |
| AMP | Adenosine monophosphate |
| AMPK | AMP-activated protein kinase |
| AR | Autosomal recessive |
| ATP | Adenosine triphosphate |
| BBMV | Brush border membrane vesicle |
| BPG | Bisphosphoglycerate |
| CASR | Calcium-sensing receptor |
| CKD | Chronic kidney disease |
| c-myb | V-myb avian myeloblastosis viral oncogene homolog |
| CrP | Creatine phosphate |
| CYP24A1 | The vitamin D 24-hydroxylase |
| CYP27B1 | The vitamin D 1-α hydroxylase |
| DMP1 | Dentin matrix acidic phosphoprotein 1 |
| DNA | Deoxyribonucleic acid |
| EGF | Epidermal growth factor |
| EGR1 | Early growth response 1 |
| Enpp1 | Ectonucleotide pyrophosphatase-phosphodiesterase family member 1 |
| ER | Endoplasmic reticulum |
| ERK1 | Extracellular signal-regulated kinase 1 |
| ERK2 | Extracellular signal-regulated kinase 2 |
| Etv5 | ETS variant 5 |
| FADH | Flavin adenine dinucleotide |
| FAM20c | Golgi-associated secretory pathway kinase |
| FAP | Fluorapatite mineral |
| FGF | Fibroblast growth factor |
| FGF23 | Fibroblast growth factor 23 |
| FGFR1 | Fibroblast growth factor receptor 1 |
| FGFR1c | FGFR1 isoform c |
| FRS2 | FGFR substrate 2 |
| GALNT3 | Polypeptide N-Acetylgalactosaminyltransferase 3 |
| GC | Glucocorticoid |
| GCMB | Glial cell missing gene |
| GI | Gastrointestinal |
| GNAS | Guanine nucleotide-binding protein, alpha stimulating |
| GRB2 | Growth factor receptor bound protein 2 |
| HVDDR | Hereditary 1,25(OH)2D-resistant rickets |
| IBGC | Idiopathic basal ganglia calcification |
| IC | Intracellular |
| ICF | Intracellular fluid |
| IU | International units |
| IP6K1 | Inositol hexakisphosphate kinase 1 |
| IP6K2 | Inositol hexakisphosphate kinase 2 |
| IP6 | Inositol hexakisphosphate |
| IP7 | 5-diphosphoinositol 1,2,3,4,6-pentakisphosphate |
| IP8 | 1,5-bisdiphosphoinositol 1,2,3,4-tetrakisphosphate |
| IV | Intravenous |
| JAM2 | Junctional adhesion molecule 2 |
| KL | α-Klotho |
| Km | Michaelis-Menten affinity constant |
| LOF | Loss of function |
| LVH | Left ventricular hypertrophy |
| MAPK | Mitogen-activated protein kinase |
| MAPK1 | Mitogen-activated protein kinase 1 |
| MAPK3 | Mitogen-activated protein kinase 3 |
| MEPE | Matrix extracellular phosphoglycoprotein |
| mPTP | Mitochondrial permeability transition pore |
| MV | Matrix vesicle |
| MYORG | Myogenesis regulating glycosidase |
| Na+ | Sodium ion |
| NAD | Nicotinamide adenine dinucleotide |
| NADH | Reduced nicotinamide adenine dinucleotide |
| NADPH | Reduced nicotinamide adenine dinucleotide phosphate |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NHE3 | Sodium/hydrogen exchanger isoform 3 |
| NHERF-1 | Na+/H+ exchanger regulatory factor |
| NOS | Nitric oxide synthase |
| NPT1 | Sodium-dependent phosphate transport protein 1 |
| NPT2a | Sodium-dependent phosphate transport protein 2a |
| NPT2b | Sodium-dependent phosphate transport protein 2b |
| NPT2c | Sodium-dependent phosphate transport protein 2c |
| NVU | Neurovascular unit |
| OPG | Osteoprotegerin |
| OPN | Osteopontin |
| P10 | Postnatal day 10 |
| PDGFB | Platelet-derived growth factor subunit B |
| PDGFRB | Platelet-derived growth factor receptor beta |
| PFA | Phosphonoformate/phosphonoformic acid |
| PFBC | Primary familial brain calcification |
| Phex | Phosphate-regulating endopeptidase homolog, X-linked |
| PHOSPHO1 | Phosphoethanolamine/phosphocholine phosphatase 1 |
| Pi | Inorganic phosphate |
| PIC | Mitochondrial phosphate carrier |
| PIT1 | Type III sodium-dependent phosphate transporter 1 |
| PIT2 | Type III sodium-dependent phosphate transporter 2 |
| PKA | Protein kinase A |
| PKC | Protein kinase C |
| PLCγ | Phospholipase C gamma isoform. |
| PPi | Pyrophosphate |
| PPIs | Proton pump inhibitors |
| PTH | Parathyroid hormone |
| PTHR1 | Parathyroid hormone 1 receptor |
| RANK | Receptor activator of NF-κB |
| RANKL | Receptor activator of NF-κB ligand |
| RDA | Recommended daily allowance |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| RXR | Retinoic acid X-receptor |
| SAMD9 | Sterile alpha motif domain containing 9 |
| SIBLING | Small integrin-binding ligand, N-linked glycoprotein |
| SLC17 | Solute carrier family 17 |
| SLC20A1 | Solute carrier family 20 member 1; gene encoding PIT1 |
| SLC20A2 | Solute carrier family 20 member 2; gene encoding PIT2 |
| SLC25A3 | Solute carrier family 25 member 3; gene encoding PIC |
| SLC34A1 | Solute carrier family 34 member 3; gene encoding NPT2a |
| SLC34A2 | Solute carrier family 34 member 2; gene encoding NPT2b |
| SLC34A3 | Solute carrier family 34 member 3; gene encoding NPT2c |
| SM | Standard medium |
| SOS | Son of sevenless |
| SPX | A protein domain named after SYG1/Pho81/XPR1 proteins |
| TEER | Transepithelial electrical resistance |
| TNAP | Tissue non-specific alkaline phosphatase |
| TNFRSF11B | TNF receptor superfamily member 11B |
| VATP | ATP flux |
| VDR | Vitamin D receptor |
| VRE | Vitamin D-reponsive elements |
| VSMC | Vascular smooth muscle cell |
| WNT | Wingless-related integration site |
| WT | Wild-type |
| XLH | X-linked hyperphosphatemia |
| XPR1 | Xenotropic and polytropic retrovirus receptor 1 |
References
- Fukumoto, S. Phosphate metabolism and vitamin d. Bonekey Rep. 2014, 3, 3. [Google Scholar] [CrossRef]
- Kher, K.; Schnaper, H.W.; Greenbaum, L.A. Clinical Pediatric Nephrology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Bevington, A.; Kemp, G.J.; Graham, R.; Russell, G. Phosphate-sensitive enzymes: Possible molecular basis for cellular disorders of phosphate metabolism. Clin. Chem. Enzym. Comms. 1992, 4, 235–257. [Google Scholar]
- Chakraborty, A.; Kim, S.; Snyder, S.H. Inositol pyrophosphates as mammalian cell signals. Sci. Signal. 2011, 4, re1. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.R.; Baev, A.Y.; Berezhnov, A.V.; Abramov, A.Y. Role of inorganic polyphosphate in mammalian cells: From signal transduction and mitochondrial metabolism to cell death. Biochem. Soc. Trans. 2016, 44, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Boullion, R. Vitamin d: From photosynthesis, metabolism, and action to clinical application. In Endocrinology; Elsevier Saunders: Philadelphia, PA, USA, 2006; pp. 1435–1463. [Google Scholar]
- Raisz, L.; Trummel, C.; Holick, M.; DeLuca, H. 1,25-dihydroxycholecalciferol: A potent stimulator of bone resorption in tissue culture. Science 1972, 175, 768–769. [Google Scholar] [CrossRef]
- Ravid, M.; Robson, M. Proximal myopathy caused by latrogenic phosphate depletion. JAMA 1976, 236, 1380–1381. [Google Scholar] [CrossRef]
- Halevy, J.; Bulvik, S. Severe hypophosphatemia in hospitalized patients. JAMA Intern. Med. 1988, 148, 153–155. [Google Scholar] [CrossRef]
- Goodman, W.G.; Goldin, J.; Kuizon, B.D.; Yoon, C.; Gales, B.; Sider, D.; Wang, Y.; Chung, J.; Emerick, A.; Greaser, L.; et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N. Engl. J. Med. 2000, 342, 1478–1483. [Google Scholar] [CrossRef]
- Hou, Y.; Li, X.; Sun, L.; Qu, Z.; Jiang, L.; Du, Y. Phosphorus and mortality risk in end-stage renal disease: A meta-analysis. Clin. Chim. Acta 2017, 474, 108–113. [Google Scholar] [CrossRef]
- O’Seaghdha, C.M.; Hwang, S.J.; Muntner, P.; Melamed, M.L.; Fox, C.S. Serum phosphorus predicts incident chronic kidney disease and end-stage renal disease. Nephrol. Dial. Transpl. 2011, 26, 2885–2890. [Google Scholar] [CrossRef]
- Sim, J.J.; Bhandari, S.K.; Smith, N.; Chung, J.; Liu, I.L.; Jacobsen, S.J.; Kalantar-Zadeh, K. Phosphorus and risk of renal failure in subjects with normal renal function. Am. J. Med. 2013, 126, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Bergwitz, C.; Juppner, H. Regulation of phosphate homeostasis by pth, vitamin d, and fgf23. Annu. Rev. Med. 2010, 61, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Manghat, P.; Sodi, R.; Swaminathan, R. Phosphate homeostasis and disorders. Ann. Clin. Biochem. 2014, 51, 631–656. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R.; Rengasamy, P. Ion interactions and constraints to plant nutrition in australian sodic soils. Aust. J. Soil Res. 1993, 31, 801–819. [Google Scholar] [CrossRef]
- Kawa, D.; Julkowska, M.M.; Sommerfeld, H.M.; Ter Horst, A.; Haring, M.A.; Testerink, C. Phosphate-dependent root system architecture responses to salt stress. Plant. Physiol. 2016, 172, 690–706. [Google Scholar] [CrossRef] [PubMed]
- Vorland, C.J.; Stremke, E.R.; Moorthi, R.N.; Hill Gallant, K.M. Effects of excessive dietary phosphorus intake on bone health. Curr. Osteoporos. Rep. 2017, 15, 473–482. [Google Scholar] [CrossRef]
- Karalis, M. Food and drug administration petition on food labeling: An update from the american dietetic association and national kidney foundation. J. Ren. Nutr. 2007, 17, 423–424. [Google Scholar] [CrossRef]
- Calvo, M.S.; Moshfegh, A.J.; Tucker, K.L. Assessing the health impact of phosphorus in the food supply: Issues and considerations. Adv. Nutr. 2014, 5, 104–113. [Google Scholar] [CrossRef]
- Carpenter, T.O.; Bergwitz, C.; Insogna, K.L. Chapter 20—Phosphorus homeostasis and related disorders. In Principles of Bone Biology, 4th ed.; Bilezikian, J.P., Martin, T.J., Clemens, T.L., Rosen, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 469–507. [Google Scholar]
- Goretti Penido, M.; Alon, U.S. Phosphate homeostasis and its role in bone health. Pediatr. Nephrol. 2012, 27, 2039–2048. [Google Scholar] [CrossRef]
- Eto, N.; Tomita, M.; Hayashi, M. Napi-mediated transcellular permeation is the dominant route in intestinal inorganic phosphate absorption in rats. Drug Metab. Pharm. 2006, 21, 217–221. [Google Scholar] [CrossRef]
- Berndt, T.; Kumar, R. Novel mechanisms in the regulation of phosphorus homeostasis. Physiology 2009, 24, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Murer, H.; Forster, I.; Biber, J. The sodium phosphate cotransporter family slc34. Pflug. Archiv. 2004, 447, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Murer, H.; Hernando, N.; Forster, I.; Biber, J. Proximal tubular phosphate reabsorption: Molecular mechanisms. Physiol. Rev. 2000, 80, 1373–1409. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.J.; Marks, J. Intestinal phosphate transport: A therapeutic target in chronic kidney disease and beyond? Pediatr. Nephrol. 2015, 30, 363–371. [Google Scholar] [CrossRef]
- Crook, M.; Swaminathan, R. Disorders of plasma phosphate and indications for its measurement. Ann. Clin. Biochem. 1996, 33, 376–396. [Google Scholar] [CrossRef]
- Benjamin, I.; Griggs, R.C.; Fitz, J.G.; Wing, E.J. Andreoli and Carpenter’s Cecil Essentials of Medicine E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Marks, J.; Lee, G.J.; Nadaraja, S.P.; Debnam, E.S.; Unwin, R.J. Experimental and regional variations in Na+-dependent and na+-independent phosphate transport along the rat small intestine and colon. Physiol. Rep. 2015, 3, e12281. [Google Scholar] [CrossRef]
- Knöpfel, T.; Himmerkus, N.; Günzel, D.; Bleich, M.; Hernando, N.; Wagner, C.A. Paracellular transport of phosphate along the intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G233–G241. [Google Scholar] [CrossRef]
- Hu, M.S.; Kayne, L.H.; Jamgotchian, N.; Ward, H.J.; Lee, D.B. Paracellular phosphate absorption in rat colon: A mechanism for enema-induced hyperphosphatemia. Miner. Electrolyte Metab. 1997, 23, 7–12. [Google Scholar]
- Marks, J. The role of slc34a2 in intestinal phosphate absorption and phosphate homeostasis. Pflug. Arch. 2019, 471, 165–173. [Google Scholar] [CrossRef]
- Hilfiker, H.; Hattenhauer, O.; Traebert, M.; Forster, I.; Murer, H.; Biber, J. Characterization of a murine type ii sodium-phosphate cotransporter expressed in mammalian small intestine. Proc. Natl. Acad. Sci. USA 1998, 95, 14564–14569. [Google Scholar] [CrossRef]
- Sabbagh, Y.; O’Brien, S.P.; Song, W.; Boulanger, J.H.; Stockmann, A.; Arbeeny, C.; Schiavi, S.C. Intestinal npt2b plays a major role in phosphate absorption and homeostasis. J. Am. Soc. Nephrol. 2009, 20, 2348–2358. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, Y.; Giral, H.; Caldas, Y.; Levi, M.; Schiavi, S.C. Intestinal phosphate transport. Adv. Chronic Kidney Dis. 2011, 18, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Giral, H.; Caldas, Y.; Sutherland, E.; Wilson, P.; Breusegem, S.; Barry, N.; Blaine, J.; Jiang, T.; Wang, X.X.; Levi, M. Regulation of rat intestinal na-dependent phosphate transporters by dietary phosphate. Am. J. Physiol. Ren. Physiol. 2009, 297, F1466–F1475. [Google Scholar] [CrossRef] [PubMed]
- Wilz, D.R.; Gray, R.W.; Dominguez, J.H.; Lemann, J., Jr. Plasma 1,25-(oh)2-vitamin d concentrations and net intestinal calcium, phosphate, and magnesium absorption in humans. Am. J. Clin. Nutr. 1979, 32, 2052–2060. [Google Scholar] [CrossRef]
- Hattenhauer, O.; Traebert, M.; Murer, H.; Biber, J. Regulation of small intestinal Na-p(i) type iib cotransporter by dietary phosphate intake. Am. J. Physiol. 1999, 277, G756–G762. [Google Scholar] [CrossRef]
- Forster, I.C.; Loo, D.D.F.; Eskandari, S. Stoichiometry and Na+ binding cooperativity of rat and flounder renal type II Na+-picotransporters. Am. J. Physiol. Ren. Physiol. 1999, 276, F644–F649. [Google Scholar] [CrossRef]
- Forster, I.C.; Virkki, L.; Bossi, E.; Murer, H.; Biber, J. Electrogenic kinetics of a mammalian intestinal type IIb Na+/Pi cotransporter. J. Membr. Biol. 2006, 212, 177–190. [Google Scholar] [CrossRef]
- Zhifeng, X.; Rejun, F.; Longchang, H.; Wenqing, S. Molecular cloning and functional characterization of swine sodium dependent phosphate cotransporter type II b (NaPi-IIb) gene. Mol. Biol. Rep. 2012, 39, 10557–10564. [Google Scholar] [CrossRef]
- Quarles, L.D. A systems biology preview of the relationships between mineral and metabolic complications in chronic kidney disease. Semin. Nephrol. 2013, 33, 130–142. [Google Scholar] [CrossRef]
- Brame, L.A.; White, K.E.; Econs, M.J. Renal phosphate wasting disorders: Clinical features and pathogenesis. Semin. Nephrol. 2004, 24, 39–47. [Google Scholar] [CrossRef]
- Lederer, E. Regulation of serum phosphate. J. Physiol. 2014, 592, 3985–3995. [Google Scholar] [CrossRef] [PubMed]
- Chande, S.; Bergwitz, C. Role of phosphate sensing in bone and mineral metabolism. Nat. Rev. Endocrinol. 2018, 14, 637–655. [Google Scholar] [CrossRef] [PubMed]
- Camalier, C.E.; Yi, M.; Yu, L.R.; Hood, B.L.; Conrads, K.A.; Lee, Y.J.; Lin, Y.; Garneys, L.M.; Bouloux, G.F.; Young, M.R.; et al. An integrated understanding of the physiological response to elevated extracellular phosphate. J. Cell Physiol. 2013, 228, 1536–1550. [Google Scholar] [CrossRef] [PubMed]
- Takashi, Y.; Fukumoto, S. Phosphate-sensing and regulatory mechanism of fgf23 production. J. Endocrinol. Investig. 2020, 43, 877–883. [Google Scholar] [CrossRef]
- Braegger, C.; Decsi, T.; Dias, J.A.; Hartman, C.; Kolacek, S.; Koletzko, B.; Koletzko, S.; Mihatsch, W.; Moreno, L.; Puntis, J.; et al. Practical approach to paediatric enteral nutrition: A comment by the espghan committee on nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 110–122. [Google Scholar] [CrossRef]
- Portale, A.A.; Halloran, B.P.; Morris, R.C., Jr. Dietary intake of phosphorus modulates the circadian rhythm in serum concentration of phosphorus. Implications for the renal production of 1,25-dihydroxyvitamin d. J. Clin. Investig. 1987, 80, 1147–1154. [Google Scholar] [CrossRef]
- Torres, P.A.U.; De Brauwere, D.P. Three feedback loops precisely regulating serum phosphate concentration. Kidney Int. 2011, 80, 443–445. [Google Scholar] [CrossRef]
- Zierold, C.; Darwish, H.M.; DeLuca, H.F. Two vitamin d response elements function in the rat 1,25-dihydroxyvitamin d 24-hydroxylase promoter. J. Biol. Chem. 1995, 270, 1675–1678. [Google Scholar] [CrossRef]
- Pike, J.W.; Meyer, M.B. The vitamin d receptor: New paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin d(3). Endocrinol. Metab. Clin. N. Am. 2010, 39, 255–269. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin d metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Kaneko, I.; Segawa, H.; Furutani, J.; Kuwahara, S.; Aranami, F.; Hanabusa, E.; Tominaga, R.; Giral, H.; Caldas, Y.; Levi, M.; et al. Hypophosphatemia in vitamin d receptor null mice: Effect of rescue diet on the developmental changes in renal Na+ -dependent phosphate cotransporters. Pflug. Arch. 2011, 461, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Marks, J.; Srai, S.K.; Biber, J.; Murer, H.; Unwin, R.J.; Debnam, E.S. Intestinal phosphate absorption and the effect of vitamin D: A comparison of rats with mice. Exp. Physiol. 2006, 91, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Segawa, H.; Kaneko, I.; Takahashi, A.; Kuwahata, M.; Ito, M.; Ohkido, I.; Tatsumi, S.; Miyamoto, K. Growth-related renal type II Na/Pi cotransporter. J. Biol. Chem. 2002, 277, 19665–19672. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Segawa, H.; Ito, M.; Kuwahata, M. Physiological regulation of renal sodium-dependent phosphate cotransporters. Jpn. J. Physiol. 2004, 54, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Urakawa, I.; Yamazaki, Y.; Hasegawa, H.; Hino, R.; Yoneya, T.; Takeuchi, Y.; Fujita, T.; Fukumoto, S.; Yamashita, T. Fgf-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type iia. Biochem. Biophys. Res. Commun. 2004, 314, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Capuano, P.; Radanovic, T.; Wagner, C.A.; Bacic, D.; Kato, S.; Uchiyama, Y.; St-Arnoud, R.; Murer, H.; Biber, J. Intestinal and renal adaptation to a low-pi diet of type II NaPi cotransporters in vitamin d receptor- and 1alphaohase-deficient mice. Am. J. Physiol. Cell Physiol. 2005, 288, C429–C434. [Google Scholar] [CrossRef]
- Xu, H.; Uno, J.K.; Inouye, M.; Xu, L.; Drees, J.B.; Collins, J.F.; Ghishan, F.K. Regulation of intestinal NaNi-IIb cotransporter gene expression by estrogen. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G1317–G1324. [Google Scholar] [CrossRef]
- Arima, K.; Hines, E.R.; Kiela, P.R.; Drees, J.B.; Collins, J.F.; Ghishan, F.K. Glucocorticoid regulation and glycosylation of mouse intestinal type IIb Na-Pi cotransporter during ontogeny. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G426–G434. [Google Scholar] [CrossRef]
- Biol, M.-C.; Lenoir, D.; Hugueny, I.; Louisot, P. Hormonal regulation of glycosylation process in rat small intestine: Responsiveness of fucosyl-transferase activity to hydrocortisone during the suckling period, unresponsiveness after weaning. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1992, 1133, 206–212. [Google Scholar] [CrossRef]
- Xu, H.; Collins, J.F.; Bai, L.; Kiela, P.R.; Ghishan, F.K. Regulation of the human sodium-phosphate cotransporter napi-iib gene promoter by epidermal growth factor. Am. J. Physiol. Cell Physiol. 2001, 280, C628–C636. [Google Scholar] [CrossRef]
- Xu, H.; Inouye, M.; Hines, E.R.; Collins, J.F.; Ghishan, F.K. Transcriptional regulation of the human NaPi-IIb cotransporter by egf in caco-2 cells involves c-myb. Am. J. Physiol. Cell Physiol. 2003, 284, C1262–C1271. [Google Scholar]
- Forster, I.C.; Hernando, N.; Biber, J.; Murer, H. Proximal tubular handling of phosphate: A molecular perspective. Kidney Int. 2006, 70, 1548–1559. [Google Scholar] [PubMed]
- Howard, G.A.; Bottemiller, B.L.; Turner, R.T.; Rader, J.I.; Baylink, D.J. Parathyroid hormone stimulates bone formation and resorption in organ culture: Evidence for a coupling mechanism. Proc. Natl. Acad. Sci. USA 1981, 78, 3204. [Google Scholar]
- Silva, B.C.; Bilezikian, J.P. Parathyroid hormone: Anabolic and catabolic actions on the skeleton. Curr. Opin. Pharm. 2015, 22, 41–50. [Google Scholar]
- Weinman, E.J.; Biswas, R.S.; Peng, Q.; Shen, L.; Turner, C.L.; E, X.; Steplock, D.; Shenolikar, S.; Cunningham, R. Parathyroid hormone inhibits renal phosphate transport by phosphorylation of serine 77 of sodium-hydrogen exchanger regulatory factor–1. J. Clin. Investig. 2007, 117, 3412–3420. [Google Scholar] [PubMed]
- Bacic, D.; Lehir, M.; Biber, J.; Kaissling, B.; Murer, H.; Wagner, C.A. The renal Na+/phosphate cotransporter napi-iia is internalized via the receptor-mediated endocytic route in response to parathyroid hormone. Kidney Int. 2006, 69, 495–503. [Google Scholar]
- Traebert, M.; Völkl, H.; Biber, J.; Murer, H.; Kaissling, B. Luminal and contraluminal action of 1–34 and 3–34 pth peptides on renal type iia na-pi cotransporter. Am. J. Physiol. Ren. Physiol. 2000, 278, F792–F798. [Google Scholar]
- Feng, J.Q.; Ward, L.M.; Liu, S.; Lu, Y.; Xie, Y.; Yuan, B.; Yu, X.; Rauch, F.; Davis, S.I.; Zhang, S.; et al. Loss of dmp1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat. Genet. 2006, 38, 1310–1315. [Google Scholar]
- Liu, S.; Guo, R.; Simpson, L.G.; Xiao, Z.-S.; Burnham, C.E.; Quarles, L.D. Regulation of fibroblastic growth factor 23 expression but not degradation by phex. J. Biol. Chem. 2003, 278, 37419–37426. [Google Scholar]
- Lu, Y.; Feng, J.Q. Fgf23 in skeletal modeling and remodeling. Curr. Osteoporos. Rep. 2011, 9, 103–108. [Google Scholar]
- Shimada, T.; Hasegawa, H.; Yamazaki, Y.; Muto, T.; Hino, R.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Fukumoto, S.; Yamashita, T. Fgf-23 is a potent regulator of vitamin d metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004, 19, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Farrow, E.G.; White, K.E. Recent advances in renal phosphate handling. Nat. Rev. Nephrol. 2010, 6, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Burnett, S.M.; Gunawardene, S.C.; Bringhurst, F.R.; Jüppner, H.; Lee, H.; Finkelstein, J.S. Regulation of c-terminal and intact fgf-23 by dietary phosphate in men and women. J. Bone Miner. Res. 2006, 21, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yokote, H.; Jing, X.; Yao, L.; Sawada, T.; Zhang, Y.; Liang, S.; Sakaguchi, K. Fibroblast growth factor 23 reduces expression of type iia na+/pi co-transporter by signaling through a receptor functionally distinct from the known fgfrs in opossum kidney cells. Genes Cells 2005, 10, 489–502. [Google Scholar] [CrossRef]
- Chen, G.; Liu, Y.; Goetz, R.; Fu, L.; Jayaraman, S.; Hu, M.C.; Moe, O.W.; Liang, G.; Li, X.; Mohammadi, M. Alpha-klotho is a non-enzymatic molecular scaffold for fgf23 hormone signalling. Nature 2018, 553, 461–466. [Google Scholar] [CrossRef]
- Urakawa, I.; Yamazaki, Y.; Shimada, T.; Iijima, K.; Hasegawa, H.; Okawa, K.; Fujita, T.; Fukumoto, S.; Yamashita, T. Klotho converts canonical fgf receptor into a specific receptor for fgf23. Nature 2006, 444, 770–774. [Google Scholar] [CrossRef]
- Andrukhova, O.; Zeitz, U.; Goetz, R.; Mohammadi, M.; Lanske, B.; Erben, R.G. Fgf23 acts directly on renal proximal tubules to induce phosphaturia through activation of the erk1/2-sgk1 signaling pathway. Bone 2012, 51, 621–628. [Google Scholar] [CrossRef]
- Liu, S.; Tang, W.; Zhou, J.; Stubbs, J.R.; Luo, Q.; Pi, M.; Quarles, L.D. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J. Am. Soc. Nephrol. 2006, 17, 1305–1315. [Google Scholar] [CrossRef]
- Lang, F.; Leibrock, C.; Pandyra, A.A.; Stournaras, C.; Wagner, C.A.; Föller, M. Phosphate homeostasis, inflammation and the regulation of fgf-23. Kidney Blood Press. Res. 2018, 43, 1742–1748. [Google Scholar] [CrossRef]
- Gattineni, J.; Friedman, P.A. Regulation of hormone-sensitive renal phosphate transport. Vitam. Horm. 2015, 98, 249–306. [Google Scholar]
- Norman, A.W. The history of the discovery of vitamin d and its daughter steroid hormone. Ann. Nutr. Metab. 2012, 61, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Petkovich, M.; Jones, G. Cyp24a1 and kidney disease. Curr. Opin. Nephrol. Hypertens. 2011, 20, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Prosser, D.E.; Kaufmann, M. 25-hydroxyvitamin d-24-hydroxylase (cyp24a1): Its important role in the degradation of vitamin D. Arch. Biochem. Biophys. 2012, 523, 9–18. [Google Scholar] [CrossRef]
- Carpenter, T.O.; Shiratori, T. Renal 25-hydroxyvitamin d-1 alpha-hydroxylase activity and mitochondrial phosphate transport in hyp mice. Am. J. Physiol. 1990, 259, E814–E821. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, M.; Lee, S.M.; Pike, J.W.; Jones, G. A high-calcium and phosphate rescue diet and vdr-expressing transgenes normalize serum vitamin d metabolite profiles and renal cyp27b1 and cyp24a1 expression in vdr null mice. Endocrinology 2015, 156, 4388–4397. [Google Scholar] [CrossRef]
- Sahay, M.; Sahay, R.K. Refractory rickets in tropics. J. Pediatr. Endocrinol. Metab. 2010, 23, 597. [Google Scholar] [CrossRef]
- Duncan, W.E. Chapter 11—Osteomalacia and rickets. In Endocrine Secrets, 5th ed.; McDermott, M.T., Ed.; Mosby: Philadelphia, PA, USA, 2009; pp. 110–116. [Google Scholar]
- Knochel, J.P.; Barcenas, C.; Cotton, J.R.; Fuller, T.J.; Haller, R.; Carter, N.W. Hypophosphatemia and rhabdomyolysis. J. Clin. Investig. 1978, 62, 1240–1246. [Google Scholar] [CrossRef]
- Darsee, J.R.; Nutter, D.O. Reversible severe congestive cardiomyopathy in three cases of hypophosphatemia. Ann. Intern. Med. 1978, 89, 867–870. [Google Scholar] [CrossRef]
- Craddock, P.R.; Yawata, Y.; VanSanten, L.; Gilberstadt, S.; Silvis, S.; Jacob, H.S. Acquired phagocyte dysfunction. A complication of the hypophosphatemia of parenteral hyperalimentation. N. Engl. J. Med. 1974, 290, 1403–1407. [Google Scholar] [CrossRef]
- Sullivan, C.; Sayre, S.S.; Leon, J.B.; Machekano, R.; Love, T.E.; Porter, D.; Marbury, M.; Sehgal, A.R. Effect of food additives on hyperphosphatemia among patients with end-stage renal disease: A randomized controlled trial. JAMA 2009, 301, 629–635. [Google Scholar] [CrossRef]
- León, J.B.; Sullivan, C.M.; Sehgal, A.R. The prevalence of phosphorus-containing food additives in top-selling foods in grocery stores. J. Ren. Nutr. 2013, 23, 265–270.e262. [Google Scholar] [CrossRef]
- Cupisti, A.; Kalantar-Zadeh, K. Management of natural and added dietary phosphorus burden in kidney disease. Semin. Nephrol. 2013, 33, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Carrigan, A.; Klinger, A.; Choquette, S.S.; Luzuriaga-McPherson, A.; Bell, E.K.; Darnell, B.; Gutiérrez, O.M. Contribution of food additives to sodium and phosphorus content of diets rich in processed foods. J. Ren. Nutr. 2014, 24, 13–19.e11. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific opinion on dietary reference values for phosphorus. EFSA J. 2015, 13, 4185. [Google Scholar]
- Hannah, J.; Roe, M.; Warthon-Medina, M.; Pinchen, H.; Barrett, M.; Perry, S. Phosphorus in food: Limitations of food composition data. J. Kidney Care 2018, 3, 362–367. [Google Scholar] [CrossRef]
- Chang, A.R.; Lazo, M.; Appel, L.J.; Gutierrez, O.M.; Grams, M.E. High dietary phosphorus intake is associated with all-cause mortality: Results from NHANES III. Am. J. Clin. Nutr. 2014, 99, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, T.O.; Imel, E.A.; Holm, I.A.; Jan de Beur, S.M.; Insogna, K.L. A clinician’s guide to x-linked hypophosphatemia. J. Bone Miner. Res. 2011, 26, 1381–1388. [Google Scholar] [CrossRef]
- Liamis, G.; Milionis, H.J.; Elisaf, M. Medication-induced hypophosphatemia: A review. QJM An. Int. J. Med. 2010, 103, 449–459. [Google Scholar] [CrossRef]
- Disorders of phosphate homeostasis. In Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism; Wiley: Hoboken, NJ, USA, 2008; pp. 674–683.
- FFass, R.; Do, S.; Hixson, L.J. Fatal hyperphosphatemia following fleet phospo-soda in a patient with colonic ileus. Am. J. Gastroenterol. 1993, 88, 929–932. [Google Scholar]
- Fine, A.; Patterson, J. Severe hyperphosphatemia following phosphate administration for bowel preparation in patients with renal failure: Two cases and a review of the literature. Am. J. Kidney Dis. 1997, 29, 103–105. [Google Scholar] [CrossRef]
- Parikh, M.J.; Dumas, G.; Silvestri, A.; Bistrian, B.R.; Driscoll, D.F. Physical compatibility of neonatal total parenteral nutrient admixtures containing organic calcium and inorganic phosphate salts. Am. J. Health Syst. Pharm. 2005, 62, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- MacKay, M.; Jackson, D.; Eggert, L.; Fitzgerald, K.; Cash, J. Practice-based validation of calcium and phosphorus solubility limits for pediatric parenteral nutrition solutions. Nutr. Clin. Pract. 2011, 26, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Ballesteros, L.F.; Ma, N.S.; Gordon, R.J.; Ward, L.; Backeljauw, P.; Wasserman, H.; Weber, D.R.; DiMeglio, L.A.; Gagne, J.; Stein, R.; et al. Unexpected widespread hypophosphatemia and bone disease associated with elemental formula use in infants and children. Bone 2017, 97, 287–292. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Candeal, E.; Caldas, Y.A.; Guillén, N.; Levi, M.; Sorribas, V. Intestinal phosphate absorption is mediated by multiple transport systems in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G355–G366. [Google Scholar] [CrossRef]
- Lotz, M.; Zisman, E.; Bartter, F.C. Evidence for a phosphorus-depletion syndrome in man. N. Engl. J. Med. 1968, 278, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Chines, A.; Pacifici, R. Antacid and sucralfate-induced hypophosphatemic osteomalacia: A case report and review of the literature. Calcif. Tissue Int. 1990, 47, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.-H.; Booth, C.J.; Choi, H.S.; Lee, J.; Kang, J.; Hur, J.; Jung, W.J.; Jung, Y.-S.; Choi, H.J.; Kim, H.; et al. High-phytate/low-calcium diet is a risk factor for crystal nephropathies, renal phosphate wasting, and bone loss. eLife 2020, 9, e52709. [Google Scholar] [CrossRef]
- Kim, O.H.; Kim, Y.O.; Shim, J.H.; Jung, Y.S.; Jung, W.J.; Choi, W.C.; Lee, H.; Lee, S.J.; Kim, K.K.; Auh, J.H.; et al. Β-propeller phytase hydrolyzes insoluble Ca(2+)-phytate salts and completely abrogates the ability of phytate to chelate metal ions. Biochemistry 2010, 49, 10216–10227. [Google Scholar] [CrossRef]
- Villa-Bellosta, R.; Sorribas, V. Role of rat sodium/phosphate cotransporters in the cell membrane transport of arsenate. Toxicol. Appl. Pharmacol. 2008, 232, 125–134. [Google Scholar] [CrossRef]
- Peerce, B.E.; Clarke, R. A phosphorylated phloretin derivative. Synthesis and effect on intestinal na+-dependent phosphate absorption. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G848–G855. [Google Scholar] [CrossRef]
- Katai, K.; Tanaka, H.; Tatsumi, S.; Fukunaga, Y.; Genjida, K.; Morita, K.; Kuboyama, N.; Suzuki, T.; Akiba, T.; Miyamoto, K.; et al. Nicotinamide inhibits sodium-dependent phosphate cotransport activity in rat small intestine. Nephrol. Dial. Transpl. 1999, 14, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Sorribas, V.; Guillén, N.; Sosa, C. Substrates and inhibitors of phosphate transporters: From experimental tools to pathophysiological relevance. Pflug. Arch. 2019, 471, 53–65. [Google Scholar] [CrossRef]
- Matsuo, A.; Negoro, T.; Seo, T.; Kitao, Y.; Shindo, M.; Segawa, H.; Miyamoto, K. Inhibitory effect of jtp-59557, a new triazole derivative, on intestinal phosphate transport in vitro and in vivo. Eur. J. Pharm. 2005, 517, 111–119. [Google Scholar] [CrossRef] [PubMed]
- King, A.J.; Siegel, M.; He, Y.; Nie, B.; Wang, J.; Koo-McCoy, S.; Minassian, N.A.; Jafri, Q.; Pan, D.; Kohler, J.; et al. Inhibition of sodium/hydrogen exchanger 3 in the gastrointestinal tract by tenapanor reduces paracellular phosphate permeability. Sci. Transl. Med. 2018, 10, eaam6474. [Google Scholar] [CrossRef]
- Del Valle, H.B.; Yaktine, A.L.; Taylor, C.L.; Ross, A.C. Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Marcinowska-Suchowierska, E.; Kupisz-Urbańska, M.; Łukaszkiewicz, J.; Płudowski, P.; Jones, G. Vitamin D toxicity-a clinical perspective. Front. Endocrinol. 2018, 9, 550. [Google Scholar] [CrossRef] [PubMed]
- Joist, H.E.; Ahya, S.N.; Giles, K.; Norwood, K.; Slatopolsky, E.; Coyne, D.W. Differential effects of very high doses of doxercalciferol and paricalcitol on serum phosphorus in hemodialysis patients. Clin. Nephrol. 2006, 65, 335–341. [Google Scholar] [CrossRef]
- Steingrimsdottir, L.; Gunnarsson, O.; Indridason, O.S.; Franzson, L.; Sigurdsson, G. Relationship between serum parathyroid hormone levels, vitamin d sufficiency, and calcium intake. JAMA 2005, 294, 2336–2341. [Google Scholar] [CrossRef]
- Vanhooke, J.L.; Prahl, J.M.; Kimmel-Jehan, C.; Mendelsohn, M.; Danielson, E.W.; Healy, K.D.; DeLuca, H.F. Cyp27b1 null mice with laczreporter gene display no 25-hydroxyvitamin d3-1alpha-hydroxylase promoter activity in the skin. Proc. Natl. Acad. Sci. USA 2006, 103, 75–80. [Google Scholar] [CrossRef]
- Jacquillet, G.; Unwin, R.J. Physiological regulation of phosphate by vitamin d, parathyroid hormone (pth) and phosphate (pi). Pflug. Arch. Eur. J. Physiol. 2019, 471, 83–98. [Google Scholar] [CrossRef]
- Fine, K.D.; Seidel, R.H.; Do, K. The prevalence, anatomic distribution, and diagnosis of colonic causes of chronic diarrhea. Gastrointest. Endosc. 2000, 51, 318–326. [Google Scholar] [CrossRef]
- Sharma, S.; Hashmi, M.F.; Castro, D. Hypophosphatemia; Statpearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Stauber, A.; Radanovic, T.; Stange, G.; Murer, H.; Wagner, C.A.; Biber, J. Regulation of intestinal phosphate transport ii. Metabolic acidosis stimulates na+-dependent phosphate absorption and expression of the na+-pi cotransporter napi-iib in small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G501–G506. [Google Scholar] [CrossRef] [PubMed]
- Nagant de Deuxchaisnes, C.; Devogelaer, J.P. Vitamin d deficiency in elderly people. BMJ 1991, 303, 718. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vervloet, M.G.; Sezer, S.; Massy, Z.A.; Johansson, L.; Cozzolino, M.; Fouque, D.; on behalf of the ERA–EDTA Working Group on Chronic Kidney Disease–Mineral and Bone Disorders and the European Renal Nutrition Working Group. The role of phosphate in kidney disease. Nat. Rev. Nephrol. 2017, 13, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Kulnigg-Dabsch, S. Autoimmune gastritis. Wien. Med. Wochenschr. 2016, 166, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Lahner, E.; Annibale, B. Pernicious anemia: New insights from a gastroenterological point of view. World J. Gastroenterol. 2009, 15, 5121–5128. [Google Scholar] [CrossRef]
- Edmonston, D.; Wolf, M. Fgf23 at the crossroads of phosphate, iron economy and erythropoiesis. Nat. Rev. Nephrol. 2020, 16, 7–19. [Google Scholar] [CrossRef]
- Basile, D.P.; Anderson, M.D.; Sutton, T.A. Pathophysiology of acute kidney injury. Compr. Physiol. 2012, 2, 1303–1353. [Google Scholar]
- Moe, O.W.; Seldin, D.W.; Baum, M. Chapter 10—The fanconi syndrome. In Genetic Diseases of the Kidney; Lifton, R.P., Somlo, S., Giebisch, G.H., Seldin, D.W., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 171–197. [Google Scholar]
- Keefe, P.; Bokhari, S.R.A. Fanconi Syndrome; Statpearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Chapter 8—Disorders of calcium, magnesium, and phosphate balance. In Pocket Companion to Brenner and Rector’s the Kidney, 8th ed.; Clarkson, M.R., Magee, C.N., Brenner, B.M., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2011; pp. 166–185. [Google Scholar]
- Bandeira, F.; Cusano, N.E.; Silva, B.C.; Cassibba, S.; Almeida, C.B.; Machado, V.C.; Bilezikian, J.P. Bone disease in primary hyperparathyroidism. Arq. Bras. Endocrinol. Metab. 2014, 58, 553–561. [Google Scholar] [CrossRef]
- Villa-Bellosta, R.; Sorribas, V. Arsenate transport by sodium/phosphate cotransporter type iib. Toxicol. Appl. Pharmacol. 2010, 247, 36–40. [Google Scholar] [CrossRef]
- Ginsberg, C.; Ix, J.H. Nicotinamide and phosphate homeostasis in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2016, 25, 285–291. [Google Scholar] [CrossRef]
- Riley, M.S.; Schade, D.S.; Eaton, R.P. Effects of insulin infusion on plasma phosphate in diabetic patients. Metabolism 1979, 28, 191–194. [Google Scholar] [CrossRef]
- Boivin, G.Y.; Chavassieux, P.M.; Santora, A.C.; Yates, J.; Meunier, P.J. Alendronate increases bone strength by increasing the mean degree of mineralization of bone tissue in osteoporotic women. Bone 2000, 27, 687–694. [Google Scholar] [CrossRef]
- Boivin, G.; Meunier, P.J. Effects of bisphosphonates on matrix mineralization. J. Musculoskelet. Neuronal Interact. 2002, 2, 538–543. [Google Scholar]
- Müller, M.; Cheneval, D.; Carafoli, E. Doxorubicin inhibits the phosphate-transport protein reconstituted in liposomes. Eur. J. Biochem. 1984, 140, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.B.; Thakkar, S.; Hix, J.K.; Bhandarkar, N.D.; Wong, A.; Schreiber, M.J. Pathophysiology, clinical consequences, and treatment of tumor lysis syndrome. Am. J. Med. 2004, 116, 546–554. [Google Scholar] [CrossRef]
- Bosch, X.; Poch, E.; Grau, J.M. Rhabdomyolysis and acute kidney injury. N. Engl. J. Med. 2009, 361, 62–72. [Google Scholar] [CrossRef]
- Waning, D.L.; Guise, T.A. Molecular mechanisms of bone metastasis and associated muscle weakness. Clin. Cancer Res. 2014, 20, 3071–3077. [Google Scholar] [CrossRef]
- Hruska, K.A.; Mathew, S.; Lund, R.; Qiu, P.; Pratt, R. Hyperphosphatemia of chronic kidney disease. Kidney Int. 2008, 74, 148–157. [Google Scholar] [CrossRef]
- Wohrle, S.; Henninger, C.; Bonny, O.; Thuery, A.; Beluch, N.; Hynes, N.E.; Guagnano, V.; Sellers, W.R.; Hofmann, F.; Kneissel, M.; et al. Pharmacological inhibition of fibroblast growth factor (fgf) receptor signaling ameliorates fgf23-mediated hypophosphatemic rickets. J. Bone Miner. Res. 2013, 28, 899–911. [Google Scholar] [CrossRef]
- Huqun; Izumi, S.; Miyazawa, H.; Ishii, K.; Uchiyama, B.; Ishida, T.; Tanaka, S.; Tazawa, R.; Fukuyama, S.; Tanaka, T.; et al. Mutations in the slc34a2 gene are associated with pulmonary alveolar microlithiasis. Am. J. Respir. Crit. Care Med. 2007, 175, 263–268. [Google Scholar] [CrossRef]
- Corut, A.; Senyigit, A.; Ugur, S.A.; Altin, S.; Ozcelik, U.; Calisir, H.; Yildirim, Z.; Gocmen, A.; Tolun, A. Mutations in SLC34A2 Cause Pulmonary Alveolar Microlithiasis and Are Possibly Associated with Testicular Microlithiasis. Am. J. Hum. Genet. 2006, 79, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Tiosano, D.; Gepstein, V. Vitamin D action: Lessons learned from hereditary 1,25-dihydroxyvitamin-d-resistant rickets patients. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, E.; Zou, M.; Al-Rijjal, R.A.; Bircan, İ.; Akçurin, S.; Meyer, B.; Shi, Y. Clinical and genetic analysis of patients with vitamin d-dependent rickets type 1a. Clin. Endocrinol. 2012, 77, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Christov, M.; Jüppner, H. Phosphate homeostasis disorders. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 685–706. [Google Scholar] [CrossRef] [PubMed]
- Topaz, O.; Shurman, D.L.; Bergman, R.; Indelman, M.; Ratajczak, P.; Mizrachi, M.; Khamaysi, Z.; Behar, D.; Petronius, D.; Friedman, V.; et al. Mutations in galnt3, encoding a protein involved in o-linked glycosylation, cause familial tumoral calcinosis. Nat. Genet. 2004, 36, 579. [Google Scholar] [CrossRef] [PubMed]
- Frishberg, Y.; Araya, K.; Rinat, C.; Topaz, O.; Yamazaki, Y.; Feinstein, Y.; Navon-Elkan, P.; Becker-Cohen, R.; Yamashita, T.; Igarashi, T.; et al. Hyperostosis-Hyperphosphatemia Syndrome Caused by Mutations in Galnt3 and Associated with Augmented Processing of fgf-23; American Society of Nephrology: Philadelphia, PA, USA, 2004; p. F-P0937. [Google Scholar]
- Benet-Pages, A.; Orlik, P.; Strom, T.M.; Lorenz-Depiereux, B. An fgf23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum. Mol. Genet. 2005, 14, 385–390. [Google Scholar] [CrossRef]
- Ichikawa, S.; Sorenson, A.H.; Imel, E.A.; Friedman, N.E.; Gertner, J.M.; Econs, M.J. Intronic deletions in the slc34a3 gene cause hereditary hypophosphatemic rickets with hypercalciuria. J. Clin. Endocrinol. Metab. 2006, 91, 4022–4027. [Google Scholar] [CrossRef]
- Ichikawa, S.; Imel, E.A.; Kreiter, M.L.; Yu, X.; Mackenzie, D.S.; Sorenson, A.H.; Goetz, R.; Mohammadi, M.; White, K.E.; Econs, M.J. A homozygous missense mutation in human klotho causes severe tumoral calcinosis. J. Clin. Investig. 2007, 117, 2684–2691. [Google Scholar] [CrossRef]
- Mankin, H.J. Hyperphosphatasia, idiopathic. In Encyclopedia of Molecular Mechanisms of Disease; Lang, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 926–927. [Google Scholar]
- Weinstein, L.S.; Gejman, P.V.; de Mazancourt, P.; American, N.; Spiegel, A.M. A heterozygous 4-bp deletion mutation in the gs alpha gene (gnas1) in a patient with albright hereditary osteodystrophy. Genomics 1992, 13, 1319–1321. [Google Scholar] [CrossRef]
- Juppner, H.; Schipani, E.; Bastepe, M.; Cole, D.E.; Lawson, M.L.; Mannstadt, M.; Hendy, G.N.; Plotkin, H.; Koshiyama, H.; Koh, T.; et al. The gene responsible for pseudohypoparathyroidism type ib is paternally imprinted and maps in four unrelated kindreds to chromosome 20q13.3. Proc. Natl. Acad. Sci. USA 1998, 95, 11798–11803. [Google Scholar] [CrossRef]
- Arnold, A.; Horst, S.A.; Gardella, T.J.; Baba, H.; Levine, M.A.; Kronenberg, H.M. Mutation of the signal peptide-encoding region of the preproparathyroid hormone gene in familial isolated hypoparathyroidism. J. Clin. Investig. 1990, 86, 1084–1087. [Google Scholar] [CrossRef]
- Pollak, M.R.; Brown, E.M.; Estep, H.L.; McLaine, P.N.; Kifor, O.; Park, J.; Hebert, S.C.; Seidman, C.E.; Seidman, J.G. Autosomal dominant hypocalcaemia caused by a Ca2+-sensing receptor gene mutation. Nat. Genet. 1994, 8, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Buckingham, B.; Levine, M.A. Familial isolated hypoparathyroidism caused by a mutation in the gene for the transcription factor gcmb. J. Clin. Investig. 2001, 108, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Jobert, A.S.; Couvineau, A.; Silve, C. A homozygous inactivating mutation in the parathyroid hormone/parathyroid hormone-related peptide receptor causing blomstrand chondrodysplasia. J. Clin. Endocrinol. Metab. 1998, 83, 3365–3368. [Google Scholar] [CrossRef] [PubMed]
- Karperien, M.C.; van der Harten, H.J.; van Schooten, R.; Farih-Sips, H.; den Hollander, N.S.; Kneppers, A.L.J.; Nijweide, P.; Papapoulos, S.E.; Löwik, C.W.G.M. A frame-shift mutation in the type i parathyroid hormone/parathyroid hormone-related peptide receptor causing blomstrand lethal osteochondrodysplasia. J. Clin. Endocrinol. Metab. 1999, 84, 3713–3720. [Google Scholar] [CrossRef] [PubMed]
- HYP-Consortium. A gene (pex) with homologies to endopeptidases is mutated in patients with x-linked hypophosphatemic rickets. Nat. Genet. 1995, 11, 130–136. [Google Scholar] [CrossRef]
- ADHR Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in fgf23. Nat. Genet. 2000, 26, 345–348. [Google Scholar] [CrossRef]
- Brownstein, C.A.; Adler, F.; Nelson-Williams, C.; Iijima, J.; Li, P.; Imura, A.; Nabeshima, Y.; Reyes-Mugica, M.; Carpenter, T.O.; Lifton, R.P. A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc. Natl. Acad. Sci. USA 2008, 105, 3455–3460. [Google Scholar] [CrossRef]
- Lorenz-Depiereux, B.; Bastepe, M.; Benet-Pages, A.; Amyere, M.; Wagenstaller, J.; Muller-Barth, U.; Badenhoop, K.; Kaiser, S.M.; Rittmaster, R.S.; Shlossberg, A.H.; et al. Dmp1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat. Genet. 2006, 38, 1248–1250. [Google Scholar] [CrossRef]
- Lorenz-Depiereux, B.; Schnabel, D.; Tiosano, D.; Häusler, G.; Strom, T.M. Loss-of-function enpp1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am. J. Hum. Genet. 2010, 86, 267–272. [Google Scholar] [CrossRef]
- Rafaelsen, S.H.; Ræder, H.; Fagerheim, A.K.; Knappskog, P.; Carpenter, T.O.; Johansson, S.; Bjerknes, R. Exome sequencing reveals fam20c mutations associated with fibroblast growth factor 23–related hypophosphatemia, dental anomalies, and ectopic calcification. J. Bone Miner. Res. 2013, 28, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Lorenz-Depiereux, B.; Benet-Pages, A.; Eckstein, G.; Tenenbaum-Rakover, Y.; Wagenstaller, J.; Tiosano, D.; Gershoni-Baruch, R.; Albers, N.; Lichtner, P.; Schnabel, D.; et al. Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene slc34a3. Am. J. Hum. Genet. 2006, 78, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Bergwitz, C.; Roslin, N.M.; Tieder, M.; Loredo-Osti, J.C.; Bastepe, M.; Abu-Zahra, H.; Frappier, D.; Burkett, K.; Carpenter, T.O.; Anderson, D.; et al. Slc34a3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter napi-iic in maintaining phosphate homeostasis. Am. J. Hum. Genet. 2006, 78, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Kitanaka, S.; Takeyama, K.; Murayama, A.; Sato, T.; Okumura, K.; Nogami, M.; Hasegawa, Y.; Niimi, H.; Yanagisawa, J.; Tanaka, T.; et al. Inactivating mutations in the 25-hydroxyvitamin d3 1alpha-hydroxylase gene in patients with pseudovitamin d-deficiency rickets. N. Engl. J. Med. 1998, 338, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.R.; Malloy, P.J.; Kieback, D.G.; Kesterson, R.A.; Pike, J.W.; Feldman, D.; O’Malley, B.W. Point mutations in the human vitamin d receptor gene associated with hypocalcemic rickets. Science 1988, 242, 1702–1705. [Google Scholar] [CrossRef]
- Pollak, M.R.; Brown, E.M.; WuChou, Y.H.; Hebert, S.C.; Marx, S.J.; Steinmann, B.; Levi, T.; Seidman, C.E.; Seidman, J.G. Mutations in the human Ca2+-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell 1993, 75, 1297–1303. [Google Scholar] [CrossRef]
- Schipani, E.; Kruse, K.; Juppner, H. A constitutively active mutant pth-pthrp receptor in jansen-type metaphyseal chondrodysplasia. Science 1995, 268, 98–100. [Google Scholar] [CrossRef]
- Brown, W.W.; Juppner, H.; Langman, C.B.; Price, H.; Farrow, E.G.; White, K.E.; McCormick, K.L. Hypophosphatemia with elevations in serum fibroblast growth factor 23 in a child with jansen’s metaphyseal chondrodysplasia. J. Clin. Endocrinol. Metab. 2009, 94, 17–20. [Google Scholar] [CrossRef]
- Topaz, O.; Indelman, M.; Chefetz, I.; Geiger, D.; Metzker, A.; Altschuler, Y.; Choder, M.; Bercovich, D.; Uitto, J.; Bergman, R.; et al. A deleterious mutation in samd9 causes normophosphatemic familial tumoral calcinosis. Am. J. Hum. Genet. 2006, 79, 759–764. [Google Scholar] [CrossRef]
- Bhoj, E.J.; Li, M.; Ahrens-Nicklas, R.; Pyle, L.C.; Wang, J.; Zhang, V.W.; Clarke, C.; Wong, L.J.; Sondheimer, N.; Ficicioglu, C.; et al. Pathologic variants of the mitochondrial phosphate carrier slc25a3: Two new patients and expansion of the cardiomyopathy/skeletal myopathy phenotype with and without lactic acidosis. JIMD Rep. 2015, 19, 59–66. [Google Scholar]
- Mayr, J.A.; Zimmermann, F.A.; Horvath, R.; Schneider, H.C.; Schoser, B.; Holinski-Feder, E.; Czermin, B.; Freisinger, P.; Sperl, W. Deficiency of the mitochondrial phosphate carrier presenting as myopathy and cardiomyopathy in a family with three affected children. Neuromuscul. Disord. 2011, 21, 803–808. [Google Scholar] [CrossRef]
- Lemos, R.R.; Ramos, E.M.; Legati, A.; Nicolas, G.; Jenkinson, E.M.; Livingston, J.H.; Crow, Y.J.; Campion, D.; Coppola, G.; Oliveira, J.R.M. Update and mutational analysis of slc20a2: A major cause of primary familial brain calcification. Hum. Mutat. 2015, 36, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Westenberger, A.; Sobrido, M.J.; García-Murias, M.; Domingo, A.; Sears, R.L.; Lemos, R.R.; Ordoñez-Ugalde, A.; Nicolas, G.; da Cunha, J.E.G.; et al. Mutations in the gene encoding pdgf-b cause brain calcifications in humans and mice. Nat. Genet. 2013, 45, 1077. [Google Scholar] [CrossRef] [PubMed]
- Legati, A.; Giovannini, D.; Nicolas, G.; Lopez-Sanchez, U.; Quintans, B.; Oliveira, J.R.; Sears, R.L.; Ramos, E.M.; Spiteri, E.; Sobrido, M.J.; et al. Mutations in xpr1 cause primary familial brain calcification associated with altered phosphate export. Nat. Genet. 2015, 47, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.-P.; Cheng, X.; Wang, C.; Zhao, M.; Guo, X.-X.; Su, H.-Z.; Lai, L.-L.; Zou, X.-H.; Chen, X.-J.; Zhao, Y.; et al. Biallelic mutations in myorg cause autosomal recessive primary familial brain calcification. Neuron 2018, 98, 1116–1123.e1115. [Google Scholar] [CrossRef] [PubMed]
- Grangeon, L.; Wallon, D.; Charbonnier, C.; Quenez, O.; Richard, A.-C.; Rousseau, S.; Budowski, C.; Lebouvier, T.; Corbille, A.-G.; Vidailhet, M.; et al. Biallelic myorg mutation carriers exhibit primary brain calcification with a distinct phenotype. Brain 2019, 142, 1573–1586. [Google Scholar] [CrossRef]
- Cen, Z.; Chen, Y.; Chen, S.; Wang, H.; Yang, D.; Zhang, H.; Wu, H.; Wang, L.; Tang, S.; Ye, J.; et al. Biallelic loss-of-function mutations in jam2 cause primary familial brain calcification. Brain 2019, 143, 491–502. [Google Scholar] [CrossRef]
- Schottlaender, L.V.; Abeti, R.; Jaunmuktane, Z.; Macmillan, C.; Chelban, V.; O’Callaghan, B.; McKinley, J.; Maroofian, R.; Efthymiou, S.; Athanasiou-Fragkouli, A.; et al. Bi-allelic jam2 variants lead to early-onset recessive primary familial brain calcification. Am. J. Hum. Genet. 2020, 106, 412–421. [Google Scholar] [CrossRef]
- Michigami, T.; Kawai, M.; Yamazaki, M.; Ozono, K. Phosphate as a signaling molecule and its sensing mechanism. Physiol. Rev. 2018, 98, 2317–2348. [Google Scholar] [CrossRef]
- Beck, L.; Beck-Cormier, S. Extracellular phosphate sensing in mammals: What do we know? J. Mol. Endocrinol. 2020, 65, R53–R63. [Google Scholar] [CrossRef]
- Chavkin, N.W.; Chia, J.J.; Crouthamel, M.H.; Giachelli, C.M. Phosphate uptake-independent signaling functions of the type iii sodium-dependent phosphate transporter, pit-1, in vascular smooth muscle cells. Exp. Cell Res. 2015, 333, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.; Leroy, C.; Salaun, C.; Margall-Ducos, G.; Desdouets, C.; Friedlander, G. Identification of a novel function of pit1 critical for cell proliferation and independent of its phosphate transport activity. J. Biol. Chem. 2009, 284, e99959. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Mosyak, L.; Kurinov, I.; Zuo, H.; Sturchler, E.; Cheng, T.C.; Subramanyam, P.; Brown, A.P.; Brennan, S.C.; Mun, H.C.; et al. Structural mechanism of ligand activation in human calcium-sensing receptor. eLife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Centeno, P.P.; Herberger, A.; Mun, H.-C.; Tu, C.; Nemeth, E.F.; Chang, W.; Conigrave, A.D.; Ward, D.T. Phosphate acts directly on the calcium-sensing receptor to stimulate parathyroid hormone secretion. Nat. Commun. 2019, 10, 4693. [Google Scholar] [CrossRef]
- Giovannini, D.; Touhami, J.; Charnet, P.; Sitbon, M.; Battini, J.-L. Inorganic phosphate export by the retrovirus receptor xpr1 in metazoans. Cell Rep. 2013, 3, 1866–1873. [Google Scholar] [CrossRef]
- Wild, R.; Gerasimaite, R.; Jung, J.-Y.; Truffault, V.; Pavlovic, I.; Schmidt, A.; Saiardi, A.; Jessen, H.J.; Poirier, Y.; Hothorn, M.; et al. Control of eukaryotic phosphate homeostasis by inositol polyphosphate sensor domains. Science 2016, 352, 986–990. [Google Scholar] [CrossRef]
- Wilson, M.S.; Jessen, H.J.; Saiardi, A. The inositol hexakisphosphate kinases ip6k1 and -2 regulate human cellular phosphate homeostasis, including xpr1-mediated phosphate export. J. Biol. Chem. 2019, 294, 11597–11608. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gu, C.; Hostachy, S.; Sahu, S.; Wittwer, C.; Jessen, H.J.; Fiedler, D.; Wang, H.; Shears, S.B. Control of xpr1-dependent cellular phosphate efflux by insp8 is an exemplar for functionally-exclusive inositol pyrophosphate signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 3568–3574. [Google Scholar] [CrossRef]
- Bergwitz, C.; Rasmussen, M.D.; Derobertis, C.; Wee, M.J.; Sinha, S.; Chen, H.H.; Huang, J.; Perrimon, N. Roles of major facilitator superfamily transporters in phosphate response in drosophila. PLoS ONE 2012, 7, e31730. [Google Scholar] [CrossRef]
- Wittrant, Y.; Bourgine, A.; Khoshniat, S.; Alliot-Licht, B.; Masson, M.; Gatius, M.; Rouillon, T.; Weiss, P.; Beck, L.; Guicheux, J. Inorganic phosphate regulates glvr-1 and -2 expression: Role of calcium and erk1/2. Biochem. Biophys. Res. Commun. 2009, 381, 259–263. [Google Scholar] [CrossRef]
- Bon, N.; Couasnay, G.; Bourgine, A.; Sourice, S.; Beck-Cormier, S.; Guicheux, J.A.-O.; Beck, L.A.-O. Phosphate (P(i))-regulated heterodimerization of the high-affinity sodium-dependent P(i) transporters PiT1/Slc20a1 and PiT2/Slc20a2 underlies extracellular P(i) sensing independently of P(i) uptake. J. Biol. Chem. 2018, 293, 2102–2214. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.-W.; Jang, H.L.; Nam, K.T.; Beck, G.R. Nano-hydroxyapatite modulates osteoblast lineage commitment by stimulation of DNA methylation and regulation of gene expression. Biomaterials 2015, 65, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.-W.; Park, J.; Habib, M.M.; Beck, G.R. Nano-hydroxyapatite stimulation of gene expression requires fgf receptor, phosphate transporter, and erk1/2 signaling. ACS Appl. Mater. Interfaces 2017, 9, 39185–39196. [Google Scholar] [CrossRef] [PubMed]
- Takashi, Y.; Kosako, H.; Sawatsubashi, S.; Kinoshita, Y.; Ito, N.; Tsoumpra, M.K.; Nangaku, M.; Abe, M.; Matsuhisa, M.; Kato, S.; et al. Activation of unliganded fgf receptor by extracellular phosphate potentiates proteolytic protection of fgf23 by its o-glycosylation. Proc. Natl. Acad. Sci. USA 2019, 116, 11418. [Google Scholar] [CrossRef] [PubMed]
- Furdui, C.M.; Lew, E.D.; Schlessinger, J.; Anderson, K.S. Autophosphorylation of fgfr1 kinase is mediated by a sequential and precisely ordered reaction. Mol. Cell 2006, 21, 711–717. [Google Scholar] [CrossRef]
- Lew, E.D.; Furdui, C.M.; Anderson, K.S.; Schlessinger, J. The precise sequence of fgf receptor autophosphorylation is kinetically driven and is disrupted by oncogenic mutations. Sci. Signal. 2009, 2, ra6. [Google Scholar] [CrossRef]
- Brown, E.M.; Gamba, G.; Riccardi, D.; Lombardi, M.; Butters, R.; Kifor, O.; Sun, A.; Hediger, M.A.; Lytton, J.; Hebert, S.C. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature 1993, 366, 575–580. [Google Scholar] [CrossRef]
- López-Sánchez, U.; Tury, S.; Nicolas, G.; Wilson, M.S.; Jurici, S.; Ayrignac, X.; Courgnaud, V.; Saiardi, A.; Sitbon, M.; Battini, J.-L. Interplay between pfbc-associated slc20a2 and xpr1 phosphate transporters requires inositol polyphosphates for control of cellular phosphate homeostasis. J. Biol. Chem. 2020. [Google Scholar] [CrossRef]
- Pauleau, A.L.; Galluzzi, L.; Scholz, S.R.; Larochette, N.; Kepp, O.; Kroemer, G. Unexpected role of the phosphate carrier in mitochondrial fragmentation. Cell Death Differ. 2008, 15, 616. [Google Scholar] [CrossRef]
- Seifert, E.L.; Ligeti, E.; Mayr, J.A.; Sondheimer, N.; Hajnoczky, G. The mitochondrial phosphate carrier: Role in oxidative metabolism, calcium handling and mitochondrial disease. Biochem. Biophys. Res. Commun. 2015, 464, 369–375. [Google Scholar] [CrossRef]
- Greig Couasnay, N.B.; Claire-Sophie, D.; Sophie, S.; Arnaud, B.; Joelle, V.; Pierre, W.; Sylvain, P.; Jerome, G.; Sarah, B.-C.; Laurent, B. Pit1/slc20a1-Mediated Endoplasmic Reticulum Homeostasis and Cell Survival in Growth Plate Chondrocytes; ASBMR: Denver, CO, USA, 2017. [Google Scholar]
- Laver, D.R.; Lenz, G.K.E.; Dulhunty, A.F. Phosphate ion channels in sarcoplasmic reticulum of rabbit skeletal muscle. J. Physiol. 2001, 535, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Takahashi, A.; Adachi, K.; Noji, H.; Yasuda, R.; Yoshida, M.; Kinosita, K., Jr. Mechanically driven atp synthesis by f1-atpase. Nature 2004, 427, 465. [Google Scholar] [CrossRef] [PubMed]
- Pesta, D.H.; Tsirigotis, D.N.; Befroy, D.E.; Caballero, D.; Jurczak, M.J.; Rahimi, Y.; Cline, G.W.; Dufour, S.; Birkenfeld, A.L.; Rothman, D.L.; et al. Hypophosphatemia promotes lower rates of muscle atp synthesis. FASEB J. 2016, 30, 3378–3387. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. Ampk: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Jeon, S.-M. Regulation and function of ampk in physiology and diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef] [PubMed]
- Nina Bon, A.B.; Greig, C.; Sophie, S.; Jérôme, G.; Sarah, B.-C.; Laurent, B. Pivotal Role of pit1/slc20a1 and pit2/slc20a2 in Phosphate-Dependent mapk/erk1/2 Signaling and fgf23 Secretion in Bone; ASBMR: Denver, CO, USA, 2017. [Google Scholar]
- Gorvin, C.M. Insights into calcium-sensing receptor trafficking and biased signalling by studies of calcium homeostasis. J. Mol. Endocrinol. 2018, 61, R1–R12. [Google Scholar] [CrossRef]
- Koyama, Y.; Rittling, S.; Tsuji, K.; Hino, K.; Salincarnboriboon, R.; Yano, T.; Taketani, Y.; Nifuji, A.; Denhardt, D.T.; Noda, M. Osteopontin deficiency suppresses high phosphate load-induced bone loss via specific modulation of osteoclasts. Endocrinology 2006, 147, 3040–3049. [Google Scholar] [CrossRef] [PubMed]
- Beck, G.R., Jr.; Zerler, B.; Moran, E. Phosphate is a specific signal for induction of osteopontin gene expression. Proc. Natl. Acad. Sci. USA 2000, 97, 8352–8357. [Google Scholar] [CrossRef]
- Giachelli, C.M. Vascular calcification: In vitro evidence for the role of inorganic phosphate. J. Am. Soc. Nephrol. 2003, 14, S300–S304. [Google Scholar] [CrossRef] [PubMed]
- Shobeiri, N.; Adams, M.A.; Holden, R.M. Phosphate: An old bone molecule but new cardiovascular risk factor. Br. J. Clin. Pharmacol. 2014, 77, 39–54. [Google Scholar] [CrossRef]
- Krabbe, S.; Christiansen, C.; Rødbro, P.; Transbøl, I. Effect of puberty on rates of bone growth and mineralisation: With observations in male delayed puberty. Arch. Dis. Child. 1979, 54, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Follet, H.; Boivin, G.; Rumelhart, C.; Meunier, P.J. The degree of mineralization is a determinant of bone strength: A study on human calcanei. Bone 2004, 34, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Monazzah, S.; Bigham-Sadegh, A. Bone injury and fracture healing biology. Biomed. Environ. Sci 2015, 28, 57–71. [Google Scholar]
- Anderson, H.C. Molecular biology of matrix vesicles. Clin. Orthop. Relat. Res. 1995, 266–280. [Google Scholar] [CrossRef]
- Anderson, H.C. Matrix vesicles and calcification. Curr. Rheumatol. Rep. 2003, 5, 222–226. [Google Scholar] [CrossRef]
- Bottini, M.; Mebarek, S.; Anderson, K.L.; Strzelecka-Kiliszek, A.; Bozycki, L.; Simao, A.M.S.; Bolean, M.; Ciancaglini, P.; Pikula, J.B.; Pikula, S.; et al. Matrix vesicles from chondrocytes and osteoblasts: Their biogenesis, properties, functions and biomimetic models. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 532–546. [Google Scholar] [CrossRef]
- Hoac, B.; Kiffer-Moreira, T.; Millán, J.L.; McKee, M.D. Polyphosphates inhibit extracellular matrix mineralization in mc3t3-e1 osteoblast cultures. Bone 2013, 53, 478–486. [Google Scholar] [CrossRef]
- Terkeltaub, R.A. Inorganic pyrophosphate generation and disposition in pathophysiology. Am. J. Physiol. Cell Physiol. 2001, 281, C1–C11. [Google Scholar] [CrossRef]
- Hessle, L.; Johnson, K.A.; Anderson, H.C.; Narisawa, S.; Sali, A.; Goding, J.W.; Terkeltaub, R.; Millan, J.L. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc. Natl. Acad. Sci. USA 2002, 99, 9445–9449. [Google Scholar] [CrossRef]
- Tiosano, D.; Hochberg, Z. Hypophosphatemia: The common denominator of all rickets. J. Bone Miner. Metab. 2009, 27, 392–401. [Google Scholar] [CrossRef]
- Mozar, A.; Haren, N.; Chasseraud, M.; Louvet, L.; Maziere, C.; Wattel, A.; Mentaverri, R.; Morliere, P.; Kamel, S.; Brazier, M.; et al. High extracellular inorganic phosphate concentration inhibits rank-rankl signaling in osteoclast-like cells. J. Cell Physiol. 2008, 215, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Kanatani, M.; Sugimoto, T.; Kano, J.; Kanzawa, M.; Chihara, K. Effect of high phosphate concentration on osteoclast differentiation as well as bone-resorbing activity. J. Cell Physiol. 2003, 196, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Takeda, E.; Yamamoto, H.; Taketani, Y.; Yamanaka-Okumura, H. Dietary phosphorus in bone health and quality of life. Nutr. Rev. 2012, 70, 311–321. [Google Scholar] [CrossRef]
- Cozzolino, M.; Pasho, S.; Fallabrino, G.; Olivi, L.; Gallieni, M.; Brancaccio, D. Pathogenesis of secondary hyperparathyroidism. Int. J. Artif. Organs. 2009, 32, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Krishnarao, G.V.G.; Draper, H.H. Influence of dietary phosphate on bone resorption in senescent mice. J. Nutr. 1972, 102, 1143–1145. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.M.; Schuch, N.J.; Genaro, P.S.; Ciconelli, R.M.; Ferraz, M.B.; Martini, L.A. Nutrient intakes related to osteoporotic fractures in men and women—The Brazilian Osteoporosis Study (BRAZOS). Nutr. J. 2009, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Murali, S.K.; Roschger, P.; Zeitz, U.; Klaushofer, K.; Andrukhova, O.; Erben, R.G. Fgf23 regulates bone mineralization in a 1,25(oh)2 d3 and klotho-independent manner. J. Bone Miner. Res. 2016, 31, 129–142. [Google Scholar] [CrossRef]
- Erben, R.G. Physiological actions of fibroblast growth factor-23. Front. Endocrinol. 2018, 9, 267. [Google Scholar] [CrossRef]
- Orfanidou, T.; Malizos, K.N.; Varitimidis, S.; Tsezou, A. 1,25-dihydroxyvitamin d(3) and extracellular inorganic phosphate activate mitogen-activated protein kinase pathway through fibroblast growth factor 23 contributing to hypertrophy and mineralization in osteoarthritic chondrocytes. Exp. Biol. Med. 2012, 237, 241–253. [Google Scholar] [CrossRef]
- Liu, E.S.; Zalutskaya, A.; Chae, B.T.; Zhu, E.D.; Gori, F.; Demay, M.B. Phosphate interacts with pthrp to regulate endochondral bone formation. Endocrinology 2014, 155, 3750–3756. [Google Scholar] [CrossRef][Green Version]
- Papaioannou, G.; Petit, E.T.; Liu, E.S.; Baccarini, M.; Pritchard, C.; Demay, M.B. Raf kinases are essential for phosphate induction of erk1/2 phosphorylation in hypertrophic chondrocytes and normal endochondral bone development. J. Biol. Chem. 2017, 292, 3164–3171. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Canaff, L.; Davidson, D.; Corluka, A.; Liu, H.; Hendy, G.N.; Henderson, J.E. Alterations in the sensing and transport of phosphate and calcium by differentiating chondrocytes. J. Biol. Chem. 2001, 276, 33995–34005. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.C.; Mansfield, K.; Hertkorn, C.; Ischiropoulos, H.; Shapiro, I.M. Phosphate-induced chondrocyte apoptosis is linked to nitric oxide generation. Am. J. Physiol. Cell Physiol. 2001, 281, C833–C839. [Google Scholar] [CrossRef] [PubMed]
- Civitelli, R. Cell-cell communication in the osteoblast/osteocyte lineage. Arch. Biochem. Biophys. 2008, 473, 188–192. [Google Scholar] [CrossRef]
- Staines, K.A.; MacRae, V.E.; Farquharson, C. The importance of the sibling family of proteins on skeletal mineralisation and bone remodelling. J. Endocrinol. 2012, 214, 241–255. [Google Scholar] [CrossRef]
- Bellido, T. Osteocyte-driven bone remodeling. Calcif. Tissue Int. 2014, 94, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Conrads, K.A.; Yu, L.R.; Lucas, D.A.; Zhou, M.; Chan, K.C.; Simpson, K.A.; Schaefer, C.F.; Issaq, H.J.; Veenstra, T.D.; Beck, G.R., Jr.; et al. Quantitative proteomic analysis of inorganic phosphate-induced murine mc3t3-e1 osteoblast cells. Electrophoresis 2004, 25, 1342–1352. [Google Scholar] [CrossRef]
- Conrads, K.A.; Yi, M.; Simpson, K.A.; Lucas, D.A.; Camalier, C.E.; Yu, L.R.; Veenstra, T.D.; Stephens, R.M.; Conrads, T.P.; Beck, G.R., Jr. A combined proteome and microarray investigation of inorganic phosphate-induced pre-osteoblast cells. Mol. Cell Proteom. 2005, 4, 1284–1296. [Google Scholar] [CrossRef]
- Kanatani, M.; Sugimoto, T.; Kano, J.; Chihara, K. Igf-i mediates the stimulatory effect of high phosphate concentration on osteoblastic cell proliferation. J. Cell Physiol. 2002, 190, 306–312. [Google Scholar] [CrossRef]
- Julien, M.; Khoshniat, S.; Lacreusette, A.; Gatius, M.; Bozec, A.; Wagner, E.F.; Wittrant, Y.; Masson, M.; Weiss, P.; Beck, L.; et al. Phosphate-dependent regulation of mgp in osteoblasts: Role of erk1/2 and fra-1. J. Bone Miner. Res. 2009, 24, 1856–1868. [Google Scholar] [CrossRef]
- Ito, N.; Findlay, D.M.; Anderson, P.H.; Bonewald, L.F.; Atkins, G.J. Extracellular phosphate modulates the effect of 1alpha,25-dihydroxy vitamin d3 (1,25d) on osteocyte like cells. J. Steroid. Biochem. Mol. Biol. 2013, 136, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Capulli, M.; Paone, R.; Rucci, N. Osteoblast and osteocyte: Games without frontiers. Arch. Biochem. Biophys. 2014, 561, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Dallas, S.L.; Prideaux, M.; Bonewald, L.F. The osteocyte: An endocrine cell. And more. Endocr Rev. 2013, 34, 658–690. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; de Crombrugghe, B. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet. 2003, 19, 458–466. [Google Scholar] [CrossRef]
- Bourgine, A.; Pilet, P.; Diouani, S.; Sourice, S.; Lesoeur, J.; Beck-Cormier, S.; Khoshniat, S.; Weiss, P.; Friedlander, G.; Guicheux, J.; et al. Mice with hypomorphic expression of the sodium-phosphate cotransporter pit1/slc20a1 have an unexpected normal bone mineralization. PLoS ONE 2013, 8, e65979. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef]
- Albano, G.; Moor, M.; Dolder, S.; Siegrist, M.; Wagner, C.A.; Biber, J.; Hernando, N.; Hofstetter, W.; Bonny, O.; Fuster, D.G. Sodium-dependent phosphate transporters in osteoclast differentiation and function. PLoS ONE 2015, 10, e0125104. [Google Scholar] [CrossRef]
- Yates, A.J.; Oreffo, R.O.; Mayor, K.; Mundy, G.R. Inhibition of bone resorption by inorganic phosphate is mediated by both reduced osteoclast formation and decreased activity of mature osteoclasts. J. Bone Miner. Res. 1991, 6, 473–478. [Google Scholar] [CrossRef]
- M’Baya-Moutoula, E.; Louvet, L.; Metzinger-Le Meuth, V.; Massy, Z.A.; Metzinger, L. High inorganic phosphate concentration inhibits osteoclastogenesis by modulating mir-223. Biochim. Biophys. Acta 2015, 1852, 2202–2212. [Google Scholar] [CrossRef]
- Beck, L.; Karaplis, A.C.; Amizuka, N.; Hewson, A.S.; Ozawa, H.; Tenenhouse, H.S. Targeted inactivation of npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc. Natl. Acad. Sci. USA 1998, 95, 5372–5377. [Google Scholar] [CrossRef]
- Hayashibara, T.; Hiraga, T.; Sugita, A.; Wang, L.; Hata, K.; Ooshima, T.; Yoneda, T. Regulation of osteoclast differentiation and function by phosphate: Potential role of osteoclasts in the skeletal abnormalities in hypophosphatemic conditions. J. Bone Miner. Res. 2007, 22, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Guo, X.L.; Alvarez, U.M.; Hruska, K.A. Regulation of sodium-dependent phosphate transport in osteoclasts. J. Clin. Investig. 1997, 100, 538–549. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, G.; Miura, K.; Kuno, M. Extracellular phosphates enhance activities of voltage-gated proton channels and production of reactive oxygen species in murine osteoclast-like cells. Pflug. Arch. 2017, 469, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Nanci, A. Ten Cate’s Oral Histology-E-Book: Development, Structure, and Function; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Abou Neel, E.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization-remineralization dynamics in teeth and bone. Int. J. Nanomed. 2016, 4743–4763. [Google Scholar] [CrossRef]
- Dietary reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academy of Sciences: Washington, DC, USA, 1997.
- Gaunt, W.E.; Irving, J.T. The influence of dietary calcium and phosphorus upon tooth formation. J. Physiol. 1940, 99, 18–29. [Google Scholar] [CrossRef]
- Ferguson, H.W.; Hartles, R.L. The effect of diets deficient in calcium or phosphorus in the presence and absence of supplements of vitamin d on the incisor teeth and bone of adult rats. Arch. Oral Biol. 1966, 11, 1345-IN31. [Google Scholar] [CrossRef]
- Schour, S.; Massler, M. The effects of dietary deficiencies upon the oral structures. Am. J. Physiol. Physiol. Rev. 1945, 41. [Google Scholar]
- Ferguson, H.W.; Hartles, R.L. The effect of vitamin d on the dentine of the incisor teeth and on the alveolar bone of young rats maintained on diets deficient in calcium or phosphorus. Arch. Oral Biol. 1964, 9, 447–460. [Google Scholar] [CrossRef]
- Becks, H.; Weber, M. The Influence of Diet on the Bone System with Special Reference to the Alveolar Process and the Labyrinthine Capsule. J. Am. Dent. Assoc. 1931, 18, 197–264. [Google Scholar]
- Jekl, V.; Krejcirova, L.; Buchtova, M.; Knotek, Z. Effect of high phosphorus diet on tooth microstructure of rodent incisors. Bone 2011, 49, 479–484. [Google Scholar] [CrossRef]
- Goodson, J.M.; Shi, P.; Mumena, C.H.; Haq, A.; Razzaque, M.S. Dietary phosphorus burden increases cariogenesis independent of vitamin d uptake. J. Steroid Biochem. Mol. Boil. 2017, 167, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Pollock, R.A.; Brown, T.W., Jr.; Rubin, D.M. “Phossy jaw” and “bis-phossy jaw” of the 19th and the 21st centuries: The diuturnity of john walker and the friction match. Craniomaxillofac Trauma Reconstr. 2015, 8, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Fantasia, J.; Carlson, E. Bisphosphonate-related osteonecrosis of the jaw: Background and guidelines for diagnosis, staging and management. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 102, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Merametdjian, L.; Beck-Cormier, S.; Bon, N.; Couasnay, G.; Sourice, S.; Guicheux, J.; Gaucher, C.; Beck, L. Expression of phosphate transporters during dental mineralization. J. Dent. Res. 2018, 97, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Nemoto, E.; Foster, B.L.; Somerman, M.J.; Shimauchi, H. Phosphate increases bone morphogenetic protein-2 expression through camp-dependent protein kinase and erk1/2 pathways in human dental pulp cells. Bone 2011, 48, 1409–1416. [Google Scholar] [CrossRef]
- Lundquist, P.; Ritchie, H.H.; Moore, K.; Lundgren, T.; Linde, A. Phosphate and calcium uptake by rat odontoblast-like mrpc-1 cells concomitant with mineralization. J. Bone Miner. Res. 2002, 17, 1801–1813. [Google Scholar] [CrossRef]
- Beck, L. Expression and function of slc34 sodium–phosphate co-transporters in skeleton and teeth. Pflug. Arch. Eur. J. Physiol. 2019, 471, 175–184. [Google Scholar] [CrossRef]
- Wright, J.T.; Carrion, I.A.; Morris, C. The molecular basis of hereditary enamel defects in humans. J. Dent. Res. 2014, 94, 52–61. [Google Scholar] [CrossRef]
- Antoniucci, D.M.; Yamashita, T.; Portale, A.A. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J. Clin. Endocrinol. Metab. 2006, 91, 3144–3149. [Google Scholar] [CrossRef]
- Coyac, B.R. Tissue-specific mineralization defects in the periodontium of the hyp mouse model of x-linked hypophosphatemia. Bone 2017, 103, 334–346. [Google Scholar] [CrossRef]
- Turan, S.; Aydin, C.; Bereket, A.; Akcay, T.; Guran, T.; Yaralioglu, B.A.; Bastepe, M.; Juppner, H. Identification of a novel dentin matrix protein-1 (dmp-1) mutation and dental anomalies in a kindred with autosomal recessive hypophosphatemia. Bone 2010, 46, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Ariyoshi, N.; Nogi, M.; Ando, A.; Watanabe, H.; Umekawa, S. Hypophosphatemia-induced cardiomyopathy. Am. J. Med. Sci. 2016, 352, 317–323. [Google Scholar] [CrossRef]
- Lichtman, M.A.; Miller, D.R.; Cohen, J.; Waterhouse, C. Reduced red cell glycolysis, 2, 3-diphosphoglycerate and adenosine triphosphate concentration, and increased hemoglobin-oxygen affinity caused by hypophosphatemia. Ann. Intern. Med. 1971, 74, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Ognibene, A.; Ciniglio, R.; Greifenstein, A.; Jarjoura, D.; Cugino, A.; Blend, D.; Whittier, F. Ventricular tachycardia in acute myocardial infarction: The role of hypophosphatemia. South. Med. J. 1994, 87, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Singhal, P.C.; Kumar, A.; Desroches, L.; Gibbons, N.; Mattana, J. Prevalence and predictors of rhabdomyolysis in patients with hypophosphatemia. Am. J. Med. 1992, 92, 458–464. [Google Scholar] [CrossRef]
- Davis, S.V.; Olichwier, K.K.; Chakko, S.C. Reversible depression of myocardial performance in hypophosphatemia. Am. J. Med. Sci. 1988, 295, 183–187. [Google Scholar] [CrossRef]
- Fuller, T.J.; Nichols, W.W.; Brenner, B.J.; Peterson, J.C. Reversible depression in myocardial performance in dogs with experimental phosphorus deficiency. J. Clin. Investig. 1978, 62, 1194–1200. [Google Scholar] [CrossRef]
- Claudius, I.; Sachs, C.; Shamji, T. Hypophosphatemia-induced heart failure. Am. J. Emerg. Med. 2002, 20, 369–370. [Google Scholar] [CrossRef]
- Neves, K.R.; Graciolli, F.G.; Dos Reis, L.M.; Pasqualucci, C.A.; MoysÉS, R.M.A.; Jorgetti, V. Adverse effects of hyperphosphatemia on myocardial hypertrophy, renal function, and bone in rats with renal failure. Kidney Int. 2004, 66, 2237–2244. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, N. Prognostic significance and therapeutic option of heart rate variability in chronic kidney disease. Int. Urol. Nephrol. 2014, 46, 19–25. [Google Scholar] [CrossRef]
- Huang, J.C.; Kuo, I.C.; Tsai, Y.C.; Lee, J.J.; Lim, L.M.; Chen, S.C.; Chiu, Y.W.; Chang, J.M.; Chen, H. Heart rate variability predicts major adverse cardiovascular events and hospitalization in maintenance hemodialysis patients. Kidney Blood Press. Res. 2017, 42, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cui, Y.; Yogendranath, P.; Wang, N. Blood pressure and heart rate variability are linked with hyperphosphatemia in chronic kidney disease patients. Chronobiol. Int. 2018, 35, 1329–1334. [Google Scholar] [CrossRef]
- Sarafidis, P.A.; Ruilope, L.M.; Loutradis, C.; Gorostidi, M.; de la Sierra, A.; de la Cruz, J.J.; Vinyoles, E.; Divisón-Garrote, J.A.; Segura, J.; Banegas, J.R. Blood pressure variability increases with advancing chronic kidney disease stage: A cross-sectional analysis of 16546 hypertensive patients. J. Hypertens. 2018, 36, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- London, G.M.; Guerin, A.P.; Marchais, S.J.; Metivier, F.; Pannier, B.; Adda, H. Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol. Dial. Transpl. 2003, 18, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Mizobuchi, M.; Towler, D.; Slatopolsky, E. Vascular calcification: The killer of patients with chronic kidney disease. J. Am. Soc. Nephrol. 2009, 20, 1453–1464. [Google Scholar] [CrossRef]
- Silswal, N.; Touchberry, C.D.; Daniel, D.R.; McCarthy, D.L.; Zhang, S.; Andresen, J.; Stubbs, J.R.; Wacker, M.J. Fgf23 directly impairs endothelium-dependent vasorelaxation by increasing superoxide levels and reducing nitric oxide bioavailability. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E426–E436. [Google Scholar] [CrossRef]
- Wohlfahrt, P.; Melenovsky, V.; Kotrc, M.; Benes, J.; Jabor, A.; Franekova, J.; Lemaire, S.; Kautzner, J.; Jarolim, P. Association of fibroblast growth factor-23 levels and angiotensin-converting enzyme inhibition in chronic systolic heart failure. JACC Heart Fail. 2015, 3, 829–839. [Google Scholar] [CrossRef]
- David, V.; Francis, C.; Babitt, J.L. Ironing out the cross talk between fgf23 and inflammation. Am. J. Physiol. Ren. Physiol. 2017, 312, F1–F8. [Google Scholar] [CrossRef]
- Desjardins, L.; Liabeuf, S.; Renard, C.; Lenglet, A.; Lemke, H.D.; Choukroun, G.; Drueke, T.B.; Massy, Z.A. Fgf23 is independently associated with vascular calcification but not bone mineral density in patients at various ckd stages. Osteoporos. Int. 2012, 23, 2017–2025. [Google Scholar] [CrossRef]
- Faul, C.; Amaral, A.P.; Oskouei, B.; Hu, M.C.; Sloan, A.; Isakova, T.; Gutierrez, O.M.; Aguillon-Prada, R.; Lincoln, J.; Hare, J.M.; et al. Fgf23 induces left ventricular hypertrophy. J. Clin. Investig. 2011, 121, 4393–4408. [Google Scholar] [CrossRef]
- Stöhr, R.; Schuh, A.; Heine, G.H.; Brandenburg, V. Fgf23 in cardiovascular disease: Innocent bystander or active mediator? Front. Endocrinol. 2018, 9, 351. [Google Scholar] [CrossRef]
- Bansal, N.; Zelnick, L.; Robinson-Cohen, C.; Hoofnagle, A.N.; Ix, J.H.; Lima, J.A.; Shoben, A.B.; Peralta, C.A.; Siscovick, D.S.; Kestenbaum, B.; et al. Serum parathyroid hormone and 25-hydroxyvitamin d concentrations and risk of incident heart failure: The multi-ethnic study of atherosclerosis. J. Am. Heart Assoc. 2014, 3, e001278. [Google Scholar] [CrossRef] [PubMed]
- Lafferty, F.W. Primary hyperparathyroidism. Changing clinical spectrum, prevalence of hypertension, and discriminant analysis of laboratory tests. Arch. Intern. Med. 1981, 141, 1761–1766. [Google Scholar] [CrossRef] [PubMed]
- Stefenelli, T.; Abela, C.; Frank, H.; Koller-Strametz, J.; Globits, S.; Bergler-Klein, J.; Niederle, B. Cardiac abnormalities in patients with primary hyperparathyroidism: Implications for follow-up. J. Clin. Endocrinol. Metab. 1997, 82, 106–112. [Google Scholar] [CrossRef]
- Brown, S.J.; Ruppe, M.D.; Tabatabai, L.S. The parathyroid gland and heart disease. Methodist. Debakey Cardiovasc. J. 2017, 13, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.L.; Pai, A.; Moe, S.M.; Giachelli, C.M. Direct effects of phosphate on vascular cell function. Advances Chronic Kidney Dis. 2011, 18, 105–112. [Google Scholar] [CrossRef]
- Shanahan, C.M.; Crouthamel, M.H.; Kapustin, A.; Giachelli, C.M. Arterial calcification in chronic kidney disease: Key roles for calcium and phosphate. Circ. Res. 2011, 109, 697–711. [Google Scholar] [CrossRef]
- Reynolds, J.L.; Joannides, A.J.; Skepper, J.N.; McNair, R.; Schurgers, L.J.; Proudfoot, D.; Jahnen-Dechent, W.; Weissberg, P.L.; Shanahan, C.M. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: A potential mechanism for accelerated vascular calcification in esrd. J. Am. Soc. Nephrol. 2004, 15, 2857. [Google Scholar] [CrossRef]
- Son, B.-K.; Kozaki, K.; Iijima, K.; Eto, M.; Nakano, T.; Akishita, M.; Ouchi, Y. Gas6/axl-pi3k/akt pathway plays a central role in the effect of statins on inorganic phosphate-induced calcification of vascular smooth muscle cells. Eur. J. Pharmacol. 2007, 556, 1–8. [Google Scholar] [CrossRef]
- Rangrez, A.Y.; M’Baya-Moutoula, E.; Metzinger-Le Meuth, V.; Henaut, L.; Djelouat, M.S.; Benchitrit, J.; Massy, Z.A.; Metzinger, L. Inorganic phosphate accelerates the migration of vascular smooth muscle cells: Evidence for the involvement of mir-223. PLoS ONE 2012, 7, e47807. [Google Scholar] [CrossRef]
- Duan, P.; Bonewald, L.F. The role of the wnt/beta-catenin signaling pathway in formation and maintenance of bone and teeth. Int. J. Biochem. Cell Biol. 2016, 77, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Gaur, T.; Lengner, C.J.; Hovhannisyan, H.; Bhat, R.A.; Bodine, P.V.; Komm, B.S.; Javed, A.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; et al. Canonical wnt signaling promotes osteogenesis by directly stimulating runx2 gene expression. J. Biol. Chem. 2005, 280, 33132–33140. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Signaling networks in runx2-dependent bone development. J. Cell Biochem. 2011, 112, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Sun, D.; Duan, Y.; Wen, P.; Dai, C.; Yang, J.; He, W. Wnt/beta-catenin signaling promotes vsmcs to osteogenic transdifferentiation and calcification through directly modulating runx2 gene expression. Exp. Cell Res. 2016, 345, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Diao, Z.; Han, X.; Liu, W. Secreted frizzled-related protein 5 attenuates high phosphate-induced calcification in vascular smooth muscle cells by inhibiting the wnt/ss-catenin pathway. Calcif. Tissue Int. 2016, 99, 66–75. [Google Scholar] [CrossRef]
- Anheim, M.; López-Sánchez, U.; Giovannini, D.; Richard, A.-C.; Touhami, J.; N’Guyen, L.; Rudolf, G.; Thibault-Stoll, A.; Frebourg, T.; Hannequin, D.; et al. Xpr1 mutations are a rare cause of primary familial brain calcification. J. Neurol. 2016, 263, 1559–1564. [Google Scholar] [CrossRef]
- Villa-Bellosta, R.; Bogaert, Y.E.; Levi, M.; Sorribas, V. Characterization of phosphate transport in rat vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1030–1036. [Google Scholar] [CrossRef]
- Hortells, L.; Guillén, N.; Sosa, C.; Sorribas, V. Several phosphate transport processes are present in vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2019, 318, H448–H460. [Google Scholar] [CrossRef]
- Shuto, E.; Taketani, Y.; Tanaka, R.; Harada, N.; Isshiki, M.; Sato, M.; Nashiki, K.; Amo, K.; Yamamoto, H.; Higashi, Y.; et al. Dietary phosphorus acutely impairs endothelial function. J. Am. Soc. Nephrol. 2009, 20, 1504–1512. [Google Scholar] [CrossRef]
- Kendrick, J.; Ix, J.H.; Targher, G.; Smits, G.; Chonchol, M. Relation of serum phosphorus levels to ankle brachial pressure index (from the third national health and nutrition examination survey). Am. J. Cardiol. 2010, 106, 564–568. [Google Scholar] [CrossRef]
- van Vuren, A.J.; Gaillard, C.A.J.M.; Eisenga, M.F.; van Wijk, R.; van Beers, E.J. The epo-fgf23 signaling pathway in erythroid progenitor cells: Opening a new area of research. Front. Physiol. 2019, 10, 304. [Google Scholar] [CrossRef] [PubMed]
- Coe, L.M.; Madathil, S.V.; Casu, C.; Lanske, B.; Rivella, S.; Sitara, D. Fgf-23 is a negative regulator of prenatal and postnatal erythropoiesis. J. Biol. Chem. 2014, 289, 9795–9810. [Google Scholar] [CrossRef] [PubMed]
- Clinkenbeard, E.L.; Hanudel, M.R.; Stayrook, K.R.; Appaiah, H.N.; Farrow, E.G.; Cass, T.A.; Summers, L.J.; Ip, C.S.; Hum, J.M.; Thomas, J.C.; et al. Erythropoietin stimulates murine and human fibroblast growth factor-23, revealing novel roles for bone and bone marrow. Haematologica 2017, 102, e427–e430. [Google Scholar] [CrossRef] [PubMed]
- Brody, T.O.M. 4-regulation of energy metabolism. In Nutritional Biochemistry, 2nd ed.; Brody, T.O.M., Ed.; Academic Press: San Diego, CA, USA, 1999; pp. 157–271. [Google Scholar]
- Smith, R.; Newman, R.J.; Radda, G.K.; Stokes, M.; Young, A. Hypophosphataemic osteomalacia and myopathy: Studies with nuclear magnetic resonance spectroscopy. Clin. Sci. 1984, 67, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Chande, S.; Caballero, D.; Ho, B.B.; Fetene, J.; Serna, J.; Pesta, D.; Nasiri, A.; Jurczak, M.; Chavkin, N.W.; Hernando, N.; et al. Slc20a1/pit1 and slc20a2/pit2 are essential for normal skeletal myofiber function and survival. Sci. Rep. 2020, 10, 3069. [Google Scholar] [CrossRef] [PubMed]
- Lotz, M.; Ney, R.; Bartter, F.C. Osteomalacia and debility resulting from phosphorus depletion. Trans. Assoc. Am. Physicians 1964, 77, 281–295. [Google Scholar]
- Chen, Y.-Y.; Kao, T.-W.; Chou, C.-W.; Wu, C.-J.; Yang, H.-F.; Lai, C.-H.; Wu, L.-W.; Chen, W.-L. Exploring the link between serum phosphate levels and low muscle strength, dynapenia, and sarcopenia. Sci. Rep. 2018, 8, 3573. [Google Scholar] [CrossRef]
- Moore, L.W.; Nolte, J.V.; Gaber, A.O.; Suki, W.N. Association of dietary phosphate and serum phosphorus concentration by levels of kidney function. Am. J. Clin. Nutr. 2015, 102, 444–453. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Yang, M.; Bao, J.-F.; Gu, L.-J.; Yu, H.-L.; Yuan, W.-J. Phosphate stimulates myotube atrophy through autophagy activation: Evidence of hyperphosphatemia contributing to skeletal muscle wasting in chronic kidney disease. BMC Nephrol. 2018, 19, 45. [Google Scholar] [CrossRef]
- Bose, S.; French, S.; Evans, F.J.; Joubert, F.; Balaban, R.S. Metabolic network control of oxidative phosphorylation: Multiple roles of inorganic phosphate. J. Biol. Chem. 2003, 278, 39155–39165. [Google Scholar] [CrossRef]
- Phillips, D.; Aponte, A.M.; French, S.A.; Chess, D.J.; Balaban, R.S. Succinyl-coa synthetase is a phosphate target for the activation of mitochondrial metabolism. Biochemistry 2009, 48, 7140–7149. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Zavala, J.S.; Pardo, J.P.; Moreno-Sanchez, R. Modulation of 2-oxoglutarate dehydrogenase complex by inorganic phosphate, Mg(2+), and other effectors. Arch. Biochem. Biophys. 2000, 379, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Hansford, R.G. Some properties of pyruvate and 2-oxoglutarate oxidation by blowfly flight-muscle mitochondria. Biochem. J. 1972, 127, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Blonde, D.J.; Kresack, E.J.; Kosicki, G.W. The effects of ions and freeze-thawing on supernatant and mitochondrial malate dehydrogenase. Can. J. Biochem. 1967, 45, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Cook, W.J.; Senkovich, O.; Chattopadhyay, D. An unexpected phosphate binding site in glyceraldehyde 3-phosphate dehydrogenase: Crystal structures of apo, holo and ternary complex of cryptosporidium parvum enzyme. BMC Struct. Biol. 2009, 9, 9. [Google Scholar] [CrossRef]
- Travis, S.F.; Sugerman, H.J.; Ruberg, R.L.; Dudrick, S.J.; Delivoria-Papadopoulos, M.; Miller, L.D.; Oski, F.A. Alterations of red-cell glycolytic intermediates and oxygen transport as a consequence of hypophosphatemia in patients receiving intravenous hyperalimentation. N. Engl. J. Med. 1971, 285, 763–768. [Google Scholar] [CrossRef]
- Boyer, C.J.C.; Baines, A.D.; Beaulieu, É.; Béliveau, R. Immunodetection of a type iii sodium-dependent phosphate cotransporter in tissues and ok cells. Biochim. Biophys. Acta (BBA) Biomembr. 1998, 1368, 73–83. [Google Scholar] [CrossRef][Green Version]
- Werner, A.; Dehmelt, L.; Nalbant, P. Na+-dependent phosphate cotransporters: The napi protein families. J. Exp. Biol. 1998, 201, 3135–3142. [Google Scholar]
- Bai, L.; Collins, J.F.; Ghishan, F.K. Cloning and characterization of a type iii na-dependent phosphate cotransporter from mouse intestine. Am. J. Physiol. Cell Physiol. 2000, 279, C1135–C1143. [Google Scholar] [CrossRef]
- Ravera, S.; Virkki, L.V.; Murer, H.; Forster, I.C. Deciphering pit transport kinetics and substrate specificity using electrophysiology and flux measurements. Am. J. Physiol. Cell Physiol. 2007, 293, C606–C620. [Google Scholar] [CrossRef]
- Fiermonte, G.; Palmieri, L.; Dolce, V.; Lasorsa, F.M.; Palmieri, F.; Runswick, M.J.; Walker, J.E. The sequence, bacterial expression, and functional reconstitution of the rat mitochondrial dicarboxylate transporter cloned via distant homologs in yeast and caenorhabditis elegans. J. Biol. Chem. 1998, 273, 24754–24759. [Google Scholar] [CrossRef]
- Fiermonte, G.; Dolce, V.; Arrigoni, R.; Runswick, M.J.; Walker, J.E.; Palmieri, F. Organization and sequence of the gene for the human mitochondrial dicarboxylate carrier: Evolution of the carrier family. Biochem. J. 1999, 344, 953–960. [Google Scholar] [CrossRef]
- Mayr, J.A.; Merkel, O.; Kohlwein, S.D.; Gebhardt, B.R.; Böhles, H.; Fötschl, U.; Koch, J.; Jaksch, M.; Lochmüller, H.; Horváth, R.; et al. Mitochondrial phosphate–carrier deficiency: A novel disorder of oxidative phosphorylation. Am. J. Hum. Genet. 2007, 80, 478–484. [Google Scholar] [CrossRef]
- Kwong, J.Q.; Davis, J.; Baines, C.P.; Sargent, M.A.; Karch, J.; Wang, X.; Huang, T.; Molkentin, J.D. Genetic deletion of the mitochondrial phosphate carrier desensitizes the mitochondrial permeability transition pore and causes cardiomyopathy. Cell Death Differ. 2014, 21, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.G.; Trajanovska, S. The multiple roles of phosphate in muscle fatigue. Front. Physiol. 2012, 3, 463. [Google Scholar] [CrossRef] [PubMed]
- Debold, E.P.; Romatowski, J.; Fitts, R.H. The depressive effect of pi on the force-pca relationship in skinned single muscle fibers is temperature dependent. Am. J. Physiol. Cell Physiol. 2006, 290, C1041–C1050. [Google Scholar] [CrossRef] [PubMed]
- Fryer, M.W.; Owen, V.J.; Lamb, G.D.; Stephenson, D.G. Effects of creatine phosphate and P(i) on Ca2+ movements and tension development in rat skinned skeletal muscle fibres. J. Physiol. 1995, 482, 123–140. [Google Scholar] [CrossRef]
- Lanza, I.R.; Wigmore, D.M.; Befroy, D.E.; Kent-Braun, J.A. In vivo atp production during free-flow and ischaemic muscle contractions in humans. J. Physiol. 2006, 577, 353–367. [Google Scholar] [CrossRef]
- Jones, D.A.; Turner, D.L.; McIntyre, D.B.; Newham, D.J. Energy turnover in relation to slowing of contractile properties during fatiguing contractions of the human anterior tibialis muscle. J. Physiol. 2009, 587, 4329–4338. [Google Scholar] [CrossRef]
- Seaberg, B.; Henslee, G.; Wang, S.; Paez-Colasante, X.; Landreth, G.E.; Rimer, M. Muscle-derived extracellular signal-regulated kinases 1 and 2 are required for the maintenance of adult myofibers and their neuromuscular junctions. Mol. Cell Biol. 2015, 35, 1238–1253. [Google Scholar] [CrossRef]
- Wang, S.; Seaberg, B.; Paez-Colasante, X.; Rimer, M. Defective acetylcholine receptor subunit switch precedes atrophy of slow-twitch skeletal muscle fibers lacking erk1/2 kinases in soleus muscle. Sci. Rep. 2016, 6, 38745. [Google Scholar] [CrossRef] [PubMed]
- Imel, E.A.; Econs, M.J. Approach to the hypophosphatemic patient. J. Clin. Endocrinol. Metab. 2012, 97, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Bergwitz, C. Dietary phosphate modifies lifespan in drosophila. Nephrol. Dial. Transpl. 2012, 27, 3399–3406. [Google Scholar] [CrossRef]
- Rose, E.; Lee, D.; Xiao, E.; Zhao, W.; Wee, M.; Cohen, J.; Bergwitz, C. Endocrine regulation of mfs2 by branchless controls phosphate excretion and stone formation in drosophila renal tubules. Sci. Rep. 2019, 9, 8798. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Tokumoto, M.; Tatsumoto, N.; Taniguchi, M.; Noguchi, H.; Nakano, T.; Masutani, K.; Ooboshi, H.; Tsuruya, K.; Kitazono, T. Phosphate overload directly induces systemic inflammation and malnutrition as well as vascular calcification in uremia. Am. J. Physiol. Ren. Physiol. 2014, 306, F1418–F1428. [Google Scholar] [CrossRef]
- Ohnishi, M.; Razzaque, M.S. Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. FASEB J. 2010, 24, 3562–3571. [Google Scholar] [CrossRef]
- Hong, S.-H.; Park, S.-J.; Lee, S.; Kim, S.; Cho, M.-H. Biological effects of inorganic phosphate: Potential signal of toxicity. J. Toxicol. Sci. 2015, 40, 55–69. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Heimbürger, O.; Paultre, F.; Diczfalusy, U.; Wang, T.; Berglund, L.; Jogestrand, T. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999, 55, 1899–1911. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Heimbürger, O.; Lindholm, B.; Kaysen, G.A.; Bergström, J. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (mia syndrome). Nephrol. Dial. Transpl. 2000, 15, 953–960. [Google Scholar] [CrossRef]
- Wang, A.Y.; Woo, J.; Lam, C.W.; Wang, M.; Chan, I.H.; Gao, P.; Lui, S.F.; Li, P.K.; Sanderson, J.E. Associations of serum fetuin-a with malnutrition, inflammation, atherosclerosis and valvular calcification syndrome and outcome in peritoneal dialysis patients. Nephrol. Dial. Transpl. 2005, 20, 1676–1685. [Google Scholar] [CrossRef]
- Jin, H.; Xu, C.-X.; Lim, H.-T.; Park, S.-J.; Shin, J.-Y.; Chung, Y.-S.; Park, S.-C.; Chang, S.-H.; Youn, H.-J.; Lee, K.-H.; et al. High Dietary Inorganic Phosphate Increases Lung Tumorigenesis and Alters Akt Signaling. Am. J. Respir. Crit. Care Med. 2008, 179, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Dai, Y.; Liu, X.; Wang, C.; Wang, A.; Chen, Z.; Heidbreder, C.E.; Kolokythas, A.; Zhou, X. Identification and experimental validation of g protein alpha inhibiting activity polypeptide 2 (gnai2) as a microrna-138 target in tongue squamous cell carcinoma. Hum. Genet. 2011, 129, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Lacerda-Abreu, M.A.; Russo-Abrahão, T.; Monteiro, R.Q.; Rumjanek, F.D.; Meyer-Fernandes, J.R. Inorganic phosphate transporters in cancer: Functions, molecular mechanisms and possible clinical applications. Biochim. Biophys. Acta (BBA) Bioenerg. 2018, 1870, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Minai-Tehrani, A.; Chang, S.H.; Jiang, H.L.; Lee, S.; Lee, A.Y.; Seo, H.W.; Chae, C.; Beck, G.R., Jr.; Cho, M.H. Knockdown of the sodium-dependent phosphate co-transporter 2b (npt2b) suppresses lung tumorigenesis. PLoS ONE 2013, 8, e77121. [Google Scholar] [CrossRef] [PubMed]
- Bobko, A.A.; Eubank, T.D.; Driesschaert, B.; Dhimitruka, I.; Evans, J.; Mohammad, R.; Tchekneva, E.E.; Dikov, M.M.; Khramtsov, V.V. Interstitial inorganic phosphate as a tumor microenvironment marker for tumor progression. Sci. Rep. 2017, 7, 41233. [Google Scholar] [CrossRef]
- Tran, T.; Bliuc, D.; Hansen, L.; Abrahamsen, B.; van den Bergh, J.; Eisman, J.A.; van Geel, T.; Geusens, P.; Vestergaard, P.; Nguyen, T.V.; et al. Persistence of excess mortality following individual nonhip fractures: A relative survival analysis. J. Clin. Endocrinol. Metab. 2018, 103, 3205–3214. [Google Scholar] [CrossRef]
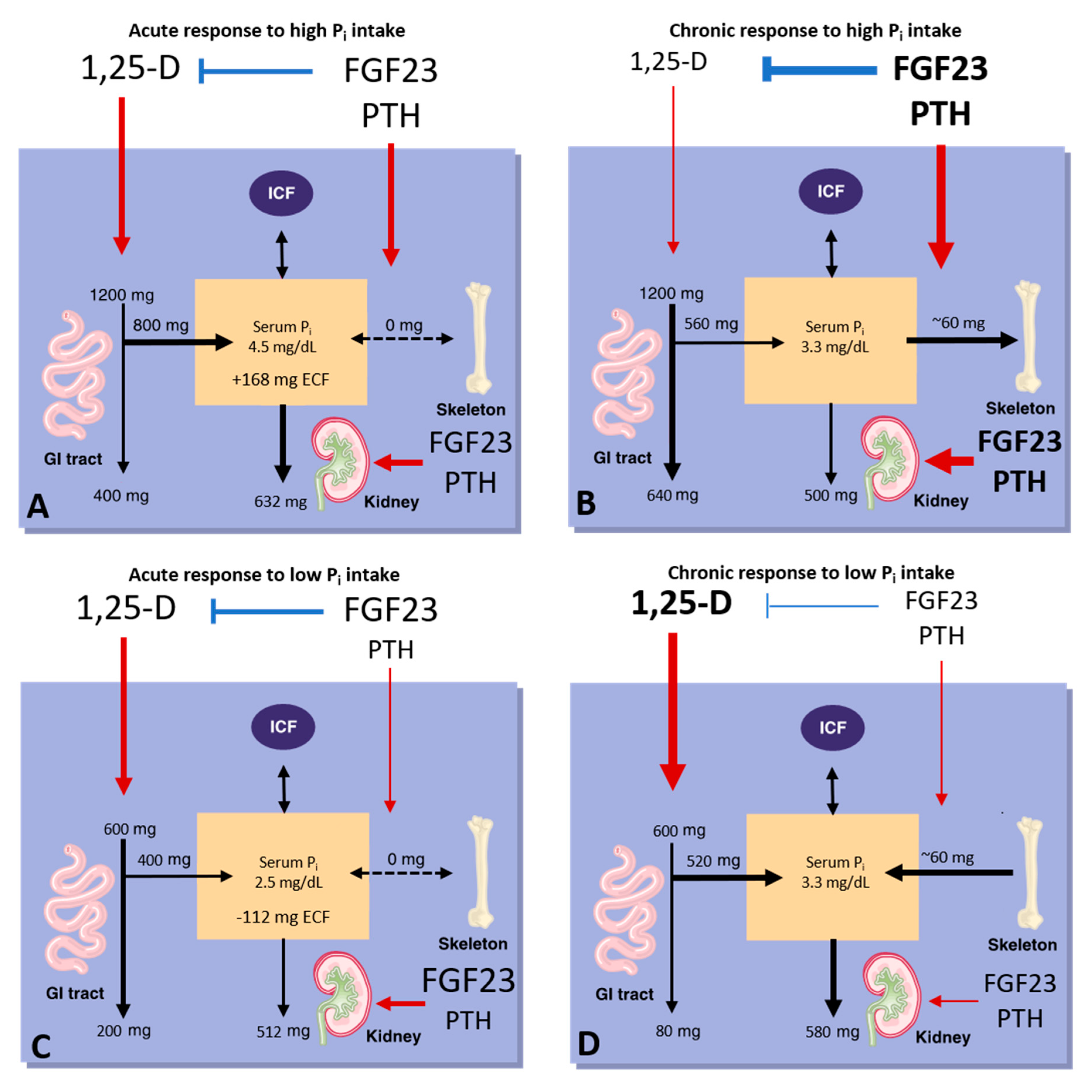

| Phosphate Preparations | Phosphorus Content | Potassium (K) Content | Sodium (Na) Content |
|---|---|---|---|
| Neutraphos-powder (for mixing with liquid) | 250 mg/packet | 270 mg/packet | 164 mg/packet |
| Neutraphos-K-powder (for mixing with liquid) | 250 mg/packet | 556 mg/packet | 0 mg/packet |
| K-Phos Original-tablet (to mix in liquid, acidifying) | 114 mg/tablet | 144 mg/tablet | 0 mg/tablet |
| K-Phos MF-tablet (mixing not required, acidifying) | 126 mg/tablet | 45 mg/tablet | 67 mg/tablet |
| K-Phos #2 (double strength of K-Phos MF) | 250 mg/tablet | 90 mg/tablet | 133 mg/tablet |
| K-Phos Neutral-tablet (non-acidifying, mixing not required) | 250 mg/tablet | 45 mg/tablet | 298 mg/tablet |
| Phospha-Soda-solution (small doses may be given undiluted) | 127 mg/mL | 0 mg/mL | 152 mg/mL |
| Joulie’s solution (prepared by compounding pharmacies) | 30 mg/mL | 0 mg/mL | 17.5–20 mg/mL |
| Vitamin D and Related Agents | Agent | Available Preparations | |
| Vitamin D | Calciferol (Drisdol) | Solution: 8000 IU/mL Tablets: 25,000 and 50,000 IU | |
| Dihydrotachysterol | DHT (Hytakerol) | Solution: 0.2 µg/5 mL Tablets: 0.125, 0.2, and 0.4 mg | |
| 1,25 dihydroxyvitamin D | Calcitriol (Rocaltrol) | 0.25 and 0.5 µg capsules and 1 µg/mL solution | |
| Calcijex | Ampules for IV use containing 1 or 2 µg of drug per mL | ||
| 1α-hydroxyvitamin D | Alfacalcidol | 0.25, 0.5, and 1 µg capsules Oral solution (drops): 2 µg/mL Solution for IV use: 2 µg/mL | |
| Vitamin D analogs | Paricalcitol (Zemplar) | 1, 2, and 4 µg capsules | |
| Doxercalciferol (Hectoral) | 0.5, 1, and 2.5 µg capsules | ||
| Disorder | Mechanism | Ref. |
|---|---|---|
| Hypophosphatemic | ||
| Dietary phosphorus deficiency | Total body deficiency, combined with an insulin-mediated cellular shift in Pi during refeeding, causes hypophosphatemia. | [50] |
| Vitamin D deficiency | Reduced intestinal Ca and Pi absorption causes rickets/osteomalacia and secondary hyperparathyroidism. | [130,131] |
| Chronic use of Pi antacids/high gastric pH (due to PPIs, autoimmune gastritis/pernicious anemia, etc.) | High gastric pH reduces Pi solubility, which potentially results in reduced mineral absorption and hypophosphatemia. | [109,111,112,132,133] |
| Reduced gastrointestinal absorption (due to Inflammatory Bowel and Celiac diseases, diarrhea, vomiting, short gut, intestinal mucosal hypoplasia, jejunal feeding, prematurity, etc.) | Chronic diarrhea and reduced gastrointestinal absorption of Pi reduce bioavailable Pi. | [127,128] |
| Parenteral iron administration | Ferric carboxymaltose blocks FGF23 cleavage, which induces renal Pi wasting. | [134] |
| Proximal tubular damage (caused by renal tubular acidosis or drugs such as theophylline, foscarnet) | Renal Pi wasting causes rickets/osteomalacia and hypercalciuria. | [135,136,137] |
| Hyperparathyroidism | Bone resorption increases serum Pi, but the net effect is to lower serum Pi due to increased renal excretion. | [138,139] |
| Drugs | ||
| Phosphonocarboxylates (e.g., PFA), phloretin derivatives (e.g., 2′-PP), arsenate | Competitively inhibit Na-Pi co-transport of Pi. | [115,116,118,140] |
| Niacin/Nicotinamide, NAD, triazole derivatives | Downregulates NPT2b, inhibits intestinal Pi transport. | [117,118,141] |
| Tenapanor | Inhibits paracellular Pi transport and downregulates NPT2b. | [118,120] |
| Insulin | Promotes Pi uptake into tissues. Can result in hypophosphatemia in the context of refeeding. | [103,142] |
| Bisphosphonates and other bone resorption blockers | Decreased bone resorption can cause hypophosphatemia along with hypocalcemia. | [143,144] |
| Adriamycin | Inhibits Pi transport by PIC in reconstituted liposomes. | [145] |
| Hyperphosphatemic | ||
| High phytate/low Ca2+ diet | Low dietary Ca2+ causes Pi hyperabsorption. The associated homeostatic response induces secondary hyperparathyroidism. | [113] |
| Tumor lysis syndrome and rhabdomyolysis | Release of intracellular Pi from lysed cells may result in hyperphosphatemia. | [146,147] |
| Bone metastases | Tumor metastasis can increase bone resorption, which may result in hyperphosphatemia and hypercalcemia. | [148] |
| Kidney failure (e.g., CKD) | Reduced number of nephrons decreases renal Pi excretion, resulting in hyperphosphatemia. | [149] |
| Lowered gastric pH | May increase Pi bioaccessibility and Pi absorption. | [107,108,110] |
| Drugs | ||
| Vitamin D | Increases intestinal absorption of Ca and Pi, increases bone resorption, suppresses PTH, and thereby reduces renal excretion of Pi, all of which contribute to hyperphosphatemia. | [7,54,55,66,124] |
| Pi supplementation | Pi-containing laxatives can induce severe hyperphosphatemia, nephrocalcinosis, and renal failure. | [105,106] |
| Pharmaceutical agents increase serum Pi | Refer to Table 1. | |
| FGFR Inhibitors | Inhibit renal FGF23 signaling. | [150] |
| Disorder | Abbreviation | Inheritance | Gene | Mechanism | Ref. |
|---|---|---|---|---|---|
| Hyperphosphatemic Disorders | |||||
| Hyperphosphatemic Familial Tumoral Calcinosis type 1 and the allelic variant Hyperostosis–Hyperphosphatemia Syndrome | HFTC1 HSS | AR AR | GALNT3 | FGF23 deficiency | [156,157] |
| Hyperphosphatemic Familial Tumoral Calcinosis Type 2 | HFTC2 | AR | FGF23 | FGF23 deficiency | [158,159] |
| Hyperphosphatemic Familial Tumoral Calcinosis Type 3 | HFTC3 | AR | KL | FGF23 resistance | [160] |
| Idiopathic Hyperphosphatasia (Juvenile Paget’s Disease) | N/A | AR | TNFRSF11B | OPG deficiency | [161] |
| Pseudohypoparathyroidism | PHP1A PHP1B | AD AD (impr.) | GNAS GNAS or up-stream regulatory region | PTH resistance, FGF23-independent | [162,163] |
| Familial Isolated Hypoparathyroidism | FIH | AD or AR | CASR GCMB PTH | PTH deficiency, FGF23-independent | [164,165,166] |
| Blomstrand disease | BOCD | AR | PTHR1 | PTH resistance, FGF23-independent | [167,168] |
| Hypophosphatemic Disorders | |||||
| X-linked hypophosphatemia | XLH | X-linked | PHEX | FGF23-dependent | [169] |
| Autosomal Dominant Hypophosphatemic Rickets | ADHR | AD | FGF23 | FGF23-dependent | [170] |
| Autosomal Dominant Hypophosphatemic Rickets | ADHR | AD | KL | FGF23-dependent | [171] |
| Autosomal Recessive Hypophosphatemic Rickets types 1, 2, and 3 | ARHR1 ARHR2 ARHR3 | AR | DMP1 ENPP1 FAM20C | FGF23-dependent | [172,173,174] |
| Hereditary Hypophosphatemic Rickets with Hypercalciuria | HHRH | AR | SLC34A3 | Proximal tubular Pi wasting, FGF23-independent | [175,176] |
| Vitamin D-resistant rickets type 1A | VDDR1A | AR | CYP27B1 | 1,25(OH)2D deficiency, FGF23-independent | [154,177] |
| Hereditary 1,25(OH)2D-resistant rickets | HVDDR | AR | VDR | 1,25(OH)2D resistance, FGF23-independent | [153,178] |
| Familial hypocalciuric hypercalcemia/neonatal severe hyperparathyroidism | FHH NSHPT | AD/AR | CASR | PTH excess, FGF23-independent | [179] |
| Jansen disease | AD | PTHR1 | Const. active PTHR1, FGF23-dependent | [180,181] | |
| Normophosphatemic disorders | |||||
| Pulmonary alveolar microlithiasis | PAM | AR | SLC34A2 | Reduced alveolar epithelial Pi uptake | [35] |
| Normophosphatemic familial tumoral calcinosis | NFTC | AR | SAMD9 | Unknown | [182] |
| Muscle dystrophy and cardiomyopathy | MDC | AR | SLC25A3 | Reduced mitochondrial Pi uptake | [183,184] |
| Primary familial basal ganglial calcification type 1 | PFBC1 or IBGC1 | AD | PIT2 | Reduced microglial Pi uptake | [185] |
| Primary familial basal ganglial calcification type 4 | PFBC4 or IBGC4 | AD | PDGFRB | Reduced PIT2 expression | [186] |
| Primary familial basal ganglial calcification type 5 | PFBC5 or IBGC5 | AD | PDGFB | Reduced PIT2 expression | [186] |
| Primary familial basal ganglial calcification type 6 | PFBC6 or IBGC6 | AD | XPR1 | Reduced vascular Pi export | [187] |
| Primary familial basal ganglial calcification type 7 | PFBC7 or IBGC7 | AR | MYORG | Unclear, astrocyte dysfunction and possible NVU disruption may be causative factors. | [188,189] |
| Primary familial basal ganglial calcification type 8 | PFBC8 or IBGC8 | AR | JAM2 | Reduced JAM2 expression | [190,191] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serna, J.; Bergwitz, C. Importance of Dietary Phosphorus for Bone Metabolism and Healthy Aging. Nutrients 2020, 12, 3001. https://doi.org/10.3390/nu12103001
Serna J, Bergwitz C. Importance of Dietary Phosphorus for Bone Metabolism and Healthy Aging. Nutrients. 2020; 12(10):3001. https://doi.org/10.3390/nu12103001
Chicago/Turabian StyleSerna, Juan, and Clemens Bergwitz. 2020. "Importance of Dietary Phosphorus for Bone Metabolism and Healthy Aging" Nutrients 12, no. 10: 3001. https://doi.org/10.3390/nu12103001
APA StyleSerna, J., & Bergwitz, C. (2020). Importance of Dietary Phosphorus for Bone Metabolism and Healthy Aging. Nutrients, 12(10), 3001. https://doi.org/10.3390/nu12103001





