Effect of a Nutrient-Rich, Food-Based Supplement Given to Rural Vietnamese Mothers Prior to or during Pregnancy on the Trajectories of Nutrient Biomarkers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Maternal Measurements
2.3. Statistical Analyses
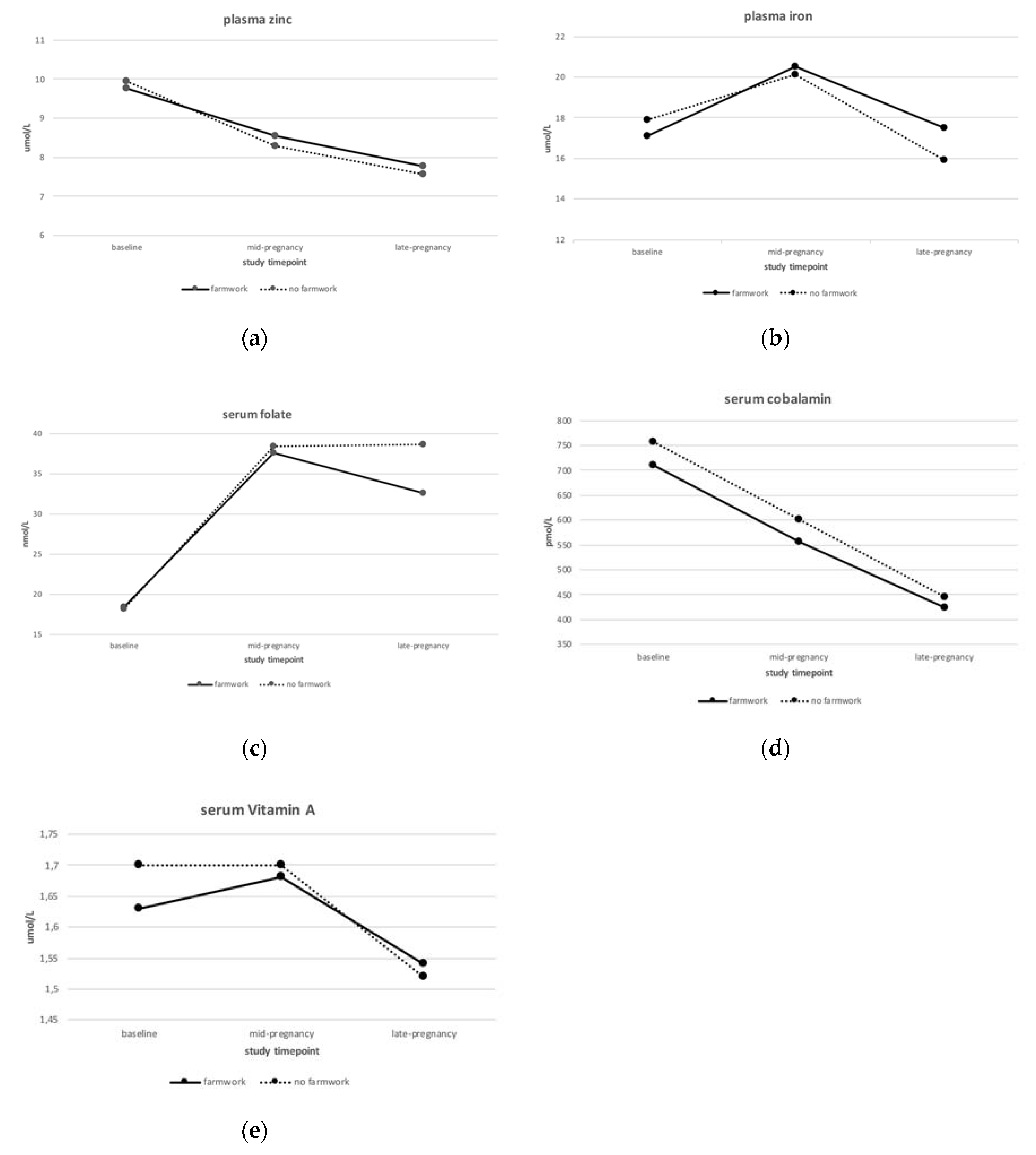
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar] [CrossRef]
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; WHO Technical Report Series 916; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Lee, S.E.; Talegawkar, S.A.; Merialdi, M.; Caulfield, L.E. Dietary intakes of women during pregnancy in low- and middle-income countries. Public Health Nutr. 2013, 16, 1340–1353. [Google Scholar] [CrossRef]
- Torheim, L.E.; Ferguson, E.L.; Penrose, K.; Arimond, M. Women in resource-poor settings are at risk of inadequate intakes of multiple micronutrients. J. Nutr. 2010, 140, 2051S–2058S. [Google Scholar] [CrossRef]
- Cole, C.R.; Grant, F.K.; Swaby-Ellis, E.D.; Smith, J.L.; Jacques, A.; Northrop-Clewes, C.A.; Caldwell, K.L.; Pfeiffer, C.M.; Ziegler, T.R. Zinc and iron deficiency and their interrelations in low-income African American and Hispanic children in Atlanta. Am. J. Clin. Nutr. 2010, 91, 1027–1034. [Google Scholar] [CrossRef]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Allen, L.H. Multiple micronutrients in pregnancy and lactation: An overview. Am. J. Clin. Nutr. 2005, 81, 1206S–1212S. [Google Scholar] [CrossRef]
- Christian, P.; Stewart, C.P. Maternal micronutrient deficiency, fetal development, and the risk of chronic disease. J. Nutr. 2010, 140, 437–445. [Google Scholar] [CrossRef]
- Zeisel, S.H. Is maternal diet supplementation beneficial? Optimal development of infant depends on mother’s diet. Am. J. Clin. Nutr. 2009, 89, 685S–687S. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Bhutta, Z.A.; Das, J.K.; Rizvi, A.; Gaffey, M.F.; Walker, N.; Horton, S.; Webb, P.; Lartey, A.; Black, R.E.; The Lancet Nutrition Interventions Review Group, and the Maternal and Child Nutrition Study Group. Evidence-based interventions for improvement of maternal and child nutrition: What can be done and at what cost? Lancet 2013, 382, 452–477. [Google Scholar] [CrossRef]
- Keats, E.C.; Haider, B.A.; Tam, E.; Bhutta, Z.A. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019, 3, CD004905. [Google Scholar] [CrossRef]
- Smith, E.R.; Shankar, A.H.; Wu, L.S.; Aboud, S.; Adu-Afarwuah, S.; Ali, H.; Agustina, R.; Arifeen, S.; Ashorn, P.; Bhutta, Z.A.; et al. Modifiers of the effect of maternal multiple micronutrient supplementation on stillbirth, birth outcomes, and infant mortality: A meta-analysis of individual patient data from 17 randomised trials in low-income and middle-income countries. Lancet Glob. Health 2017, 5, e1090–e1100. [Google Scholar] [CrossRef]
- Looman, M.; Geelen, A.; Samlal, R.A.K.; Heijligenberg, R.; Klein Gunnewiek, J.M.T.; Balvers, M.G.J.; Wijnberger, L.D.E.; Brouwer-Brolsma, E.M.; Feskens, E.J.M. Changes in Micronutrient Intake and Status, Diet Quality and Glucose Tolerance from Preconception to the Second Trimester of Pregnancy. Nutrients 2019, 11, 460. [Google Scholar] [CrossRef] [PubMed]
- Looman, M.; van den Berg, C.; Geelen, A.; Samlal, R.A.K.; Heijligenberg, R.; Klein Gunnewiek, J.M.T.; Balvers, M.G.J.; Leendertz-Eggen, C.L.; Wijnberger, L.D.E.; Feskens, E.J.M.; et al. Supplement Use and Dietary Sources of Folate, Vitamin D, and n-3 Fatty Acids during Preconception: The GLIMP2 Study. Nutrients 2018, 10, 962. [Google Scholar] [CrossRef] [PubMed]
- Schulze, K.J.; Mehra, S.; Shaikh, S.; Ali, H.; Shamim, A.A.; Wu, L.S.; Mitra, M.; Arguello, M.A.; Kmush, B.; Sungpuag, P.; et al. Antenatal Multiple Micronutrient Supplementation Compared to Iron-Folic Acid Affects Micronutrient Status but Does Not Eliminate Deficiencies in a Randomized Controlled Trial Among Pregnant Women of Rural Bangladesh. J. Nutr. 2019, 149, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Ziaei, S.; Rahman, A.; Raqib, R.; Lonnerdal, B.; Ekstrom, E.C. A Prenatal Multiple Micronutrient Supplement Produces Higher Maternal Vitamin B-12 Concentrations and Similar Folate, Ferritin, and Zinc Concentrations as the Standard 60-mg Iron Plus 400-mug Folic Acid Supplement in Rural Bangladeshi Women. J. Nutr. 2016, 146, 2520–2529. [Google Scholar] [CrossRef]
- Potdar, R.D.; Sahariah, S.A.; Gandhi, M.; Kehoe, S.H.; Brown, N.; Sane, H.; Dayama, M.; Jha, S.; Lawande, A.; Coakley, P.J.; et al. Improving women’s diet quality preconceptionally and during gestation: Effects on birth weight and prevalence of low birth weight—A randomized controlled efficacy trial in India (Mumbai Maternal Nutrition Project). Am. J. Clin. Nutr. 2014, 100, 1257–1268. [Google Scholar] [CrossRef]
- Ramakrishnan, U.; Grant, F.; Goldenberg, T.; Zongrone, A.; Martorell, R. Effect of women’s nutrition before and during early pregnancy on maternal and infant outcomes: A systematic review. Paediatr. Perinat. Epidemiol 2012, 26 (Suppl. 1), 285–301. [Google Scholar] [CrossRef]
- Nga, H.T.; Quyen, P.N.; Chaffee, B.W.; Diep Anh, N.T.; Ngu, T.; King, J.C. Effect of a nutrient-rich, food-based supplement given to rural Vietnamese mothers prior to and/or during pregnancy on birth outcomes: A randomized controlled trial. PLoS ONE 2020, 15, e0232197. [Google Scholar] [CrossRef]
- Tu, N.; King, J.C.; Dirren, H.; Thu, H.N.; Ngoc, Q.P.; Diep, A.N. Effect of animal-source food supplement prior to and during pregnancy on birthweight and prematurity in rural Vietnam: A brief study description. Food Nutr. Bull. 2014, 35, S205–S208. [Google Scholar] [CrossRef]
- Ministry of Health; National Institute of Nutrition. Vietnamese Food Composition Tables; Medical Publishing House: Hanoi, Vietnam, 2007. [Google Scholar]
- Chung, C.S.; Stookey, J.; Dare, D.; Welch, R.; Nguyen, T.Q.; Roehl, R.; Peerson, J.M.; King, J.C.; Brown, K.H. Current dietary zinc intake has a greater effect on fractional zinc absorption than does longer term zinc consumption in healthy adult men. Am. J. Clin. Nutr. 2008, 87, 1224–1229. [Google Scholar] [CrossRef]
- O’Broin, S.; Kelleher, B. Microbiological assay on microtitre plates of folate in serum and red cells. J. Clin. Pathol. 1992, 45, 344–347. [Google Scholar] [CrossRef]
- Association of Official Agricultural Chemists; Horwitz, W. Official Methods of Analysis, 16th ed.; AOAC International: Gaithersburg, MD, USA, 1995. [Google Scholar]
- World Health Organization and Food and Agriculture Organization of the United Nations. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; Report of a Joint FAO/WHO Expert Consultation; Food and Agriculture Organization: Rome, Italy, 2004. [Google Scholar]
- World Health Organization/Centers for Disease Control and Prevention. Assessing the Iron Status of Populations: Report of a Joint World Health Organization/Centers for Disease Control and Prevention Technical Consultation on the Assessment of Iron Status at the Population Level; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- de Benoist, B. Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies. Food Nutr. Bull. 2008, 29, S238–S244. [Google Scholar] [CrossRef]
- King, J.C.; Brown, K.H.; Gibson, R.S.; Krebs, N.F.; Lowe, N.M.; Siekmann, J.H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)-Zinc Review. J. Nutr. 2015, 146, 858S–885S. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005; WHO global database on vitamin A deficiency; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- World Health Organization. Serum and Red Blood Cell Folate Concentrations for Assessing Folate Status in Populations Vitamin and Mineral Nutrition Information System. Geneva (Switzerland). Available online: http://apps.who.int/iris/bitstream/10665/162114/1/WHO_NMH_NHD_EPG_15.01.pdf?ua=1 (accessed on 27 August 2020).
- King, J.C. A Summary of Pathways or Mechanisms Linking Preconception Maternal Nutrition with Birth Outcomes. J. Nutr. 2016, 146, 1437S–1444S. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.H.; Young, M.; Gonzalez-Casanova, I.; Pham, H.Q.; Nguyen, H.; Truong, T.V.; Nguyen, S.V.; Harding, K.B.; Reinhart, G.A.; Martorell, R.; et al. Impact of Preconception Micronutrient Supplementation on Anemia and Iron Status during Pregnancy and Postpartum: A Randomized Controlled Trial in Rural Vietnam. PLoS ONE 2016, 11, e0167416. [Google Scholar] [CrossRef] [PubMed]
- Hambidge, K.M.; Westcott, J.E.; Garces, A.; Figueroa, L.; Goudar, S.S.; Dhaded, S.M.; Pasha, O.; Ali, S.A.; Tshefu, A.; Lokangaka, A.; et al. A multicountry randomized controlled trial of comprehensive maternal nutrition supplementation initiated before conception: The Women First trial. Am. J. Clin. Nutr. 2019, 109, 457–469. [Google Scholar] [CrossRef]
- Dhaded, S.M.; Hambidge, K.M.; Ali, S.A.; Somannavar, M.; Saleem, S.; Pasha, O.; Khan, U.; Herekar, V.; Vernekar, S.; Kumar, S.Y.; et al. Preconception nutrition intervention improved birth length and reduced stunting and wasting in newborns in South Asia: The Women First Randomized Controlled Trial. PLoS ONE 2020, 15, e0218960. [Google Scholar] [CrossRef]
- Christian, P.; Jiang, T.; Khatry, S.K.; LeClerq, S.C.; Shrestha, S.R.; West, K.P., Jr. Antenatal supplementation with micronutrients and biochemical indicators of status and subclinical infection in rural Nepal. Am. J. Clin. Nutr. 2006, 83, 788–794. [Google Scholar] [CrossRef]
- Berti, C.; Fekete, K.; Dullemeijer, C.; Trovato, M.; Souverein, O.W.; Cavelaars, A.; Dhonukshe-Rutten, R.; Massari, M.; Decsi, T.; Van’t Veer, P.; et al. Folate intake and markers of folate status in women of reproductive age, pregnant and lactating women: A meta-analysis. J. Nutr. Metab. 2012, 2012, 470656. [Google Scholar] [CrossRef]
- Arimond, M.; Vitta, B.S.; Martin-Prevel, Y.; Moursi, M.; Dewey, K.G. Local foods can meet micronutrient needs for women in urban Burkina Faso, but only if rarely consumed micronutrient-dense foods are included in daily diets: A linear programming exercise. Matern. Child. Nutr. 2018, 14, e12461. [Google Scholar] [CrossRef]
- Neggers, Y.; Goldenberg, R.L. Some thoughts on body mass index, micronutrient intakes and pregnancy outcome. J. Nutr. 2003, 133, 1737S–1740S. [Google Scholar] [CrossRef]
- Swanson, C.A.; King, J.C. Reduced serum zinc concentration during pregnancy. Obstet. Gynecol. 1983, 62, 313–318. [Google Scholar] [CrossRef] [PubMed]

| Mean (SD)/Median (25th, 75th Percentile) | |
|---|---|
| Age at Random Assignment, Year | 21.4 (2.85) |
| Highest educational level, % | |
| elementary school | 2.3% |
| middle school | 56.6% |
| high school | 20.6% |
| occupational school or higher | 20.6% |
| Living arrangement, % | |
| with parents-in-law | 71.4% |
| with husband only | 10.5% |
| with parents | 18.1% |
| Household latrine, % | |
| non/field/bush | 1.0% |
| uncovered | 5.1% |
| covered | 63.8% |
| flush | 30.1% |
| Work as farmers, % | 77.9% |
| Anthropometry | |
| weight, kg | 45,9 (4.85) |
| height, cm | 152.8 (5.09) |
| MUAC, cm | 24.02 (1.80) |
| AMA, cm2 | 23.3 (4.83) |
| AFA, cm2 | 16.4 (4.38) |
| BMI, kg/m2 | 19.7 (1.77) |
| Nutrient intakes 1 | |
| energy intake, kcal/day | 1748 (355) |
| carbohydrate intake, en% | 65.5 (6.90) |
| fat intake, en% | 18.8 (6.14) |
| protein intake, en% | 16.0 (2.01) |
| iron intake, mg/day | 12.7 (3.69) |
| zinc intake, mg/day | 9.14 (2.26) |
| vitamin A intake, μg/day | 476 (284, 681) |
| folate intake, μg/day | 314 (144) |
| vitamin B12 intake, μg/day | 1.78 (0.79, 2.71) |
| Nutritional status measurements 2 | |
| hemoglobin, g/dL | 12.9 (1.20) |
| hematocrit, % | 40.3 (2.93) |
| ferritin, μg/L | 43.3 (25.71, 83.34) |
| plasma iron, μmol/L | 17.2 (6.61) |
| plasma zinc, μmol/L | 9.79 (1.41) |
| serum vitamin A, μmol/L | 1.64 (0.40) |
| serum folate, nmol/L | 21.7 (10.7) |
| serum cobalamin, pmol/L | 723 (572, 895) |
| CRP, mg/L | 0.20 (0.10, 0.50) |
| Farmworkers n = 247 (77.9%) | Non-Farmworkers n = 70 (22.1%) | p2 for Difference | |
|---|---|---|---|
| age at random assignment, year | 21.0 (2.84) | 22.7 (2.54) | <0.0001 |
| Highest educational level, % | <0.0001 | ||
| elementary school | 2.9% | 0% | |
| middle school | 68.2% | 14.7% | |
| high school | 19.8% | 23.5% | |
| occupational school or higher | 9.1% | 61.8% | |
| Living arrangement, % | 0.002 | ||
| with parents-in-law | 75.5% | 56.5% | |
| with husband only | 10.6% | 10.1% | |
| with parents | 13.9% | 33.3% | |
| Household latrine, % | <0.0001 | ||
| non/field/bush | 1.2% | 0% | |
| uncovered | 5.8% | 2.9% | |
| covered | 69.6% | 42.7% | |
| flush | 23.5% | 54.4% | |
| Anthropometry | |||
| weight, kg | 46.0 (4.80) | 45.6 (4.94) | 0.3 |
| height, cm | 153 (5.07) | 153 (5.16) | 0.3 |
| MUAC, cm | 24.1 (1.77) | 23.8 (1.90) | 0.3 |
| AMA, cm2 | 23.5 (4.69) | 22.6 (5.29) | 0.07 |
| AFA, cm2 | 16.3 (4.38) | 16.4 (4.44) | 0.7 |
| BMI, kg/m2 | 19.7 (1.72) | 19.4 (1.92) | 0.1 |
| gestational weight gain, kg | 6.67 (2.96) | 9.65 (4.28) | <0.0001 |
| Nutrient intakes 3 | |||
| energy intake, kcal/day | 1751 (375) | 1739 (286) | 0.7 |
| carbohydrate intake, en% | 66.1 (6.99) | 63.5 (6.33) | 0.003 |
| fat intake, en% | 18.4 (6.22) | 20.3 (5.71) | 0.02 |
| protein intake, en% | 15.8 (1.93) | 16.6 (2.18) | 0.007 |
| iron intake, mg/day | 12.5 (3.21) | 13.3 (5.00) | 0.3 |
| zinc intake, mg/day | 9.06 (2.33) | 9.4 (2.02) | 0.09 |
| vitamin A intake, μg/day | 476 (291, 677) | 462 (264, 793) | 0.6 |
| folate intake, μg/day | 315 (146) | 309 (138) | 0.9 |
| vitamin B12 intake, μg/day | 1.75 (0.75, 2.66) | 1.98 (0.83, 3.26) | 0.1 |
| Nutritional status measurements 4 | |||
| hemoglobin, g/dL | 12.8 (1.21) | 13.1 (1.14) | 0.08 |
| hematocrit, % | 40.3 (2.94) | 40.1 (2.95) | 0.5 |
| ferritin, μg/L | 44.2 (25.1, 88.6) | 42.8 (26.4, 78.2) | 0.7 |
| plasma iron, μmol/L | 17.1 (6.13) | 17.9 (8.59) | 0.9 |
| plasma zinc, μmol/L | 9.8 (1.42) | 10.0 (1.34) | 0.4 |
| serum vitamin A, μmol/L | 1.6 (0.40) | 1.70 (0.39) | 0.2 |
| serum folate, nmol/L | 21.4 (10.7) | 22.3 (11.0) | 0.6 |
| serum cobalamin, pmol/L | 711 (574, 895) | 757 (575, 896) | 0.5 |
| CRP, mg/L | 0.20 (0.10, 0.60) | 0.20 (0.10, 0.50) | 0.8 |
| 1. Plasma Zinc Trajectories | ||||
| (a) Mixed Model | (b) Mixed Model Including Baseline Plasma Zinc | |||
| ß (SE) | p-Value | ß (SE) | p-Value | |
| baseline dietary zinc intake, mg/day | 0.0813 (0.0601) | 0.2 | 0.0385 (0.0407) | 0.3 |
| concurrent change in dietary zinc intake, mg/day | −0.0057 (0.0254) | 0.8 | −0.0059 (0.0171) | 0.7 |
| baseline energy intake, kcal/day | −0.0007 (0.0003) | 0.045 | −0.0003 (0.0002) | 0.3 |
| intervention group | 0.0264 (0.2034) | 0.9 | 0.1321 (0.1403) | 0.3 |
| maternal age, years | 0.0262 (0.0283) | 0.4 | 0.0081 (0.0193) | 0.7 |
| maternal BMI before pregnancy, kg/m2 | 0.0076 (0.0460) | 0.9 | −0.0305 (0.0320) | 0.3 |
| farm work (Ref group: farm work) | −0.2745 (0.1909) | 0.2 | −0.2962 (0.1294) | 0.02 |
| household latrine (no vs. flush latrine, flush = Ref) | −0.7587 (0.6840) | 0.3 | 0.0465 (0.4595) | 0.9 |
| gestational weight gain, kg | −0.0266 (0.0254) | 0.3 | −0.0236 (0.0171) | 0.2 |
| baseline CRP, mg/L | −0.0438 (0.0620) | 0.5 | 0.0374 (0.0562) | 0.5 |
| baseline plasma zinc levels, mmol/L | 0.5135 (0.0375) | <0.0001 | ||
| 2. Plasma Iron Trajectories | ||||
| (a) Mixed Model | (b) Mixed Model Including Baseline Plasma Iron | |||
| baseline dietary iron intake, mg/day | −0.0775 (0.1088) | 0.5 | −0.0125 (0.0796) | 0.9 |
| concurrent change in dietary iron intake, mg/day | 0.0221 (0.0535) | 0.7 | 0.0103 (0.0396) | 0.8 |
| baseline energy intake, kcal/day | 0.0009 (0.0012) | 0.5 | 0.0004 (0.0009) | 0.7 |
| intervention group | −0.8230 (1.0093) | 0.4 | 0.8653 (0.7723) | 0.3 |
| maternal age, years | 0.0660 (0.1351) | 0.6 | 0.0918 (0.1004) | 0.4 |
| maternal BMI before pregnancy, kg/m2 | −0.0725 (0.2211) | 0.7 | −0.1478 (0.1661) | 0.4 |
| farm work (Ref group: farm work) | −0.3270 (0.9178) | 0.7 | −0.7109 (0.6788) | 0.3 |
| household latrine (no vs. flush latrine, flush = Ref) | −2.1156 (3.2385) | 0.5 | −1.1767 (2.3558) | 0.5 |
| gestational weight gain, kg | 0.0137 (0.1201) | 0.9 | 0.0572 (0.0884) | 0.5 |
| baseline CRP, mg/L | −0.1726 (0.2983) | 0.6 | 0.4588 (0.3002) | 0.1 |
| baseline plasma iron levels, mmol/L | 0.5106 (0.0419) | <0.0001 | ||
| 3. Serum Folate Trajectories | ||||
| (a) Mixed Model | (b) Mixed Model Including Baseline Serum Folate | |||
| ß (SE) | p-Value | ß (SE) | p-value | |
| baseline dietary folate intake, mg/day | −0.0003 (0.0003) | 0.2 | −0.0002 (0.0002) | 0.3 |
| concurrent change in dietary folate intake, mg/day | 0.0001 (0.0002) | 0.8 | 0.0001 (0.0001) | 0.4 |
| baseline energy intake, kcal/day | 0.000004 (0.0001) | 1 | 0.00002 (0.0001) | 0.8 |
| intervention group | 0.0979 (0.0871) | 0.3 | 0.0390 (0.0652) | 0.6 |
| maternal age, years | 0.0213 (0.0116) | 0.07 | 0.0091 (0.0087) | 0.3 |
| maternal BMI before pregnancy, kg/m2 | 0.0044 (0.0190) | 0.8 | 0.0026 (0.0141) | 0.9 |
| farm work (Ref group: farm work) | 0.0355 (0.0777) | 0.6 | 0.0520 (0.0577) | 0.4 |
| household latrine (no vs. flush latrine, flush = Ref) | 0.0799 (0.2829) | 0.8 | 0.1731 (0.2071) | 0.4 |
| gestational weight gain, kg | −0.0074 (0.0102) | 0.5 | −0.0043 (0.0076) | 0.6 |
| baseline CRP, mg/L | 0.0493 (0.0242) | 0.04 | 0.0297 (0.0179) | 0.099 |
| baseline serum folate levels, nmol/L | 0.5507 (0.0492) | <0.0001 | ||
| 4. Serum Cobalamin Trajectories | ||||
| (a) Mixed Model | (b) Mixed Model Including Baseline Serum Cobalamin | |||
| baseline dietary cobalamin intake, mg/day | 0.0157 (0.0148) | 0.3 | −0.0044 (0.0064) | 0.5 |
| concurrent change in dietary cobalamin intake, mg/day | 0.0046 (0.0071) | 0.5 | −0.0016 (0.0031) | 0.6 |
| baseline energy intake, kcal/day | −0.0002 (0.0001) | 0.06 | 0.00002 (0.00004) | 0.6 |
| intervention group | 0.0344 (0.0762) | 0.7 | 0.0009 (0.0337) | 1 |
| maternal age, years | 0.0086 (0.0106) | 0.4 | −0.0014 (0.0046) | 0.8 |
| maternal BMI before pregnancy, kg/m2 | −0.0194 (0.0176) | 0.3 | −0.0124 (0.0076) | 0.1 |
| farm work (Ref group: farm work) | −0.0129 (0.0718) | 0.9 | 0.0116 (0.0311) | 0.7 |
| household latrine (no vs. flush latrine, flush = Ref) | 0.3166 (0.2569) | 0.2 | 0.1519 (0.1089) | 0.2 |
| gestational weight gain, kg | −0.0097 (0.0096) | 0.3 | −0.0125 (0.0041) | 0.003 |
| baseline CRP, mg/L | −0.0198 (0.0224) | 0.4 | −0.0067 (0.0096) | 0.5 |
| baseline serum cobalamin levels, pmol/L | 0.7128 (0.0311) | <0.0001 | ||
| 5. Serum Vitamin A Trajectories | ||||
| (a) Mixed Model | (b) Mixed Model Including Baseline Serum Vitamin A | |||
| baseline dietary vitamin A intake, mg/day | 0.0001 (0.0001) | 0.1 | 0.0001 (0.0001) | 0.03 |
| concurrent change in dietary vitamin A intake, mg/day | 0.00003 (0.00003) | 0.3 | 0.00003 (0.00002) | 0.1 |
| baseline energy intake, kcal/day | −0.0001 (0.0001) | 0.2 | −0.00003 (0.00004) | 0.4 |
| intervention group | −0.0194 (0.0666) | 0.8 | 0.0102 (0.0440) | 0.8 |
| maternal age, years | −0.0063 (0.0086) | 0.5 | 0.0011 (0.0057) | 0.8 |
| maternal BMI before pregnancy, kg/m2 | 0.0073 (0.0142) | 0.6 | −0.0071 (0.0094) | 0.4 |
| farm work (Ref group: farm work) | −0.0103 (0.0579) | 0.9 | −0.0411 (0.0380) | 0.3 |
| household latrine (no vs. flush latrine, flush = Ref) | 0.0714 (0.2064) | 0.7 | 0.1157 (0.1329) | 0.4 |
| gestational weight gain, kg | 0.0224 (0.0077) | 0.004 | 0.0107 (0.0051) | 0.04 |
| baseline CRP, mg/L | −0.0122 (0.0182) | 0.5 | 0.0062 (0.0119) | 0.6 |
| baseline serum vitamin A levels, mmol/L | 0.5641 (0.0395) | <0.0001 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goletzke, J.; Nga, H.T.; Quyen, P.N.; Ngu, T.; King, J.C. Effect of a Nutrient-Rich, Food-Based Supplement Given to Rural Vietnamese Mothers Prior to or during Pregnancy on the Trajectories of Nutrient Biomarkers. Nutrients 2020, 12, 2913. https://doi.org/10.3390/nu12102913
Goletzke J, Nga HT, Quyen PN, Ngu T, King JC. Effect of a Nutrient-Rich, Food-Based Supplement Given to Rural Vietnamese Mothers Prior to or during Pregnancy on the Trajectories of Nutrient Biomarkers. Nutrients. 2020; 12(10):2913. https://doi.org/10.3390/nu12102913
Chicago/Turabian StyleGoletzke, Janina, Hoang T. Nga, Phi N. Quyen, Tu Ngu, and Janet C. King. 2020. "Effect of a Nutrient-Rich, Food-Based Supplement Given to Rural Vietnamese Mothers Prior to or during Pregnancy on the Trajectories of Nutrient Biomarkers" Nutrients 12, no. 10: 2913. https://doi.org/10.3390/nu12102913
APA StyleGoletzke, J., Nga, H. T., Quyen, P. N., Ngu, T., & King, J. C. (2020). Effect of a Nutrient-Rich, Food-Based Supplement Given to Rural Vietnamese Mothers Prior to or during Pregnancy on the Trajectories of Nutrient Biomarkers. Nutrients, 12(10), 2913. https://doi.org/10.3390/nu12102913




