Food Addiction Is Associated with Binge Eating and Psychiatric Distress among Post-Operative Bariatric Surgery Patients and May Improve in Response to Cognitive Behavioural Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Study Procedures
2.3. Study Measures
2.4. Statistical Analysis
3. Results
3.1. Participant Flow and Characteristics
3.2. Correlates of Food Addiction 1 Year Post-Surgery
3.3. Comparison of Participants with versus without Food Addiction 1 Year Post-Surgery
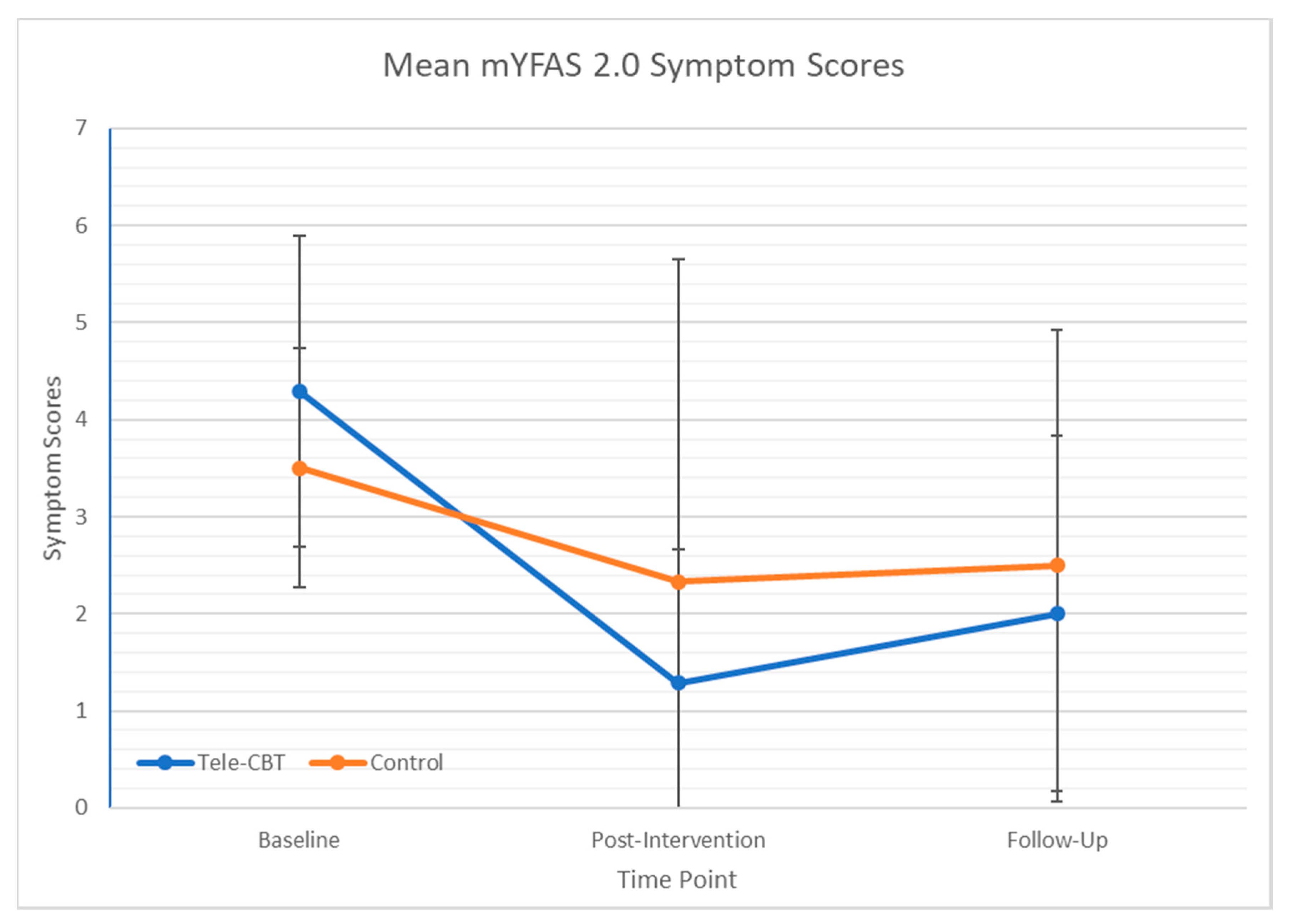
3.4. Changes in Food Addiction Following Tele-CBT
4. Discussion
5. Limitations and Future Research Directions
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arroyo-Johnson, C.; Mincey, K.D. Obesity epidemiology worldwide. Gastroenterol. Clin. N. Am. 2016, 45, 571–579. [Google Scholar] [CrossRef]
- Karlsson, J.; Taft, C.; Ryden, A.; Sjostrom, L.; Sullivan, M. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: The SOS intervention study. Int. J. Obes. 2007, 31, 1248–1261. [Google Scholar] [CrossRef]
- Puzziferri, N.; Roshek, T.B., III; Mayo, H.G.; Gallagher, R.; Belle, S.H.; Livingston, E.H. Long-term follow-up after bariatric surgery: A systematic review. JAMA 2014, 312, 934–942. [Google Scholar] [CrossRef]
- Sjöström, L.; Lindroos, A.K.; Peltonen, M.; Torgerson, J.; Bouchard, C.; Carlsson, B.; Dahlgren, S.; Larsson, B.; Narbro, K.; Sjöström, C.D.; et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N. Engl. J. Med. 2004, 351, 2683–2693. [Google Scholar] [CrossRef]
- Courcoulas, A.P.; Christian, N.J.; Belle, S.H.; Berk, P.D.; Flum, D.R.; Garcia, L.; Horlick, M.; Kalarchian, M.A.; King, W.C.; Mitchell, J.E.; et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA 2013, 310, 2416–2425. [Google Scholar] [CrossRef]
- Courcoulas, A.P.; King, W.C.; Belle, S.H.; Berk, P.; Flum, D.R.; Garcia, L.; Gourash, W.; Horlick, M.; Mitchell, J.E.; Pomp, A.; et al. Seven-Year Weight Trajectories and Health Outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. 2018, 153, 427–434. [Google Scholar] [CrossRef]
- Dimeglio, C.; Becouarn, G.; Topart, P.; Bodin, R.; Buisson, J.C.; Ritz, P. Weight Loss Trajectories After Bariatric Surgery for Obesity: Mathematical Model and Proof-of-Concept Study. JMIR Med. Inform. 2020, 8, e13672. [Google Scholar] [CrossRef]
- Monaco-Ferreira, D.V.; Leandro-Merhi, V.A. Weight Regain 10 Years After Roux-en-Y Gastric Bypass. Obes. Surg. 2017, 27, 1137–1144. [Google Scholar] [CrossRef]
- Magro, D.O.; Geloneze, B.; Delfini, R.; Pereja, B.C.; Callejas, F.; Pereja, J.C. Longterm weight regain after gastric bypass: A 5-year prospective study. Obes. Surg. 2008, 18, 648–651. [Google Scholar] [CrossRef]
- Davis, J.A.; Saunders, R. Impact of weight trajectory after bariatric surgery on co-morbidity evolution and burden. BMC Health Serv. Res. 2020, 20, 278. [Google Scholar] [CrossRef]
- Devlin, M.J.; King, W.C.; Kalarchian, M.A.; Hinerman, A.; Marcus, M.D.; Yanovski, S.Z.; Mitchell, J.E. Eating pathology and associations with long-term changes in weight and quality of life in the longitudinal assessment of bariatric surgery study. Int. J. Eat. Disord. 2018, 51, 1322–1330. [Google Scholar] [CrossRef]
- Meany, G.; Conceiҫão, E.; Mitchell, J.E. Binge eating, binge eating disorder and loss of control eating: Effects on weight outcomes after bariatric surgery. Eur. Eat. Disord. Rev. 2014, 22, 87–91. [Google Scholar] [CrossRef]
- Pizato, N.; Botelho, P.B.; Gonçalves, V.S.S.; Dutra, E.S.; de Carvalho, K.M.B. Effect of grazing behavior on weight regain post-bariatric surgery: A systematic review. Nutrients 2017, 9, 1322. [Google Scholar] [CrossRef]
- Schulte, E.M.; Avena, N.M.; Gearhardt, A.N. Which foods may be addictive? The roles of processing, fat content, and glycemic load. PLoS ONE 2015, 10, e0117959. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; Davis, C.; Kuschner, R.; Brownell, K.D. The addiction potential of hyperpalatable foods. Curr. Drug Abuse Rev. 2011, 4, 140–145. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Randolph, T.G. The descriptive features of food addiction; addictive eating and drinking. Q. J. Stud. Alcohol. 1956, 17, 198–224. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; Corbin, W.R.; Brownell, K.D. Preliminary validation of the Yale Food Addition Scale. Appetite 2009, 52, 430–436. [Google Scholar] [CrossRef]
- Ivezaj, V.; Wiedemann, A.A.; Grilo, C.M. Food addiction and bariatric surgery: A systematic review of the literature. Obes. Rev. 2017, 18, 1386–1397. [Google Scholar] [CrossRef]
- Davis, C.; Curtis, C.; Levitan, R.D.; Carter, J.C.; Kaplan, A.S.; Kennedy, J.L. Evidence that ‘food addiction’ is a valid phenotype of obesity. Appetite 2011, 57, 711–717. [Google Scholar] [CrossRef]
- Ivezaj, V.; White, M.A.; Grilo, C.M. Examining binge-eating disorder and food addiction in adults with overweight and obesity. Obesity 2016, 24, 2064–2069. [Google Scholar] [CrossRef]
- Benzerouk, F.; Gierski, F.; Ducluzeau, P.-H.; Bourbao-Tournois, C.; Gaubil-Kaladjian, I.; Bertin E’ Kaladjian, A.; Ballon, N.; Brunault, P. Food addiction, in obese patients seeking bariatric surgery, is associated with higher prevalence of current mood and anxiety disorders and past mood disorders. Pyschiatry Res. 2018, 267, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Meule, A.; Heckel, D.; Jurowich, C.F.; Vogele, C.; Kubler, A. Correlates of food addiction in obese individuals seeking bariatric surgery. Clin. Obes. 2014, 4, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Koball, A.M.; Clark, M.M.; Collazo-Clavell, M.; Kellogg, T.; Ames, G.; Ebbert, J.; Grothe, K.B. The relationship among food addiction, negative mood, and eating-disordered behaviors in patients seeking to have bariatric surgery. Surg. Obes. Relat. Dis. 2016, 12, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Burrows, T.; Skinner, J.; McKenna, R.; Rollo, M. Food addition, binge eating disorder, and obesity: Is there a relationship? Behav. Sci. 2017, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Davis, C. From passive overeating to “food addiction”: A spectrum of compulsion and severity. ISRN Obes. 2013, 2013, 435027. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; Boswell, R.G.; White, M.A. The association of “food addiction” with disordered eating and body mass index. Eat. Behav. 2014, 15, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.M.; Saules, K.K. Validation of the Yale Food Addiction Scale among a weight-loss surgery population. Eat. Behav. 2013, 14, 216–219. [Google Scholar] [CrossRef]
- Koball, A.M.; Ames, G.; Goetze, R.E.; Grothe, K. Bariatric Surgery as a Treatment for Food Addiction? A Review of the Literature. Curr. Addict. Rep. 2000, 7, 1–8. [Google Scholar] [CrossRef]
- Cassin, S.E.; Sijercic, I.; Montemarano, V. Psychosocial Interventions for Food Addiction: A Systematic Review. Curr. Addict. Rep. 2020, 7, 9–19. [Google Scholar] [CrossRef]
- Cassin, S.E.; Sockalingam, S.; Du, C.; Wnuk, S.; Hawa, R.; Parikh, S.V. A pilot randomized controlled trial of telephone-based cognitive behavioural therapy for preoperative bariatric surgery patients. Behav. Res. Ther. 2016, 80, 17–22. [Google Scholar] [CrossRef]
- Sockalingam, S.; Cassin, S.E.; Wnuk, S.; Du, C.; Jackson, T.; Hawa, R.; Parikh, S.V. A Pilot Study on Telephone Cognitive Behavioral Therapy for Patients Six-Months Post-Bariatric Surgery. Obes. Surg. 2017, 27, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Sockalingam, S.; Leung, S.E.; Hawa, R.; Wnuk, S.; Parikh, S.V.; Jackson, T.; Cassin, S.E. Telephone-based cognitive behavioural therapy for female patients 1-year post-bariatric surgery: A pitlo study. Obes. Res. Clin. Pract. 2019, 13, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Cassin, S.E.; Sockalingam, S.; Wnuk, S.; Strimas, R.; Royal, S.; Hawa, R.; Parikh, S.V. Cognitive behavioral therapy for bariatric surgery patients: Preliminary evidence for feasibility, acceptability, and effectiveness. Cogn. Behav. Pract. 2013, 20, 529–543. [Google Scholar] [CrossRef]
- Schulte, E.; Gearhardt, A.N. Development of the Modified Yale Food Addiction Scale Version 2.0. Eur. Eat. Disord. Rev. 2017, 25, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.M.; Martens, K.; Smith-Mason, C.E.; Hamann, A.; Miller-Matero, L.R. Validation of the Yale Food Addiction Scale 2.0 among a Bariatric Surgery Population. Obes. Surg. 2019, 29, 2923–2928. [Google Scholar] [CrossRef] [PubMed]
- Gormally, J.; Black, S.; Daston, S.; Rardin, D. The assessment of binge eating severity among obese persons. Addict. Behav. 1982, 7, 47–55. [Google Scholar] [CrossRef]
- Hood, M.M.; Grupski, A.E.; Hall, B.J.; Ivan, I.; Corsica, J. Factor structure and predictive utility of the Binge Eating Scale in bariatric surgery candidates. Surg. Obes. Relat. Dis. 2013, 9, 942–948. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.; Lowe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef]
- Cassin, S.; Sockalingam, S.; Hawa, R.; Wnuk, S.; Royal, S.; Taube-Schiff, M.; Okrainec, A. Psychometric properties of the Patient Health Questionnaire (PHQ-9) as a depression screening tool for bariatric surgery candidates. Psychosomatics 2013, 54, 352–358. [Google Scholar] [CrossRef]
- Sockalingam, S.; Wnuk, S.; Kantarovich, K.; Meaney, C.; Okrainec, A.; Hawa, R.; Cassin, S. Employment Outcomes One Year after Bariatric Surgery: The Role of Patient and Psychosocial Factors. Obes. Surg. 2015, 25, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Sockalingam, S.; Hawa, R.; Wnuk, S.; Santiago, V.; Kowgier, M.; Jackson, T.; Okrainec, A.; Cassin, S. Psychosocial predictors of quality of life and weight loss two years after bariatric surgery: Results from the Toronto Bari-PSYCH study. Gen. Hosp. Psychiatry 2017, 47, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.E.; Harris, G.T. Comparing effect sizes in follow-up studies: ROC area, Cohen’s d, and r. Law Hum. Behav. 2005, 29, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Pepino, M.Y.; Stein, R.I.; Eagon, J.C.; Klein, S. Bariatric surgery-induced weight loss causes remission of food addiction in extreme obesity. Obesity 2014, 22, 1792–1798. [Google Scholar] [CrossRef]
- Sevincer, G.M.; Konuk, N.; Bozkurt, S.; Coskun, H. Food addiction and the outcome of bariatric surgery at 1-year: Prospective observational study. Psychiatry Res. 2016, 244, 159–164. [Google Scholar] [CrossRef]
- Leahey, T.M.; Bond, D.S.; Raynor, H.; Roye, D.; Vithiananthan, S.; Ryder, B.A.; Sax, H.C.; Wing, R.R. Effects of bariatric surgery on food cravings: Do food cravings and the consumption of craved foods “normalize” after surgery? Surg. Obes. Relat. Dis. 2012, 8, 84–91. [Google Scholar] [CrossRef]
- Berthoud, H.R.; Zheng, H.; Shin, A.C. Food reward in the obese and after weight loss induced by calorie restriction and bariatric surgery. Ann. N. Y. Acad. Sci. 2012, 1264, 36–48. [Google Scholar] [CrossRef]
- Le Roux, C.W.; Bueter, M.; Theis, N.; Werling, M.; Ashrafian, H.; Löwenstein, C.; Athanasiou, T.; Bloom, S.R.; Spector, A.C.; Olbers, T.; et al. Gastric bypass reduces fat intake and preference. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1057–R1066. [Google Scholar] [CrossRef]
- Thomas, J.R.; Marcus, E. High and low fat food selection with reported frequency intolerance following Roux-en-Y gastric bypass. Obes. Surg. 2008, 18, 282–287. [Google Scholar] [CrossRef]
- Schultes, B.; Ernst, B.; Wilms, B.; Thurnheer, M.; Hallschmid, M. Hedonic hunger is increased in severely obese patients and is reduced after gastric bypass surgery. Am. J. Clin. Nutr. 2010, 92, 277–283. [Google Scholar] [CrossRef]
- Thirlby, R.C.; Bahiraei, F.; Randall, J.; Drewnoski, A. Effect of Roux-en-Y gastric bypass on satiety and food likes: The role of genetics. J. Gastrointest. Surg. 2006, 10, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Yanos, B.R.; Saules, K.K.; Schuh, L.M.; Sogg, S. Predictors of lowest weight and long-term weight regain among Roux-en-Y gastric bypass patients. Obes. Surg. 2015, 25, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Schulte, E.M.; Joyner, M.A.; Potenza, M.N.; Grilo, C.M.; Gearhardt, A.N. Current conditions regarding food addiction. Curr. Psychiatry Rep. 2015, 17, 563. [Google Scholar] [CrossRef] [PubMed]
- Costa-Dookhan, K.A.; Leung, S.E.; Cassin, S.E.; Sockalingam, S. Psychosocial predictors of response to telephone-based cognitive behavioural therapy in bariatric surgery patients. Can. J. Diabetes 2020, 44, 236–240. [Google Scholar] [CrossRef]
- Wiss, D.A.; Brewerton, T.D. Incorporating food addiction into disordered eating: The disordered eating food addiction nutrition guide (DEFANG). Eat. Weight Disord. 2017, 22, 49–59. [Google Scholar] [CrossRef]
- Treasure, J.; Leslie, M.; Chami, R.; Fernandez-Arand, F. Are trans diagnostic models of eating disorders fit for purpose? A consideration of the evidence for food addiction. Eur. Eat. Disord. Rev. 2018, 26, 83–91. [Google Scholar] [CrossRef]
| Variable | M (SD) or n (%) |
|---|---|
| Age (years) | 48.40 (8.51) |
| Gender (female) | 82 (82%) |
| Race/Ethnicity | |
| Black | 4 (4%) |
| East Asian | 1 (1%) |
| Latin American | 3 (3%) |
| South Asian | 1 (1%) |
| White (Caucasian) | 84 (84%) |
| Other | 7 (7%) |
| Relationship Status | |
| Married/Common-Law | 62 (62%) |
| Divorced/Separated | 13 (13%) |
| Single | 23 (23%) |
| Widowed | 1 (1%) |
| Occupational status | |
| Full-Time | 74 (74%) |
| Part-Time | 6 (6%) |
| Retired | 7 (7%) |
| Disability | 7 (7%) |
| Unemployed | 6 (6%) |
| Education | |
| Some High School | 3 (3%) |
| High School Graduate | 7 (7%) |
| Some College/University | 22 (22%) |
| College or University Graduate | 68 (67%) |
| Measure | mYFAS 2.0 Symptom Scores | mYFAS 2.0 Diagnosis Scores | ||
|---|---|---|---|---|
| r | p | r | p | |
| BES | 0.633 | <0.001 | 0.365 | <0.001 |
| PHQ-9 | 0.459 | <0.001 | 0.217 | 0.030 |
| GAD-7 | 0.372 | <0.001 | 0.239 | 0.016 |
| %TWL | −0.293 | 0.003 | −0.229 | 0.022 |
| Measure | No Food Addiction (n = 87) | Food Addiction (n = 13) | Total Sample (n = 100) |
|---|---|---|---|
| BES | 11.86 ± 7.85 | 20.46 ± 5.78 | 12.980 ± 8.12 |
| PHQ-9 | 5.22 ± 4.60 | 8.54 ± 3.36 | 5.65 ± 4.59 |
| GAD-7 | 4.40 ± 3.96 | 7.15 ± 4.20 | 4.76 ± 4.07 |
| %TWL | 29.91 ± 9.44 | 22.09 ± 14.91 | 28.89 ± 10.55 |
| mYFAS 2.0 Symptomatology | 0.72 ± 1.13 | 3.92 ± 1.44 | 1.14 ± 1.59 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cassin, S.; Leung, S.; Hawa, R.; Wnuk, S.; Jackson, T.; Sockalingam, S. Food Addiction Is Associated with Binge Eating and Psychiatric Distress among Post-Operative Bariatric Surgery Patients and May Improve in Response to Cognitive Behavioural Therapy. Nutrients 2020, 12, 2905. https://doi.org/10.3390/nu12102905
Cassin S, Leung S, Hawa R, Wnuk S, Jackson T, Sockalingam S. Food Addiction Is Associated with Binge Eating and Psychiatric Distress among Post-Operative Bariatric Surgery Patients and May Improve in Response to Cognitive Behavioural Therapy. Nutrients. 2020; 12(10):2905. https://doi.org/10.3390/nu12102905
Chicago/Turabian StyleCassin, Stephanie, Samantha Leung, Raed Hawa, Susan Wnuk, Timothy Jackson, and Sanjeev Sockalingam. 2020. "Food Addiction Is Associated with Binge Eating and Psychiatric Distress among Post-Operative Bariatric Surgery Patients and May Improve in Response to Cognitive Behavioural Therapy" Nutrients 12, no. 10: 2905. https://doi.org/10.3390/nu12102905
APA StyleCassin, S., Leung, S., Hawa, R., Wnuk, S., Jackson, T., & Sockalingam, S. (2020). Food Addiction Is Associated with Binge Eating and Psychiatric Distress among Post-Operative Bariatric Surgery Patients and May Improve in Response to Cognitive Behavioural Therapy. Nutrients, 12(10), 2905. https://doi.org/10.3390/nu12102905







