The Influence of Lycopene, [6]-Gingerol, and Silymarin on the Apoptosis on U-118MG Glioblastoma Cells In Vitro Model
Abstract
1. Introduction
2. Materials and Methods
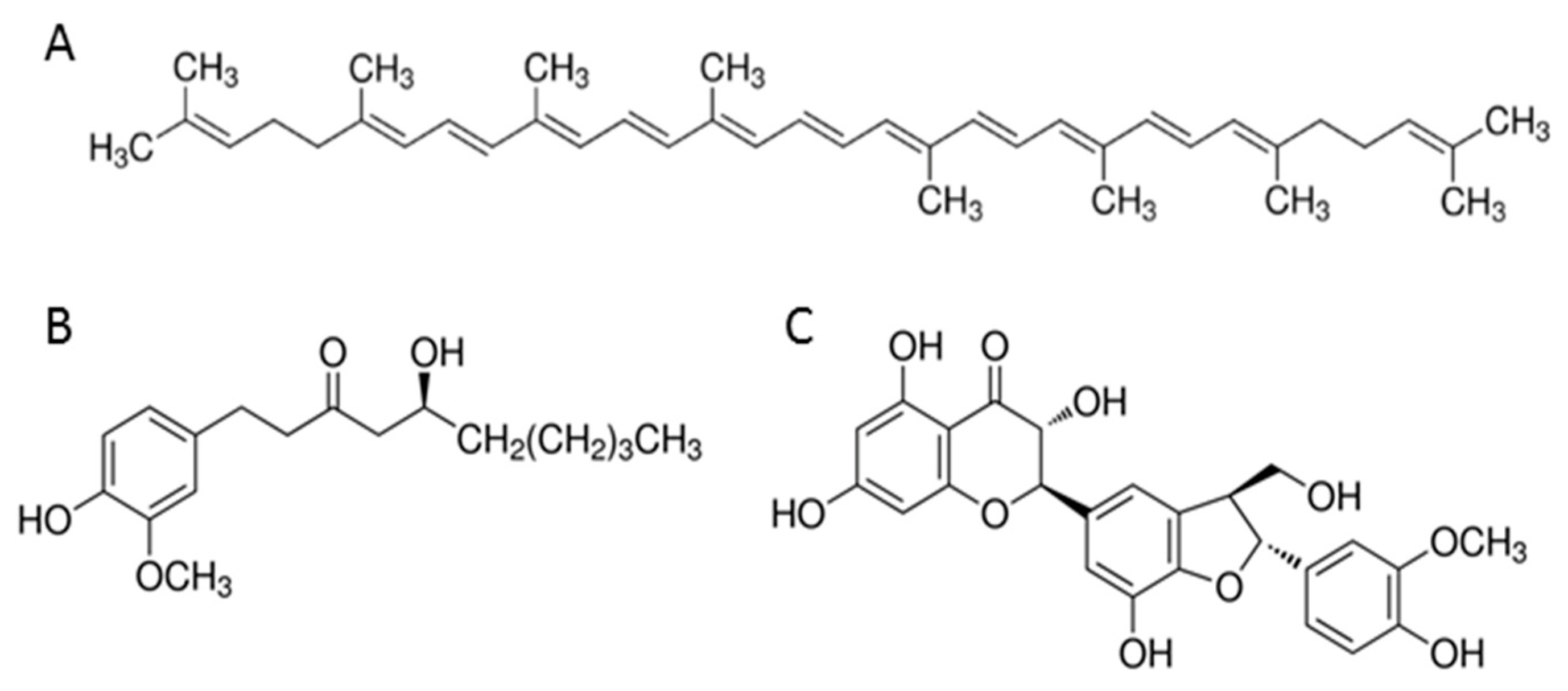
2.1. Cell Culture and Chemicals
2.2. Treatment of Cells
2.3. Determination of Apoptosis by Annexin V Staining
2.4. Detection of the Mitochondrial Membrane Potential
2.5. Quantification of Caspase-3/7 Activity and DNA Electrophoresis
2.6. Statistical Analysis
3. Results
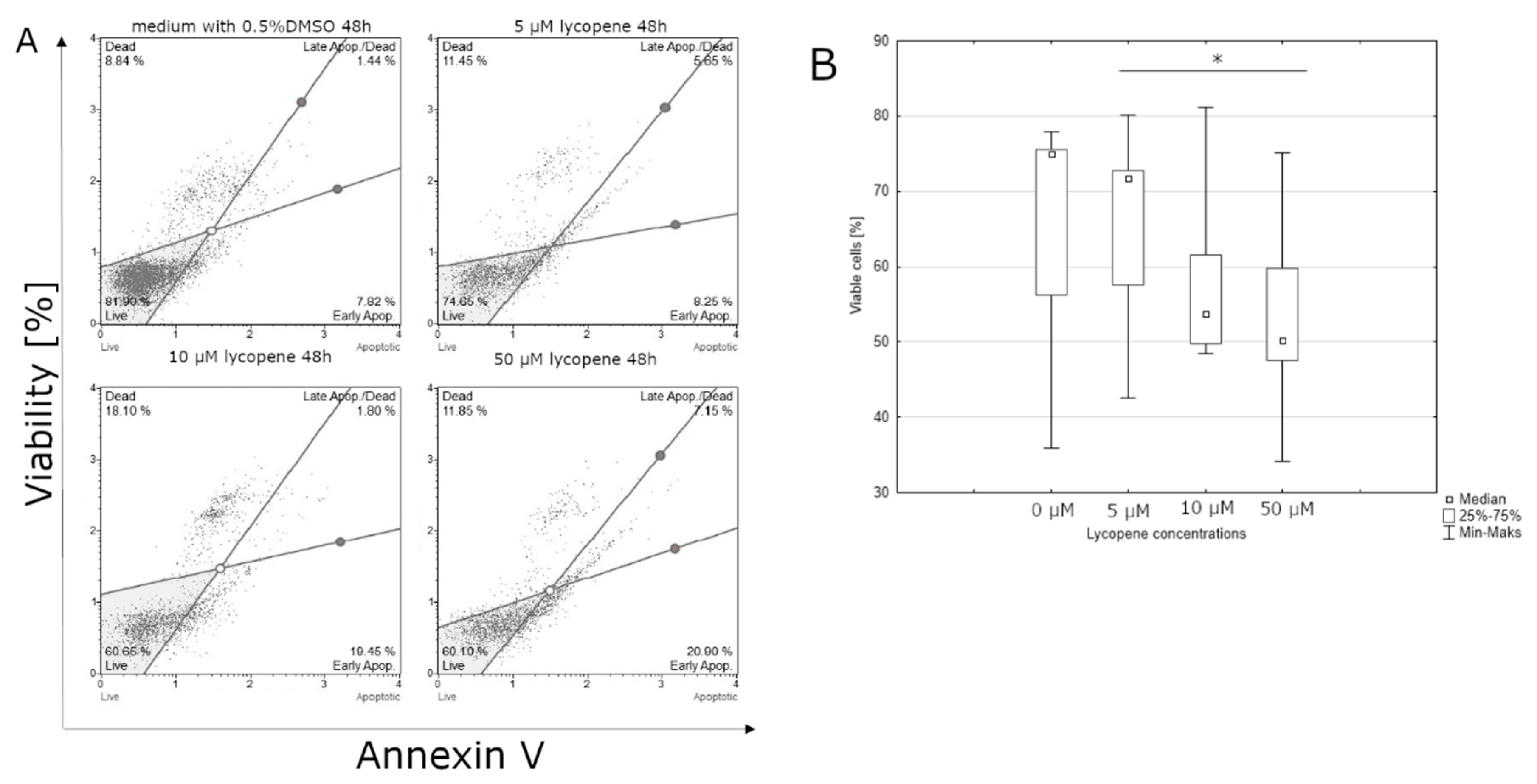
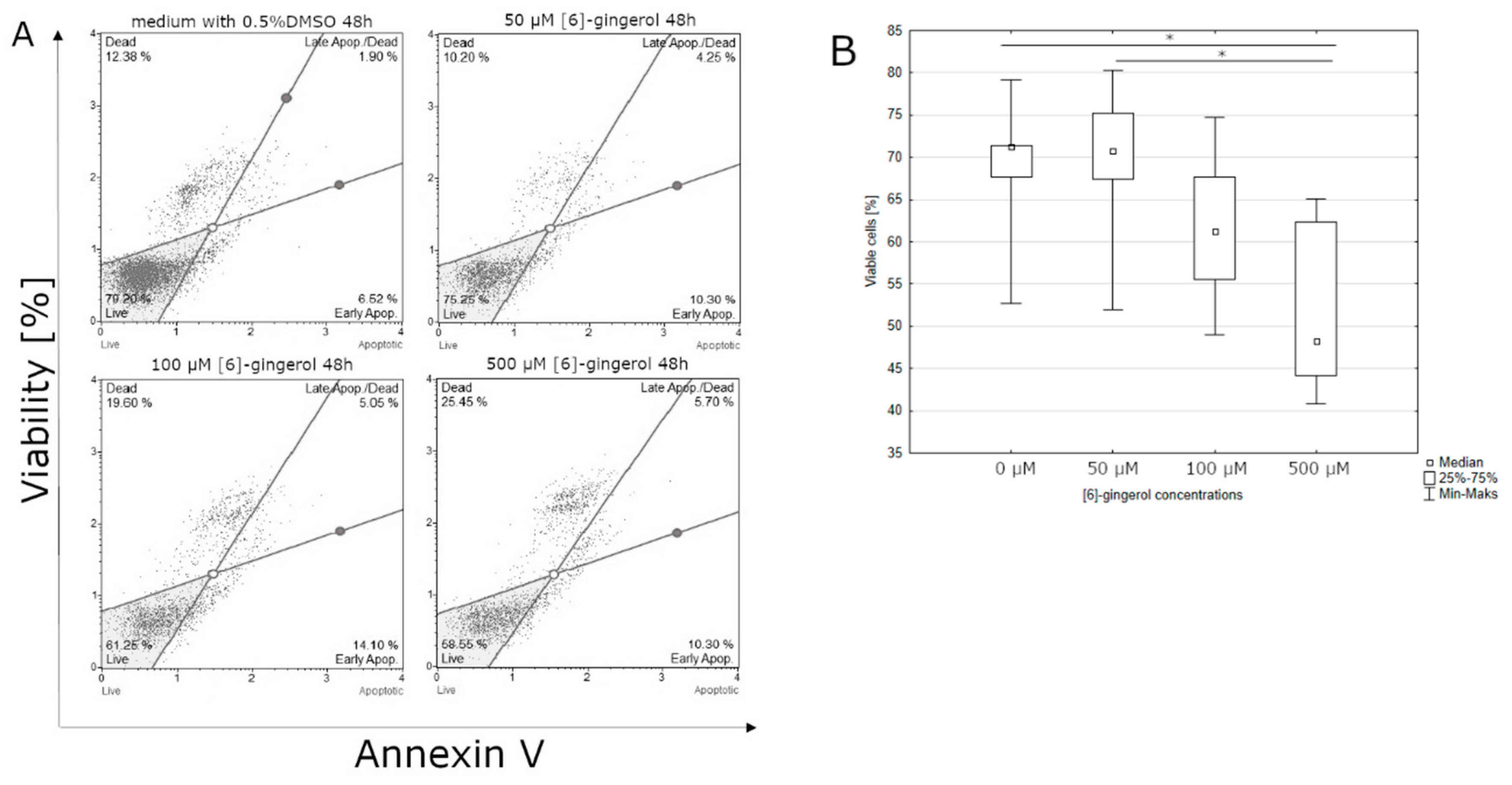
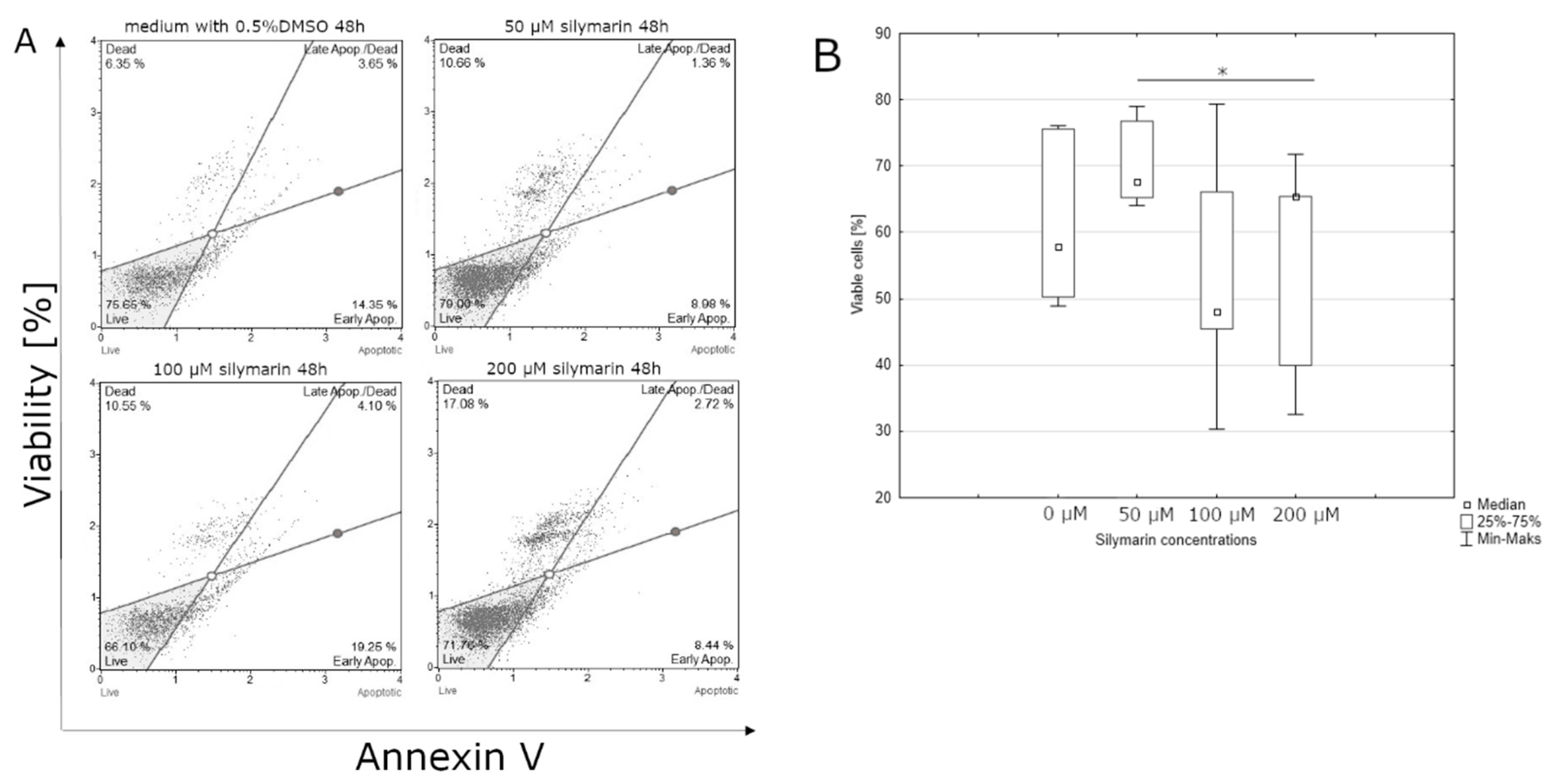
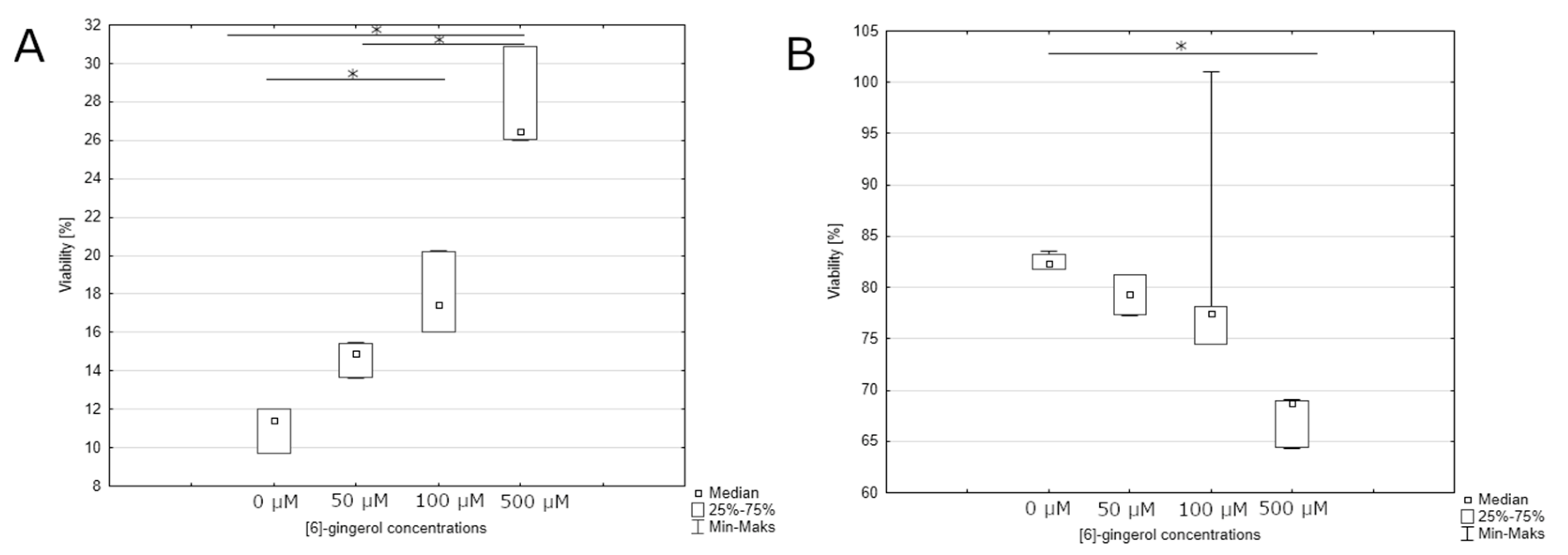
3.1. Effect of Lycopene, [6]-Gingerol, and Silymarin on Apoptosis of U118-MG Glioblastoma Cells Evaluated by an Annexin V Dead Cell Kit
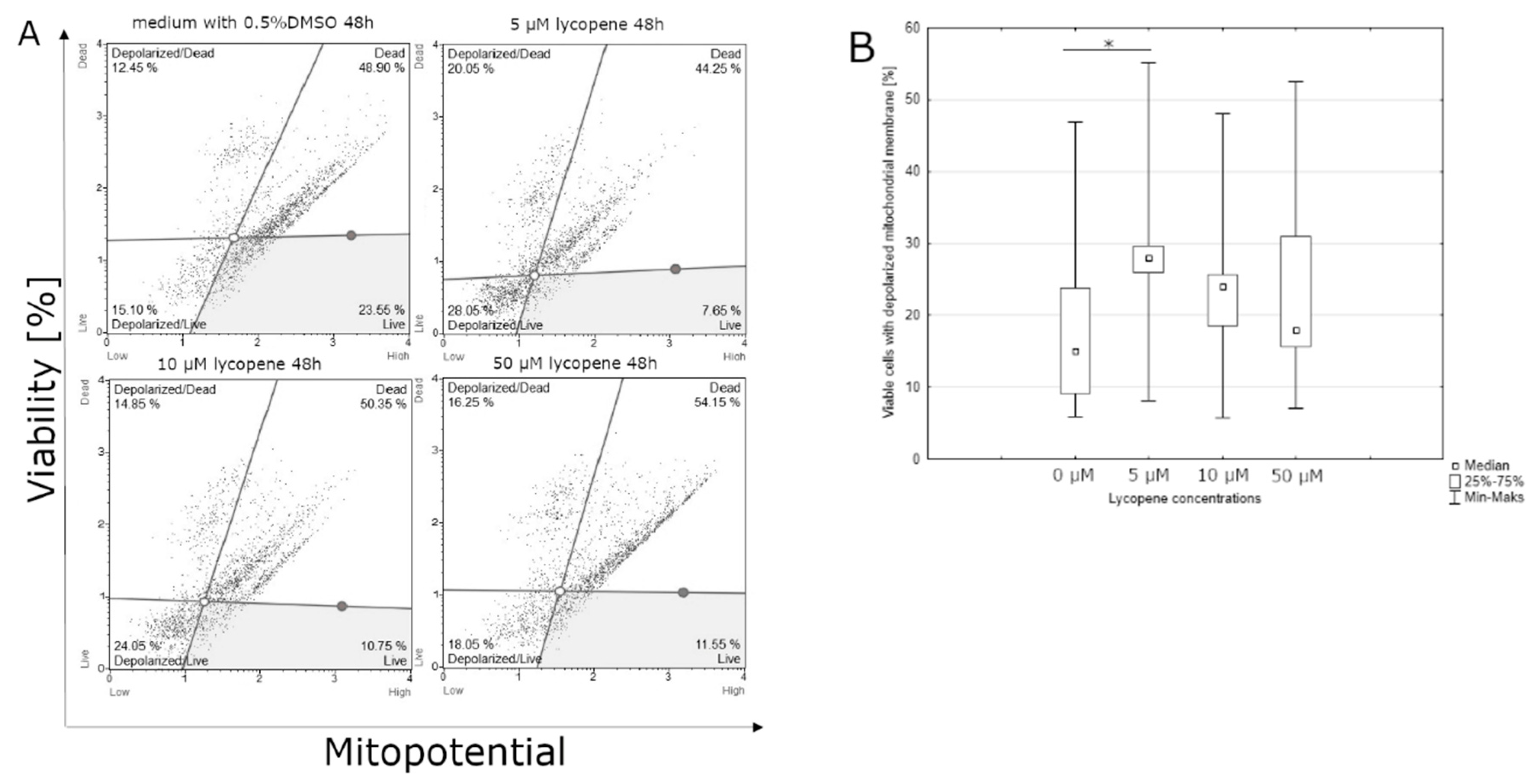
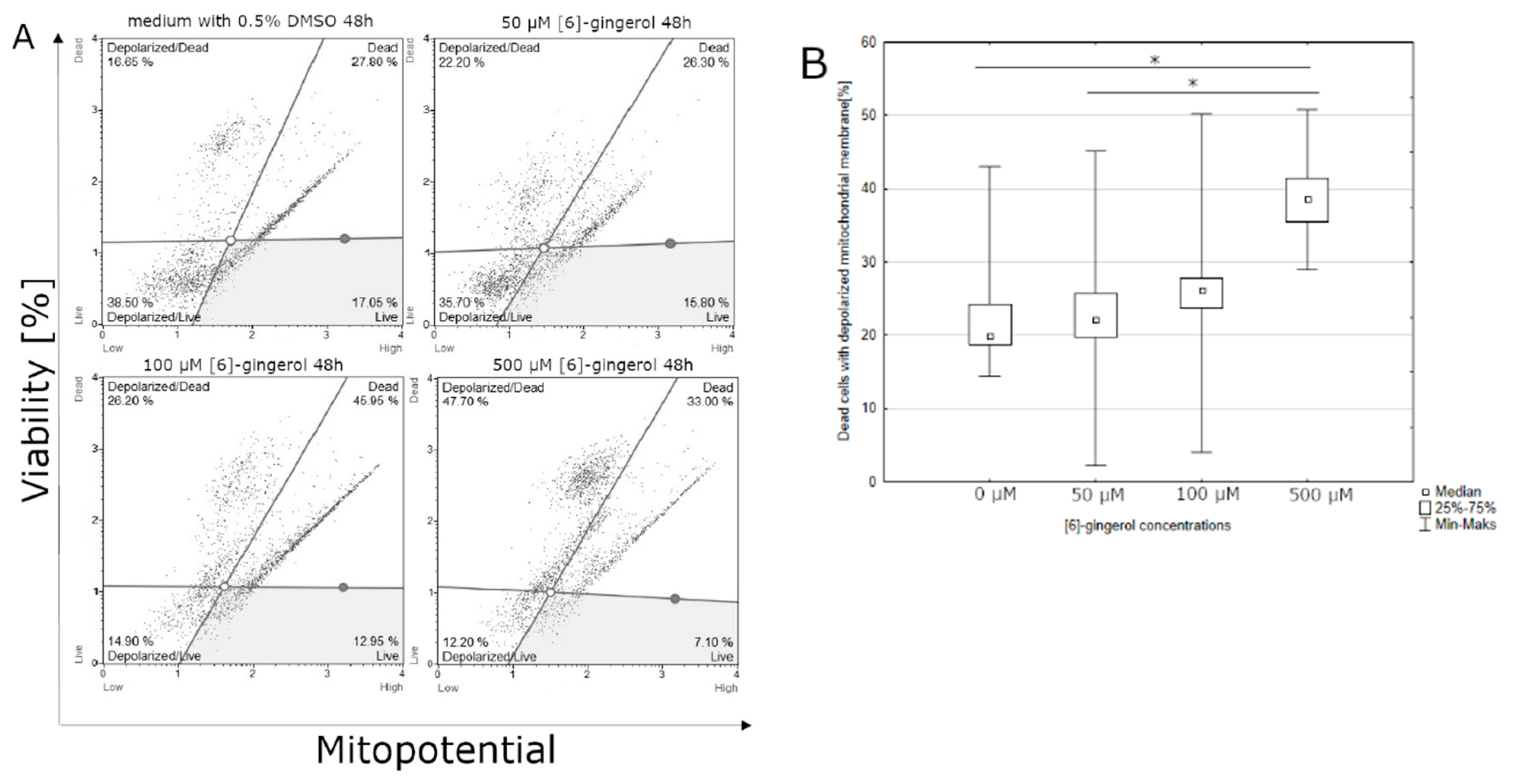
3.2. Effect of Lycopene, [6]-Gingerol and Silymarin on Apoptosis of U118-MG Glioblastoma Cells Evaluated by a Mitopotential Assay
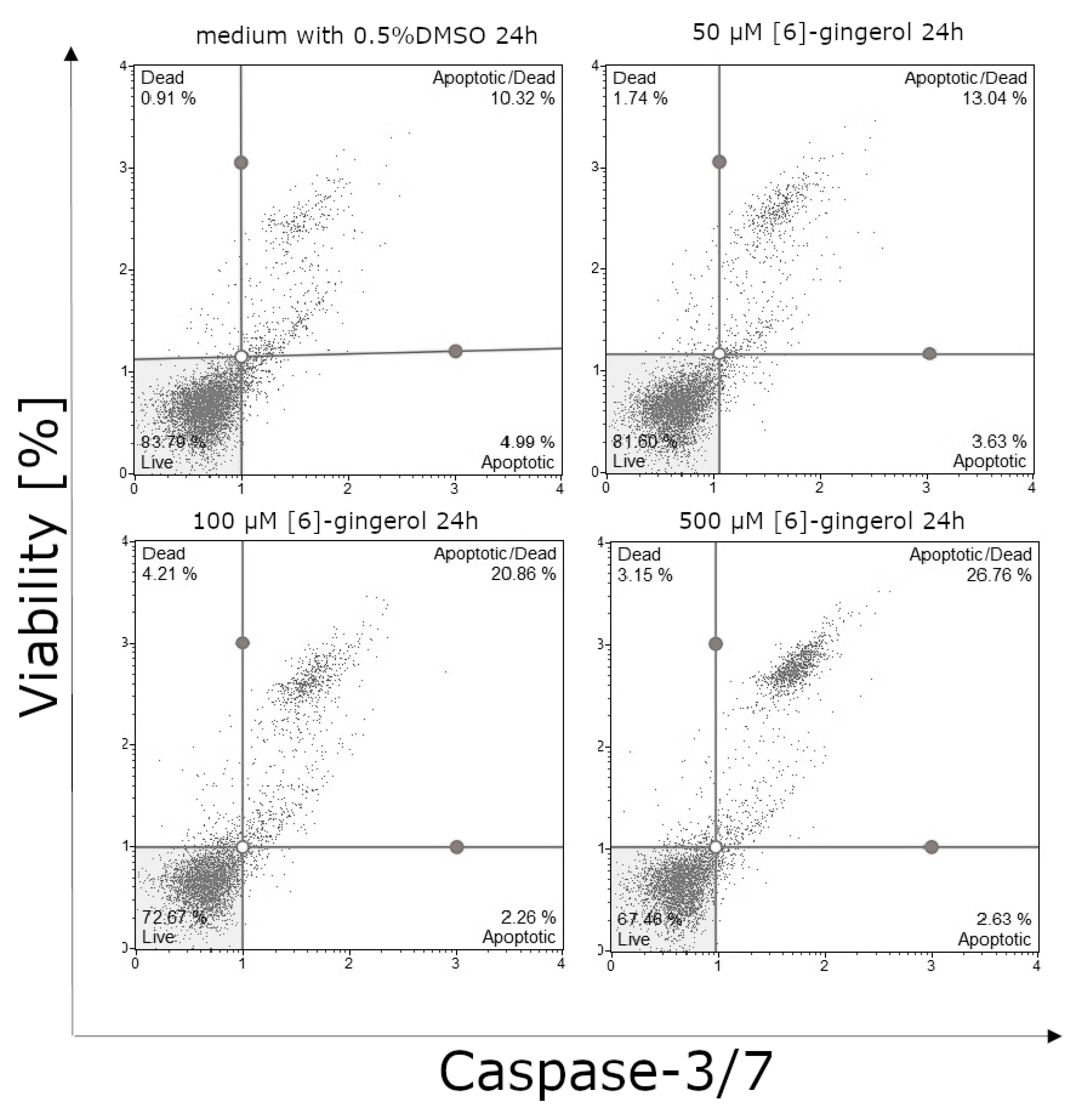
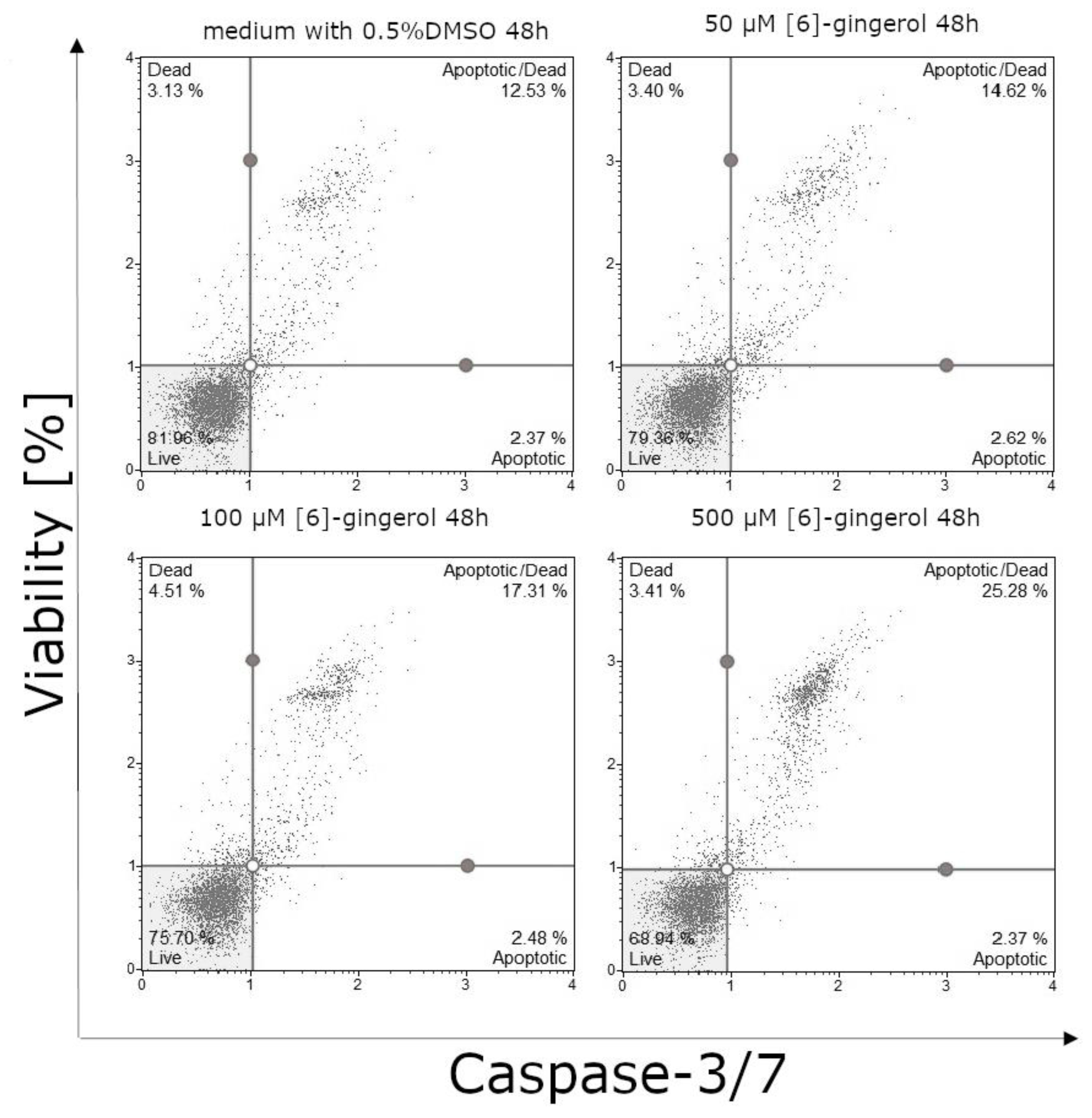
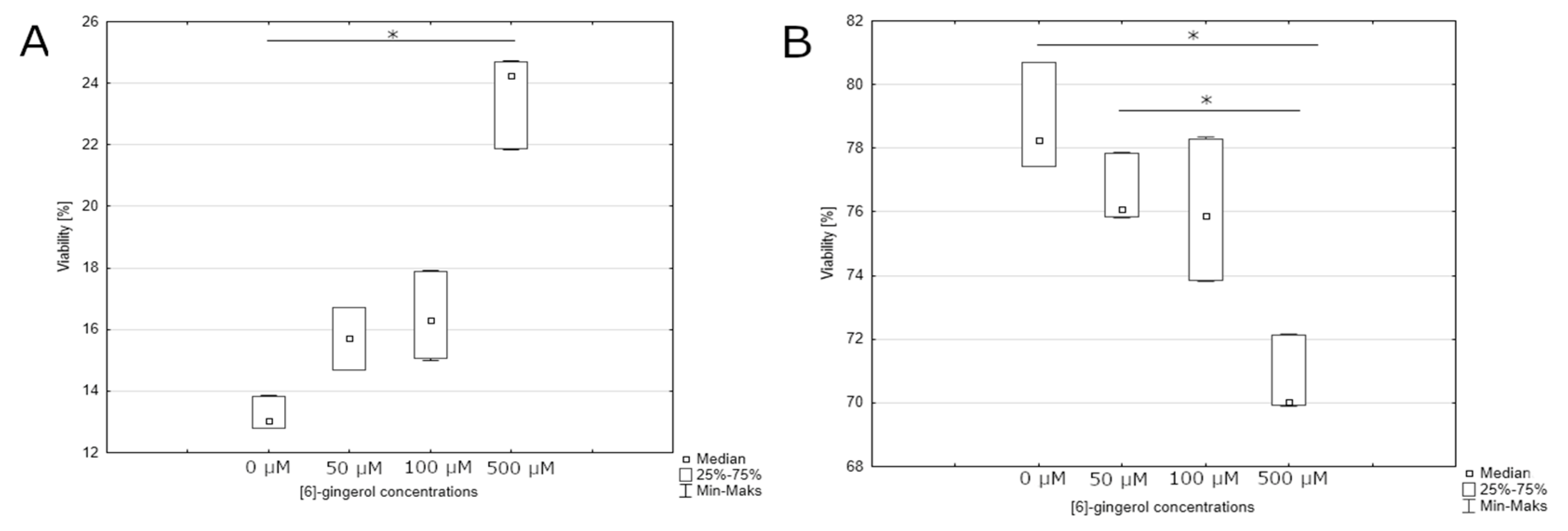
3.3. Effect of Lycopene, [6]-Gingerol and Silymarin on Caspase-3/7 Activity of U118-MG Glioblastoma Cells Evaluated by Caspase-3/7 Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Okła, K.; Wawruszak, A.; Bilska, S. Glejaki—Epidemiologia, klasyfikacja i etiologia Gliomas—Epidemiology, classification and etiology. Rew. Res. Cancer Treat. 2015, 1, 5–16. [Google Scholar]
- Cook, K.M.; Figg, W.D. Angiogenesis Inhibitors-Current Strategies and Future Prospects. CA Cancer J. Clin. 2010, 60, 222–243. [Google Scholar] [CrossRef] [PubMed]
- Lowe, S.W.; Lin, A.W. Apoptosis in cancer. Carcinogenesis 2000, 21, 485–495. [Google Scholar] [CrossRef]
- Willett, W. Diet and cancer. Oncologist 2000, 1, 41–54. [Google Scholar] [CrossRef]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L.; Valanis, B.; Williams, J.H.; et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1150–1155. [Google Scholar] [CrossRef]
- Frusciante, L.; Carli, P.; Ercolano, M.R.; Pernice, R.; Di Matteo, A.; Fogliano, V.; Pellegrini, N. Antioxidant nutritional quality of tomato. Mol. Nutr. Food Res. 2007, 51, 609–617. [Google Scholar] [CrossRef]
- Zborowska, K.; Holecki, T. Medycyna alternatywna jako uzupełniająca forma leczenia chorób nowotworowych w opinii pacjentów onkologicznych. Psychoonkologia 2010, 100, 21–28. [Google Scholar]
- Agarwal, S.; Rao, A.V. Tomato lycopene and its role in human health and chronic diseases. CMAJ 2000, 163, 739–744. [Google Scholar]
- Giovannucci, E. Tomatoes, tomato-based products, lycopene, and cancer: Review of the epidemiologic literature. J. Natl. Cancer Inst. 1999, 91, 317–331. [Google Scholar] [CrossRef]
- Paduch, R.; Klatka, M.; Klatka, J. Rodzaje śmierci komórki. Pomeranian J. Life Sci. 2015, 61. [Google Scholar] [CrossRef]
- Gręda, A.; Jantas, D. Dysfunkcje mitochondriów w chorobach neurodegeneracyjnych: Potencjalny punkt uchwytu dla leków neuroprotekcyjnych. Postęp. Biol. Komórki 2012, 39, 3. [Google Scholar]
- Altman, R.D.; Marcussen, K.C. Effects of a ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheum. 2001, 44, 2531–2538. [Google Scholar] [CrossRef]
- Kim, E.-C.; Min, J.-K.; Kim, T.-Y.; Lee, S.-J.; Yang, H.-O.; Han, S.; Kim, Y.-M.; Kwon, Y.-G. [6]-Gingerol, a pungent ingredient of ginger, inhibits angiogenesis in vitro and in vivo. Biochem. Biophys. Res. Commun. 2005, 335, 300–308. [Google Scholar] [CrossRef]
- Lee, H.S.; Seo, E.Y.; Kang, N.E.; Kim, W.K. [6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer cells. J. Nutr. Biochem. 2008, 19, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, N.; Zhao, J.; Wolf, D.M.; Agarwal, R. Inhibition of human carcinoma cell growth and DNA synthesis by silibinin, an active constituent of milk thistle: Comparison with silymarin. Cancer Lett. 1999, 147, 77–84. [Google Scholar] [CrossRef]
- Giovannucci, E.; Rimm, E.B.; Liu, Y.; Stampfer, M.J.; Willett, W.C. A Prospective Study of Tomato Products, Lycopene, and Prostate Cancer Risk. CancerSpectrum Knowl. Environ. 2002, 94, 391–398. [Google Scholar] [CrossRef]
- Rafi, M.M.; Kanakasabai, S.; Reyes, M.D.; Bright, J.J. Lycopene modulates growth and survival associated genes in prostate cancer. J. Nutr. Biochem. 2013, 24, 1724–1734. [Google Scholar] [CrossRef]
- Ramakrishnan, G.; Lo Muzio, L.; Elinos-Báez, C.M.; Jagan, S.; Augustine, T.A.; Kamaraj, S.; Anandakumar, P.; Devaki, T. Silymarin inhibited proliferation and induced apoptosis in hepatic cancer cells. Cell Prolif. 2009, 42, 229–240. [Google Scholar] [CrossRef]
- Herrmann, M.; Lorenz, H.M.; Voll, R.; Griinke, M.; Woith, W.; Kalden, J.R. A rapid and simple method for the isolation of apoptotic DNA fragments. Nucleic Acids Res. 1994, 22, 5506–5507. [Google Scholar] [CrossRef]
- Barisic, K.; Petrik Jozsef, R.L. Biochemistry of apoptotic cell death. Acta Pharm. 2003, 53, 161–164. [Google Scholar]
- Rupniewska, Z.; Bojarska-Junak, A. Apoptoza: Przepuszczalność błony mitochondrialnej i rola pełniona przez białka z rodziny Bcl-2 Apoptosis: Mitochondrial membrane permeabilization and the role played by Bcl-2 family proteins. Postep. Hig. Med. Dosw. 2004, 58, 538–547. [Google Scholar]
- Bhola, P.D.; Mattheyses, A.L.; Simon, S.M. Spatial and Temporal Dynamics of Mitochondrial Membrane Permeability Waves during Apoptosis. Biophys. J. 2009, 97, 2222–2231. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Colangelo, M.; Simone, R.; Catalano, A.; Boninsegna, A.; Lanza, P.; Monego, G.; Ranelletti, F.O. Lycopene induces cell growth inhibition by altering mevalonate pathway and Ras signaling in cancer cell lines. Carcinogenesis 2010, 31, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Puri, T.; Goyal, S.; Julka, P.; Nair, O.; Sharma, D.; Rath, G. Lycopene in treatment of high-grade gliomas: A pilot study. Neurol. India 2010, 58, 20. [Google Scholar] [CrossRef]
- Giovannucci, E.; Ascherio, A.; Rimm, E.B.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C. Intake of Carotenoids and Retino in Relation to Risk of Prostate Cancer. JNCI J. Natl. Cancer Inst. 1995, 87, 1767–1776. [Google Scholar] [CrossRef]
- Tang, F.-Y.; Cho, H.-J.; Pai, M.-H.; Chen, Y.-H. Concomitant supplementation of lycopene and eicosapentaenoic acid inhibits the proliferation of human colon cancer cells. J. Nutr. Biochem. 2009, 20, 426–434. [Google Scholar] [CrossRef]
- Burgess, L.C.; Rice, E.; Fischer, T.; Seekins, J.R.; Burgess, T.P.; Sticka, S.J.; Klatt, K. Lycopene has limited effect on cell proliferation in only two of seven human cell lines (both cancerous and noncancerous) in an in vitro system with doses across the physiological range. Toxicol. In Vitro 2008, 22, 1297–1300. [Google Scholar] [CrossRef] [PubMed]
- Karas, M.; Amir, H.; Fishman, D.; Danilenko, M.; Segal, S.; Nahum, A.; Koifmann, A.; Giat, Y.; Levy, J.; Sharoni, Y. Lycopene Interferes with Cell Cycle Progression and Insulin-Like Growth Factor I Signaling in Mammary Cancer Cells. Nutr. Cancer 2000, 36, 101–111. [Google Scholar] [CrossRef]
- Salman, H.; Bergman, M.; Djaldetti, M.; Bessler, H. Lycopene affects proliferation and apoptosis of four malignant cell lines. Biomed. Pharmacother. 2007, 61, 366–369. [Google Scholar] [CrossRef]
- Lee, D.-H.; Kim, D.-W.; Jung, C.-H.; Lee, Y.J.; Park, D. Gingerol sensitizes TRAIL-induced apoptotic cell death of glioblastoma cells. Toxicol. Appl. Pharmacol. 2014, 279, 253–265. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, J.-G.; Qu, J.; Zhang, Q.; Prasad, C.; Wei, Z.-J. Assessment of anti-cancerous potential of 6-gingerol ( Tongling White Ginger) and its synergy with drugs on human cervical adenocarcinoma cells. Food Chem. Toxicol. 2017, 109, 910–922. [Google Scholar] [CrossRef] [PubMed]
- Eren, D.; Betul, Y.M. Revealing the effect of 6-gingerol, 6-shogaol and curcumin on mPGES-1, GSK-3β and β-catenin pathway in A549 cell line. Chem. Biol. Interact. 2016, 258, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Elkady, A.I.; Abuzinadah, O.A.; Baeshen, N.A.; Rahmy, T.R. Differential Control of Growth, Apoptotic Activity, and Gene Expression in Human Breast Cancer Cells by Extracts Derived from Medicinal Herbs Zingiber officinale. J. Biomed. Biotechnol. 2012, 2012, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-M.; Kao, C.-L.; Tseng, Y.-T.; Lo, Y.-C.; Chen, C.-Y. Ginger Phytochemicals Inhibit Cell Growth and Modulate Drug Resistance Factors in Docetaxel Resistant Prostate Cancer Cell. Molecules 2017, 22, 1477. [Google Scholar] [CrossRef]
- Meng, B.; Ii, H.; Qu, W.; Yuan, H. Anticancer Effects of Gingerol in Retinoblastoma Cancer Cells (RB355 Cell Line) Are Mediated via Apoptosis Induction, Cell Cycle Arrest and Upregulation of PI3K/Akt Signaling Pathway. Med. Sci. Monit. 2018, 24, 1980–1987. [Google Scholar] [CrossRef]
- Won, D.-H.; Kim, L.-H.; Jang, B.; Yang, I.-H.; Kwon, H.-J.; Jin, B.; Oh, S.H.; Kang, J.-H.; Hong, S.-D.; Shin, J.-A.; et al. In vitro and in vivo anti-cancer activity of silymarin on oral cancer. Tumor Biol. 2018, 40, 101042831877617. [Google Scholar] [CrossRef]
- Ranjbar, N.; Saravani, R.; Faezizadeh, Z. Silymarin inhibits Toll-like receptor 8 gene expression and apoptosis in Ramos cancer cell line. Avicenna J. Phytomed. 2019, 9, 1–9. [Google Scholar]
- Montgomery, A.; Adeyeni, T.; San, K.; Heuertz, R.M.; Ezekiel, U.R. Curcumin Sensitizes Silymarin to Exert Synergistic Anticancer Activity in Colon Cancer Cells. J. Cancer 2016, 7, 1250–1257. [Google Scholar] [CrossRef]
- Fan, L.; Ma, Y.; Liu, Y.; Zheng, D.; Huang, G. Silymarin induces cell cycle arrest and apoptosis in ovarian cancer cells. Eur. J. Pharmacol. 2014, 743, 79–88. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Ray, S.K. Anti-tumor activities of luteolin and silibinin in glioblastoma cells: Overexpression of miR-7-1-3p augmented luteolin and silibinin to inhibit autophagy and induce apoptosis in glioblastoma in vivo. Apoptosis 2016, 21, 312–328. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czarnik-Kwaśniak, J.; Kwaśniak, K.; Kwasek, P.; Świerzowska, E.; Strojewska, A.; Tabarkiewicz, J. The Influence of Lycopene, [6]-Gingerol, and Silymarin on the Apoptosis on U-118MG Glioblastoma Cells In Vitro Model. Nutrients 2020, 12, 96. https://doi.org/10.3390/nu12010096
Czarnik-Kwaśniak J, Kwaśniak K, Kwasek P, Świerzowska E, Strojewska A, Tabarkiewicz J. The Influence of Lycopene, [6]-Gingerol, and Silymarin on the Apoptosis on U-118MG Glioblastoma Cells In Vitro Model. Nutrients. 2020; 12(1):96. https://doi.org/10.3390/nu12010096
Chicago/Turabian StyleCzarnik-Kwaśniak, Justyna, Konrad Kwaśniak, Paulina Kwasek, Elżbieta Świerzowska, Agata Strojewska, and Jacek Tabarkiewicz. 2020. "The Influence of Lycopene, [6]-Gingerol, and Silymarin on the Apoptosis on U-118MG Glioblastoma Cells In Vitro Model" Nutrients 12, no. 1: 96. https://doi.org/10.3390/nu12010096
APA StyleCzarnik-Kwaśniak, J., Kwaśniak, K., Kwasek, P., Świerzowska, E., Strojewska, A., & Tabarkiewicz, J. (2020). The Influence of Lycopene, [6]-Gingerol, and Silymarin on the Apoptosis on U-118MG Glioblastoma Cells In Vitro Model. Nutrients, 12(1), 96. https://doi.org/10.3390/nu12010096





