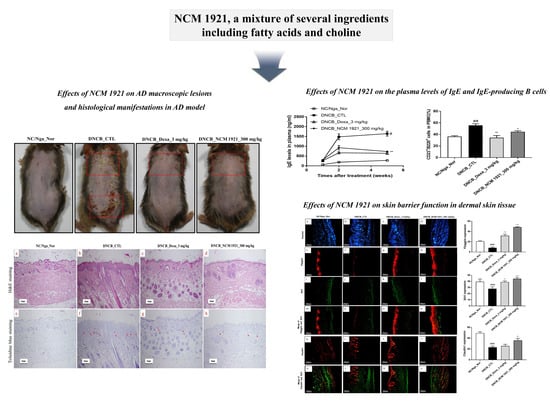
NCM 1921, a Mixture of Several Ingredients, Including Fatty Acids and Choline, Attenuates Atopic Dermatitis in 1-Chloro-2,4-Dinitrobenzene-Treated NC/Nga Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Supplement
2.2. Animal Experiments
2.3. Histological Examination of AD-Like Dermal Pathology
2.4. Isolation of White Blood Cells from Peripheral Blood
2.5. Isolation of Axillary Lymph Nodes, the Spleen, and Dorsal Skin Cells
2.6. Splenocyte Isolation and Culture
2.7. Fluorescence-Activated Cell Sorting
2.8. Enzyme-Linked Immunosorbent Assay
2.9. Statistical Analysis
3. Results
3.1. The Effects of NCM 1921 on Macroscopic AD Lesions
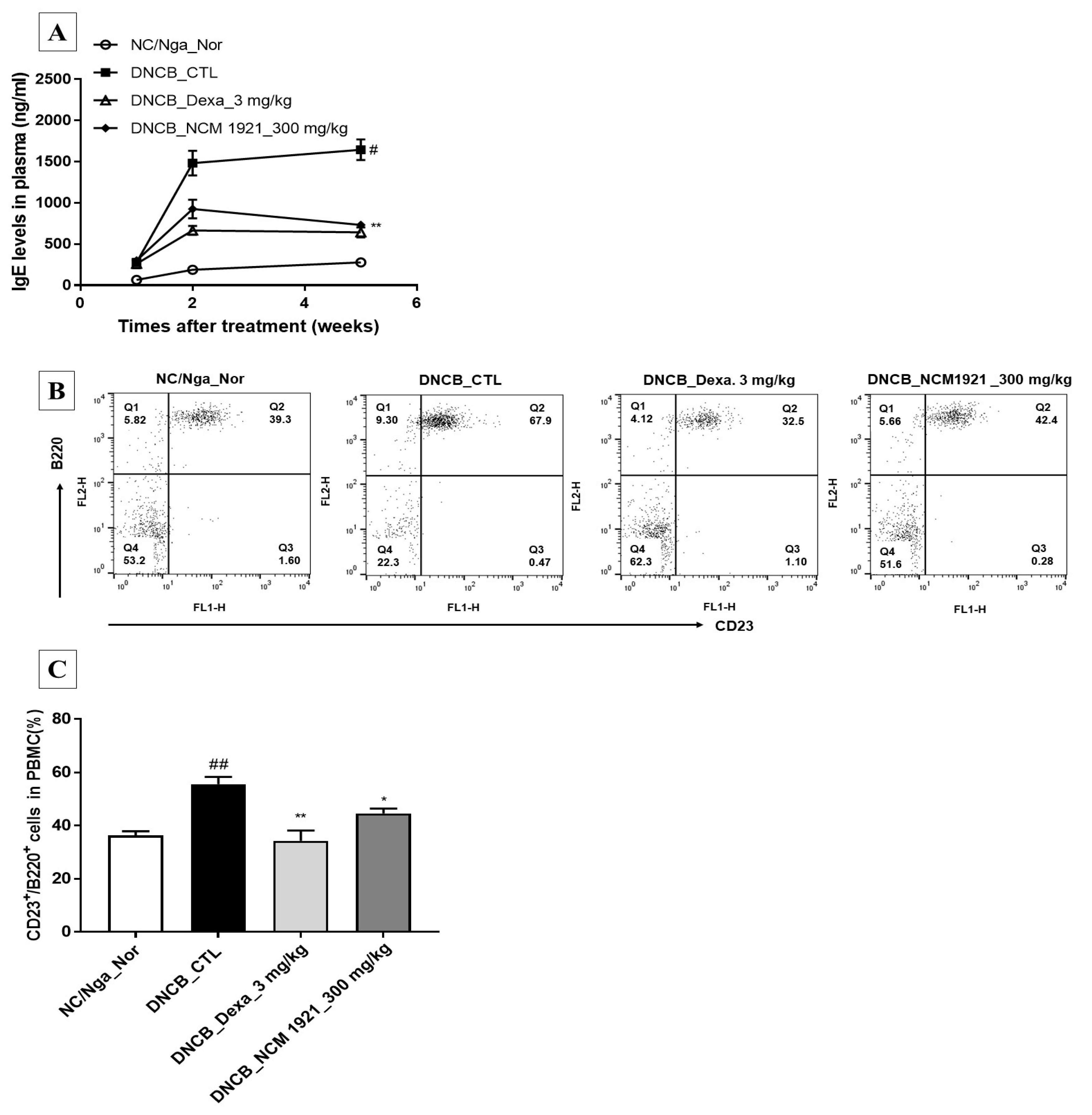
3.2. The Effects of NCM 1921 on the Plasma Levels of IgE and IgE-Producing B Cells
3.3. The Effects of NCM 1921 on Blood Cell Populations and the Absolute Numbers of Immune Cell Subtypes in ALNs, the Spleen, and Dorsal Skin
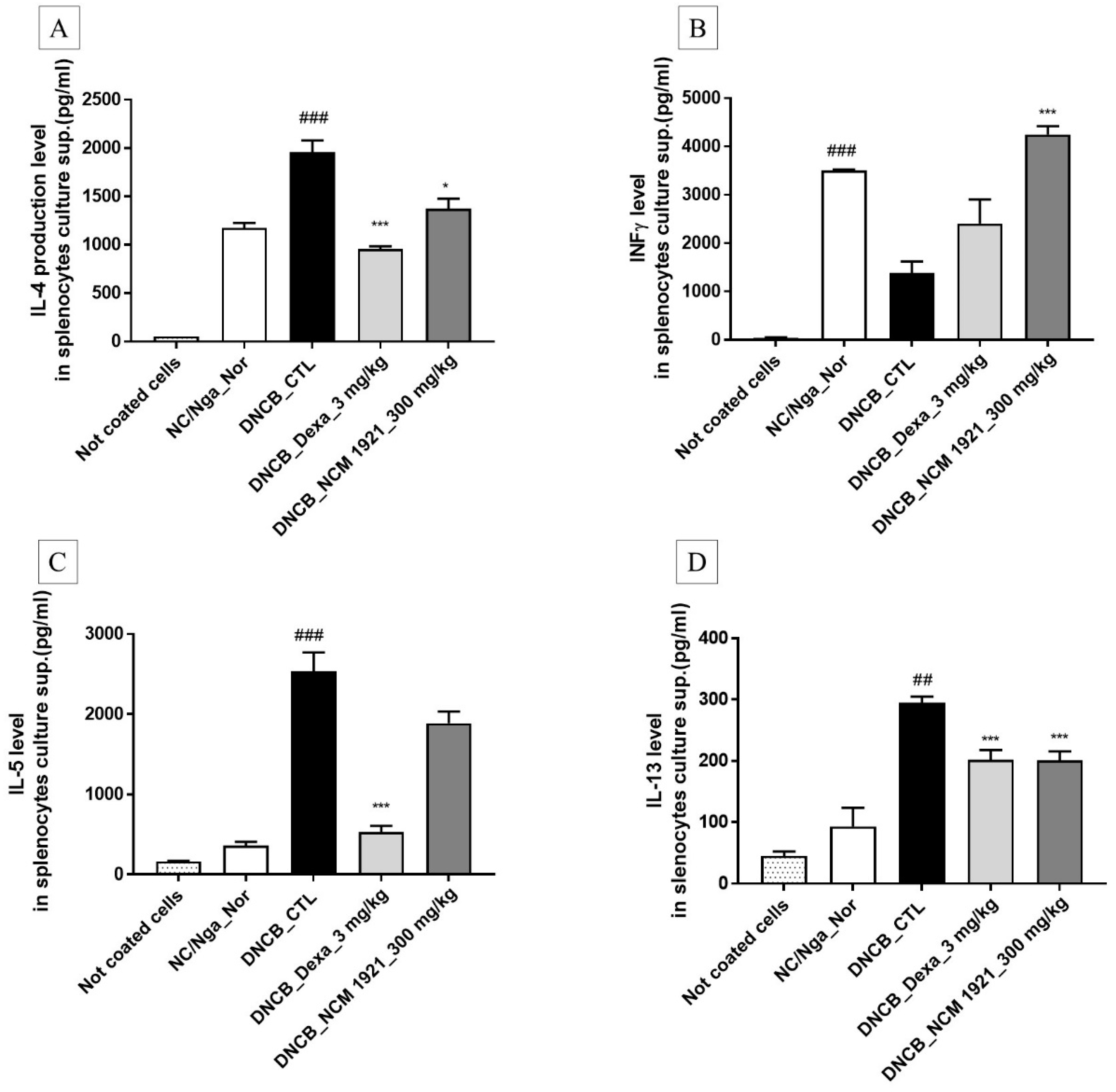
3.4. The Effects of NCM 1921 on the Production of Th2 Cytokines (IL-4, IL-5, and IL-13) and Th1 Cytokines (IFN-γ) by Cultured Splenocytes
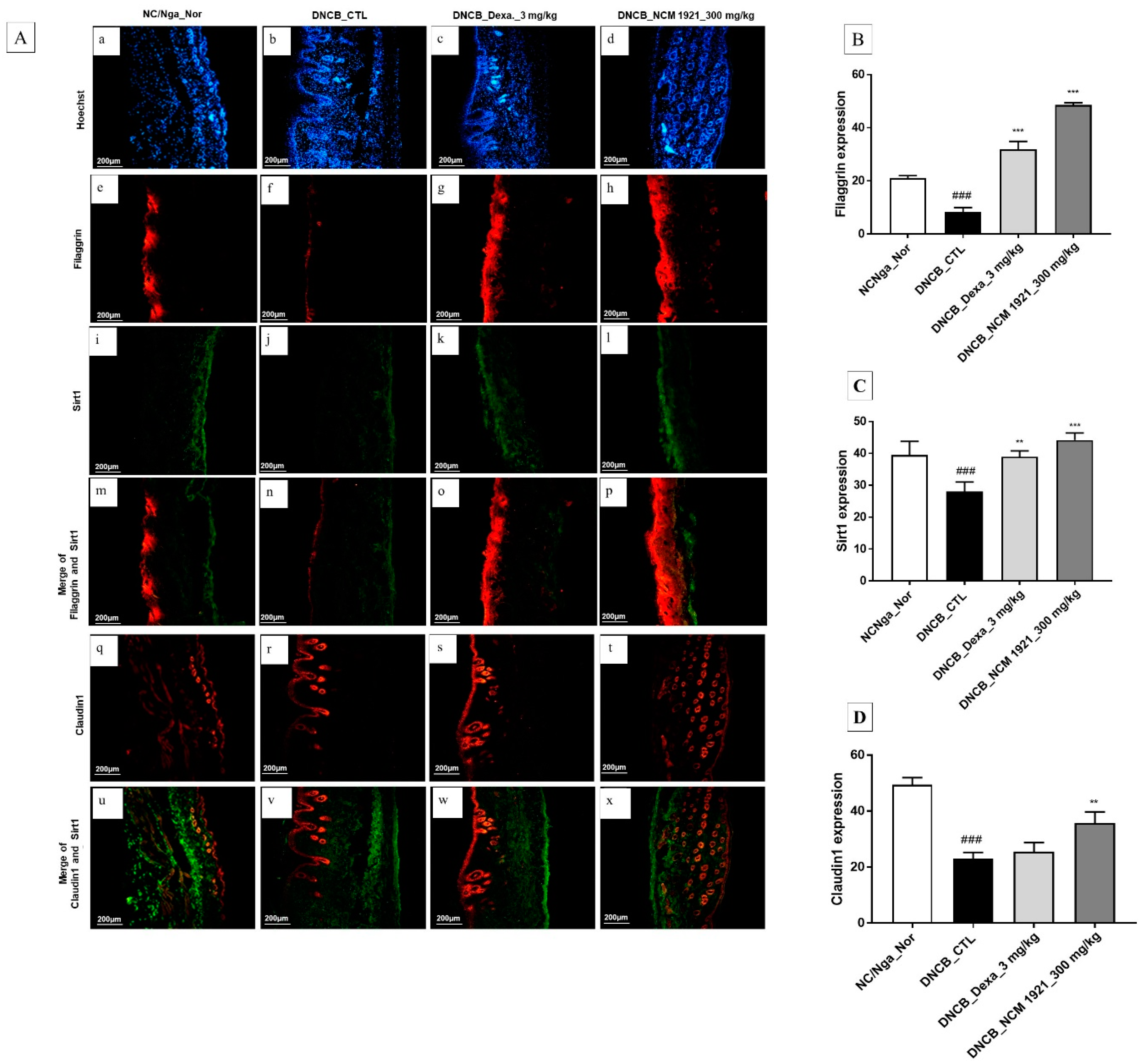
3.5. The Effects of NCM 1921 on the Expression of Claudin1, Filaggrin, and Sirt1 in Dermal Skin Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mansouri, Y.; Guttman-Yassky, E. Immune pathways in atopic dermatitis, and definition of biomarkers through broad and targeted therapeutics. J. Clin. Med. 2015, 4, 858–873. [Google Scholar] [CrossRef] [PubMed]
- Pastore, S.; Mascia, F.; Giustizieri, M.L.; Giannetti, A.; Girolomoni, G. Pathogenetic mechanisms of atopic dermatitis. Arch. Immunol. Ther. Exp. 2000, 48, 497–504. [Google Scholar]
- Leung, D.Y.; Boguniewicz, M.; Howell, M.D.; Nomura, I.; Hamid, Q.A. New insights into atopic dermatitis. J. Clin. Investig. 2004, 113, 651–657. [Google Scholar] [CrossRef]
- Lin, T.K.; Zhong, L.; Santiago, J.L. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Wahlgren, C.F. Itch and atopic dermatitis: An overview. J. Dermatol. 1999, 26, 770–779. [Google Scholar] [CrossRef]
- Tokumasu, R.; Yamaga, K.; Yamazaki, Y.; Murota, H.; Suzuki, K.; Tamura, A.; Bando, K.; Furuta, Y.; Katayama, I.; Tsukita, S. Dose-dependent role of claudin-1 in vivo in orchestrating features of atopic dermatitis. Proc. Natl. Acad. Sci. USA 2016, 113, E4061–E4068. [Google Scholar] [CrossRef]
- Kim, K. Influences of environmental chemicals on atopic dermatitis. Toxicol. Res. 2015, 31, 89–96. [Google Scholar] [CrossRef]
- Oikarinen, A.; Haapasaari, K.M.; Sutinen, M.; Tasanen, K. The molecular basis of glucocorticoid-induced skin atrophy: Topical glucocorticoid apparently decreases both collagen synthesis and the corresponding collagen mRNA level in human skin in vivo. Br. J. Dermatol. 1998, 139, 1106–1110. [Google Scholar] [CrossRef]
- Seki, H.; Sasaki, T.; Ueda, T.; Arita, M. Resolvins as regulators of the immune system. Sci. World J. 2010, 10, 818–831. [Google Scholar] [CrossRef]
- Kremmyda, L.S.; Vlachava, M.; Noakes, P.S.; Diaper, N.D.; Miles, E.A.; Calder, P.C. Atopy Risk in infants and children in relation to early exposure to fish, oily fish, or long-chain omega-3 fatty acids: A systematic review. Clin. Rev. Allerg. Immunol. 2011, 41, 36–66. [Google Scholar] [CrossRef]
- Krauss-Etschmann, S.; Hartl, D.; Rzehak, P.; Heinrich, J.; Shadid, R.; Del Carmen Ramirez-Tortosa, M.; Campoy, C.; Pardillo, S.; Schendel, D.J.; Decsi, T.; et al. Nutraceuticals for Healthier Life Study Group: Decreased cord blood IL-4, IL-13, and CCR4 and increased TGF-beta levels after fish oil supplementation of pregnant women. J. Allergy Clin. Immunol. 2008, 121, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, M.T.; Abad-Casintahan, F.; Lopez-Villafuerte, L. The effect of topical virgin coconut oil on SCORAD index, transepidermal water loss, and skin capacitance in mild to moderate pediatric atopic dermatitis: A randomized, double-blind, clinical trial. Int. J. Dermatol. 2014, 53, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jang, J.E.; Kim, J.; Lee, Y.I.; Lee, D.W.; Song, S.Y.; Lee, J.H. Enhanced barrier functions and anti-inflammatory effect of cultured coconut extract on human skin. Food Chem. Toxicol. 2017, 106, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Varma, S.R.; Sivaprakasam, T.O.; Arumugam, I.; Dilip, N.; Raghuraman, M.; Pavan, K.B.; Rafiq, M.; Paramesh, R. In vitro anti-inflammatory and skin protective properties of Virgin coconut oil. J. Tradit. Complement. Med. 2018, 9, 5–14. [Google Scholar] [CrossRef]
- Yang, H.T.; Chen, J.W.; Rathod, J.; Jiang, Y.Z.; Tsai, P.J.; Hung, Y.P.; Ko, W.C.; Paredes-Sabja, D.; Huang, I.H. Lauric acid is an inhibitor of Clostridium difficile growth in vitro and reduces inflammation in a mouse infection model. Front. Microbiol. 2018, 8, 2635. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, C.; Xu, Q.; Zhang, Y.; Li, H.; Li, F.; Liu, Y.; Guo, C. Caprylic acid suppresses inflammation via TLR4/NF-κB signaling and improves atherosclerosis in ApoE-deficient mice. Nutr. Metab. 2019, 16, 40. [Google Scholar] [CrossRef]
- Watson, A.L.; Fray, T.R.; Bailey, J.; Baker, C.B.; Beyer, S.A.; Markwell, P.J. Dietary constituents are able to play a beneficial role in canine epidermal barrier function. Exp. Dermatol. 2006, 15, 74–81. [Google Scholar] [CrossRef]
- Abdel Latif, M.; Abdul-Hamid, M.; Galaly, S.R. Effect of diethylcarbamazine citrate and omega-3 fatty acids on trimellitic anhydride-induced rat skin allergy. Asian Pac. J. Allergy Immunol. 2015, 33, 33–41. [Google Scholar]
- Farooq, M.A.; Gaertner, S.; Amoura, L.; Niazi, Z.R.; Park, S.H.; Qureshi, A.W.; Oak, M.H.; Toti, F.; Schini-Kerth, V.B.; Auger, C. Intake of omega-3 formulation EPA: DHA 6:1 by old rats for 2 weeks improved endothelium-dependent relaxations and normalized the expression level of ACE/AT1R/NADPH oxidase and the formation of ROS in the mesenteric artery. Biochem. Pharmacol. 2019, 10, 113749. [Google Scholar] [CrossRef]
- Mueller, R.S.; Fettman, M.J.; Richardson, K.; Hansen, R.A.; Miller, A.; Magowitz, J.; Ogilvie, G.K. Plasma and skin concentrations of polyunsaturated fatty acids before and after supplementation with n-3 fatty acids in dogs with atopic dermatitis. Am. J. Vet. Res. 2005, 66, 868–873. [Google Scholar] [CrossRef]
- Roth-Walter, F.; Pacios, L.F.; Bianchini, R.; Jensen-Jarolim, E. Linking iron-deficiency with allergy: Role of molecular allergens and the microbiome. Metallomics 2017, 9, 1676–1692. [Google Scholar] [CrossRef] [PubMed]
- Park, B.K.; Park, Y.C.; Jung, I.C.; Kim, S.H.; Choi, J.J.; Do, M.; Kim, S.Y.; Jin, M. Gamisasangja-tang suppresses pruritus and atopic skin inflammation in the NC/Nga murine model of atopic dermatitis. J. Ethnopharmacol. 2015, 165, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Saunders, S.P.; Moran, T.; Floudas, A.; Wurlod, F.; Kaszlikowska, A.; Salimi, M.; Quinn, E.M.; Oliphant, C.J.; Núñez, G.; McManus, R.; et al. Spontaneous atopic dermatitis is mediated by innate immunity, with the secondary lung inflammation of the atopic march requiring adaptive immunity. J. Allergy Clin. Immunol. 2016, 137, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Ming, M.; Zhao, B.; Shea, C.R.; Shah, P.; Qiang, L.; White, S.R.; Sims, D.M.; He, Y.Y. Loss of sirtuin 1 (SIRT1) disrupts skin barrier integrity and sensitizes mice to epicutaneous allergen challenge. J. Allergy Clin. Immunol. 2015, 135, 936–945. [Google Scholar] [CrossRef]
- Morar, N.; Cookson, W.O.; Harper, J.I.; Moffatt, M.F. Filaggrin mutations in children with severe atopic dermatitis. J. Investig. Dermatol. 2007, 127, 1667–1672. [Google Scholar] [CrossRef]
- Sandilands, A.; Terron-Kwiatkowski, A.; Hull, P.R.; O’Regan, G.M.; Clayton, T.H.; Watson, R.M.; Carrick, T.; Evans, A.T.; Liao, H.; Zhao, Y.; et al. Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat. Genet. 2007, 39, 650–654. [Google Scholar] [CrossRef]
- Gruber, R.; Börnchen, C.; Rose, K.; Daubmann, A.; Volksdorf, T.; Wladykowski, E.; Vidal-Y-Sy, S.; Peters, E.M.; Danso, M.; Bouwstra, J.A.; et al. Diverse regulation of Claudin-1 and Claudin-4 in atopic dermatitis. Am. J. Pathol. 2015, 185, 2777–2789. [Google Scholar] [CrossRef]
- Park, H.S.; Hwang, Y.H.; Kim, M.K.; Hong, G.E.; Lee, H.J.; Nagappan, A.; Yumnam, S.; Kim, E.H.; Heo, J.D.; Lee, S.J.; et al. Functional polysaccharides from Grifola frondosa aqueous extract inhibit atopic dermatitis-like skin lesions in NC/Nga mice. Biosci. Biotechnol. Biochem. 2015, 79, 147–154. [Google Scholar] [CrossRef]
- Asad, S.; Winge, M.C.; Wahlgren, C.F.; Bilcha, K.D.; Nordenskjöld, M.; Taylan, F.; Bradley, M. The tight junction gene Claudin-1 is associated with atopic dermatitis among Ethiopians. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1939–1941. [Google Scholar] [CrossRef]
- Oyoshi, M.K.; He, R.; Li, Y.; Mondal, S.; Yoon, J.; Afshar, R.; Chen, M.; Lee, D.M.; Luo, H.R.; Luster, A.D.; et al. Leukotriene B4-driven neutrophil recruitment to the skin is essential for allergic skin inflammation. Immunity 2012, 37, 747–758. [Google Scholar] [CrossRef]
- Liu, F.T.; Goodarzi, H.; Chen, H.Y. IgE, mast cells, and eosinophils in atopic dermatitis. Clin. Rev. Allergy Immunol. 2011, 41, 298–310. [Google Scholar] [CrossRef]
- Jankovic, D.; Feng, C.G. CD4(+) T Cell differentiation in infection: Amendments to the Th1/Th2 Axiom. Front. Immunol. 2015, 6, 198. [Google Scholar] [CrossRef] [PubMed]
- Yagi, R.; Nagai, H.; Iigo, Y.; Akimoto, T.; Arai, T.; Kubo, M. Development of atopic dermatitis-like skin lesions in STAT6-deficient NC/Nga mice. J. Immunol. 2002, 168, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.Y.; Gu, L.; Dou, X. Serum levels of thymus and activation-regulated chemokine can be used in the clinical evaluation of atopic dermatitis. Int. J. Dermatol. 2015, 54, e261–e265. [Google Scholar] [CrossRef] [PubMed]
- Blander, G.; Bhimavarapu, A.; Mammone, T.; Maes, D.; Elliston, K.; Reich, C.; Matsui, M.S.; Guarente, L.; Loureiro, J.J. SIRT1 promotes differentiation of normal human keratinocytes. J. Investig. Dermatol. 2009, 129, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Park, K.Y.; Seo, S.J. Adiponectin upregulates filaggrin expression via SIRT1-mediated signaling in human normal keratinocytes. Ann. Dermatol. 2017, 29, 407–413. [Google Scholar] [CrossRef]

| Cell Phenotypes | DNCB | ||||
|---|---|---|---|---|---|
| NC/Nga_Nor | CTL | Dexa_3 mg/kg | NCM1921_300 mg/kg | ||
| WBC | Total WBCs cells (×103/µL) | 3.6 ± 0.5 | 1.7 ± 0.1 ## | 3.3 ± 0.5 ** | 3.1 ± 0.5 ** |
| WBCs differential counting (%) | |||||
| Neutrophils | 16.7 ± 1.4 | 36.7 ± 2.7 | 25.8 ± 0.7 | 25.4 ± 2.2 | |
| Lymphocytes | 79.1 ± 1.1 | 58.5 ± 2.3 | 70.3 ± 0.6 | 69.5 ± 2.3 | |
| Monocytes | 0.48 ± 0.1 | 0.43 ± 0.1 | 0.65 ± 0.1 | 0.40 ± 0.0 | |
| Eosinophils | 1.28 ± 0.3 | 1.17 ± 0.2 | 1.65 ± 0.2 | 2.58 ± 0.5 | |
| Basophils | 0.28 ± 0.1 | 0.33 ± 0.0 | 0.33 ± 0.0 | 0.25 ± 0.1 | |
| ALN | Total ALN cells (×104/mL) | 5.3 ± 1.6 | 184. ± 22.5 | 38.3 ± 12.2 | 104.3 ± 10.9 |
| CD3+/CD19− (×105 Cells) | 0.93 ± 0.27 | 30.9 ± 4.57 ### | 6.54 ± 2.11 *** | 30.2 ± 5.85 | |
| CD4+/CD8+ (×105 Cells) | 0.66 ± 0.13 | 22.2 ± 2.85 ### | 6.28 ± 1.87 *** | 19.5 ± 3.95 | |
| CD4+/CD69+ (×105 Cells) | 0.59 ± 0.15 | 7.50 ± 1.42 ### | 1.65 ± 0.52 ** | 5.84 ± 1.14 | |
| CD23+/B220+ (×105 Cells) | 0.95 ± 0.24 | 130.2 ± 19.8 ### | 27.0 ± 10.2 *** | 62.6 ± 7.95 ** | |
| Spleen | Total spleen cells (×104/mL) | 665.0 ± 99.1 | 1585.0 ± 231.0 | 810.0 ± 144.0 | 850.0 ± 103.0 |
| CD3+/CD19− (×105 Cells) | 265.4 ± 35.8 | 561.8 ± 136.2 # | 372.5 ± 71.7 | 3720 ± 41.7 | |
| CD4+/CD8+ (× 105 Cells) | 119.2 ± 25.5 | 226.0 ± 34.5 # | 162.7 ± 30.7 | 154.9 ± 25.6 | |
| CD4+/CD69+ (×105 Cells) | 7.52 ± 1.33 | 20.9 ± 7.3 | 13.7 ± 4.74 | 11.9 ± 1.96 | |
| CD23+/B220+ (×105 Cells) | 248.5 ± 80.7 | 509.36 ± 110.0 # | 294. ± 69.3 | 399.4 ± 71.4 | |
| Dorsal skin | Total dorsal skin cells (×104/mL) | 3.5 ± 0.5 | 27.5 ± 2.5 | 9.0 ± 1.0 | 10.5 ± 2.5 |
| CD3+(×105 Cells) | 0.2 ± 0.12 | 3.03 ± 0.28 ### | 1.53 ± 0.39 ** | 2.42 ± 0.01 * | |
| CCR3+/CD11b+ (×105 Cells) | 0.89 ± 0.06 | 3.79 ± 1.12 ## | 2.07 ± 0.03 | 1.69 ± 0.27 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-S.; Yang, W.-K.; Jo, E.-H.; Ho Shin, S.; Lee, Y.-C.; Park, M.-C.; Kim, S.-H. NCM 1921, a Mixture of Several Ingredients, Including Fatty Acids and Choline, Attenuates Atopic Dermatitis in 1-Chloro-2,4-Dinitrobenzene-Treated NC/Nga Mice. Nutrients 2020, 12, 165. https://doi.org/10.3390/nu12010165
Lee Y-S, Yang W-K, Jo E-H, Ho Shin S, Lee Y-C, Park M-C, Kim S-H. NCM 1921, a Mixture of Several Ingredients, Including Fatty Acids and Choline, Attenuates Atopic Dermatitis in 1-Chloro-2,4-Dinitrobenzene-Treated NC/Nga Mice. Nutrients. 2020; 12(1):165. https://doi.org/10.3390/nu12010165
Chicago/Turabian StyleLee, Young-Sil, Won-Kyung Yang, Eun-Hee Jo, Seung Ho Shin, Young-Cheol Lee, Min-Cheol Park, and Seung-Hyung Kim. 2020. "NCM 1921, a Mixture of Several Ingredients, Including Fatty Acids and Choline, Attenuates Atopic Dermatitis in 1-Chloro-2,4-Dinitrobenzene-Treated NC/Nga Mice" Nutrients 12, no. 1: 165. https://doi.org/10.3390/nu12010165
APA StyleLee, Y.-S., Yang, W.-K., Jo, E.-H., Ho Shin, S., Lee, Y.-C., Park, M.-C., & Kim, S.-H. (2020). NCM 1921, a Mixture of Several Ingredients, Including Fatty Acids and Choline, Attenuates Atopic Dermatitis in 1-Chloro-2,4-Dinitrobenzene-Treated NC/Nga Mice. Nutrients, 12(1), 165. https://doi.org/10.3390/nu12010165