Subclinical Mastitis in a European Multicenter Cohort: Prevalence, Impact on Human Milk (HM) Composition, and Association with Infant HM Intake and Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Maternal and Infant Characteristics
2.3. Collection of HM Samples and Diagnosis of SCM
2.4. Macronutrient Composition of HM
2.5. Mineral and Trace Elements Concentration of HM
2.6. Estimation of Milk Intake by Test Weighing Technique
2.7. Infant Growth Parameters
2.8. Statistical Analysis
3. Results
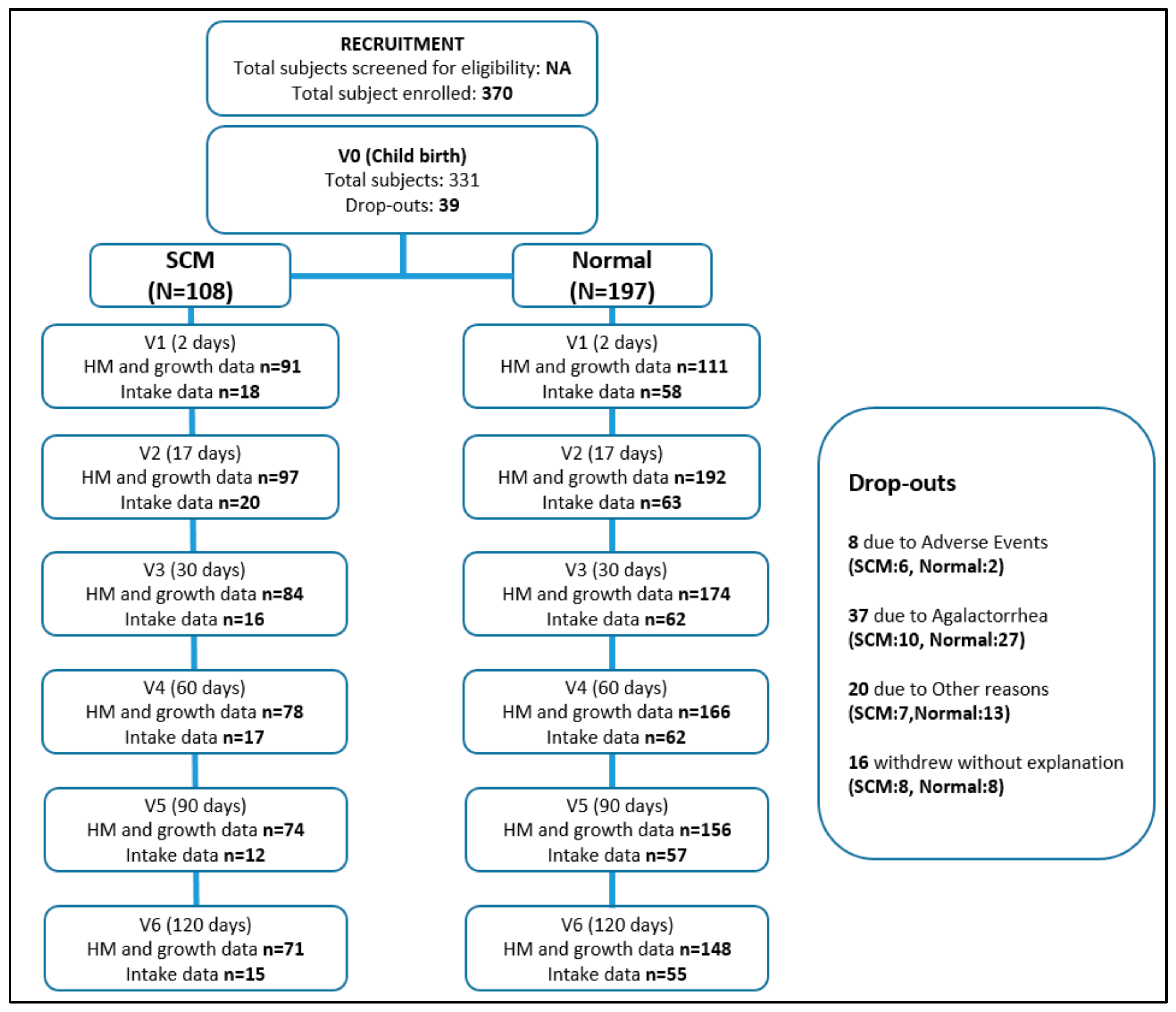
3.1. Study Population
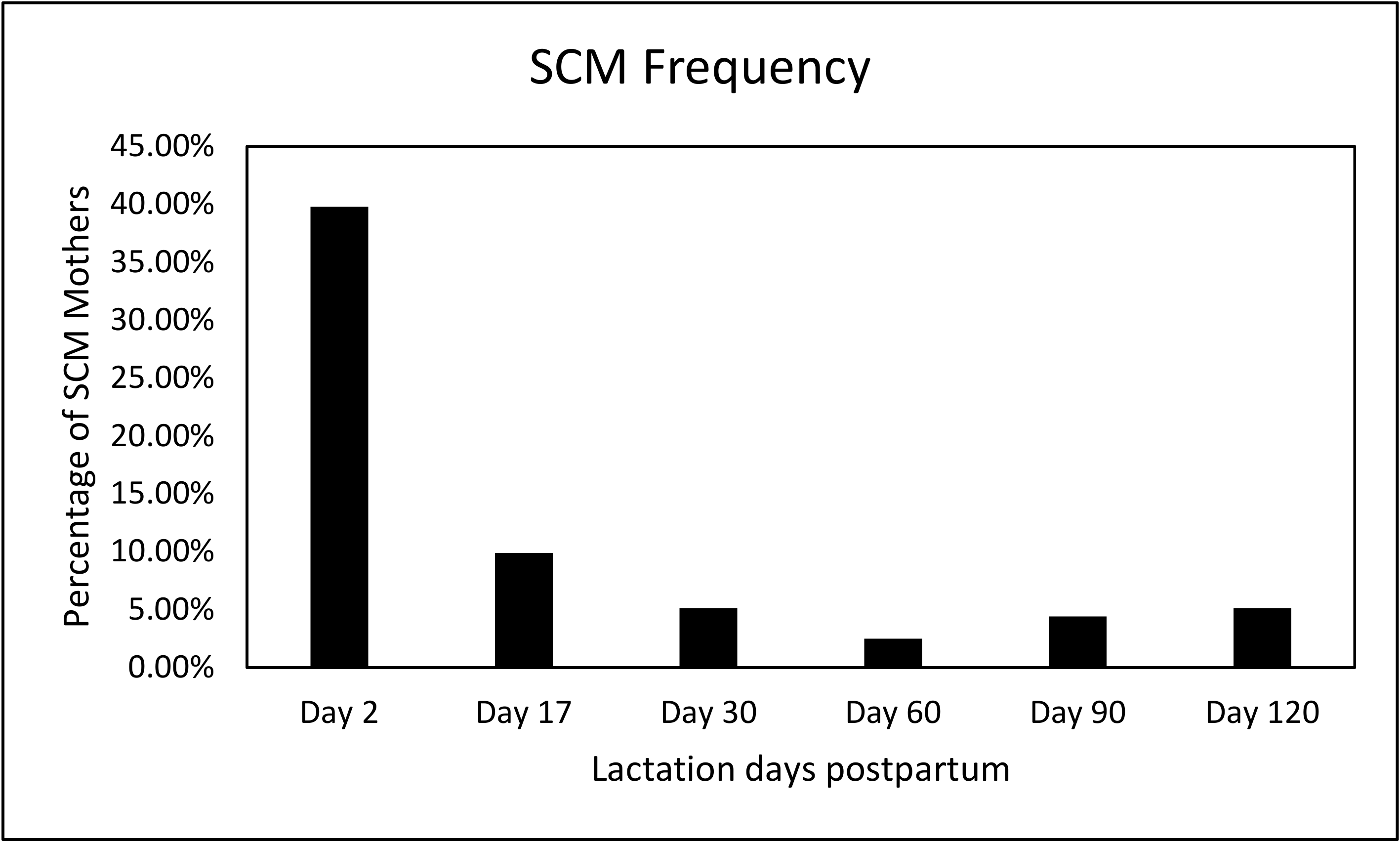
3.2. SCM Prevalence
3.3. Maternal and Infant Characteristics
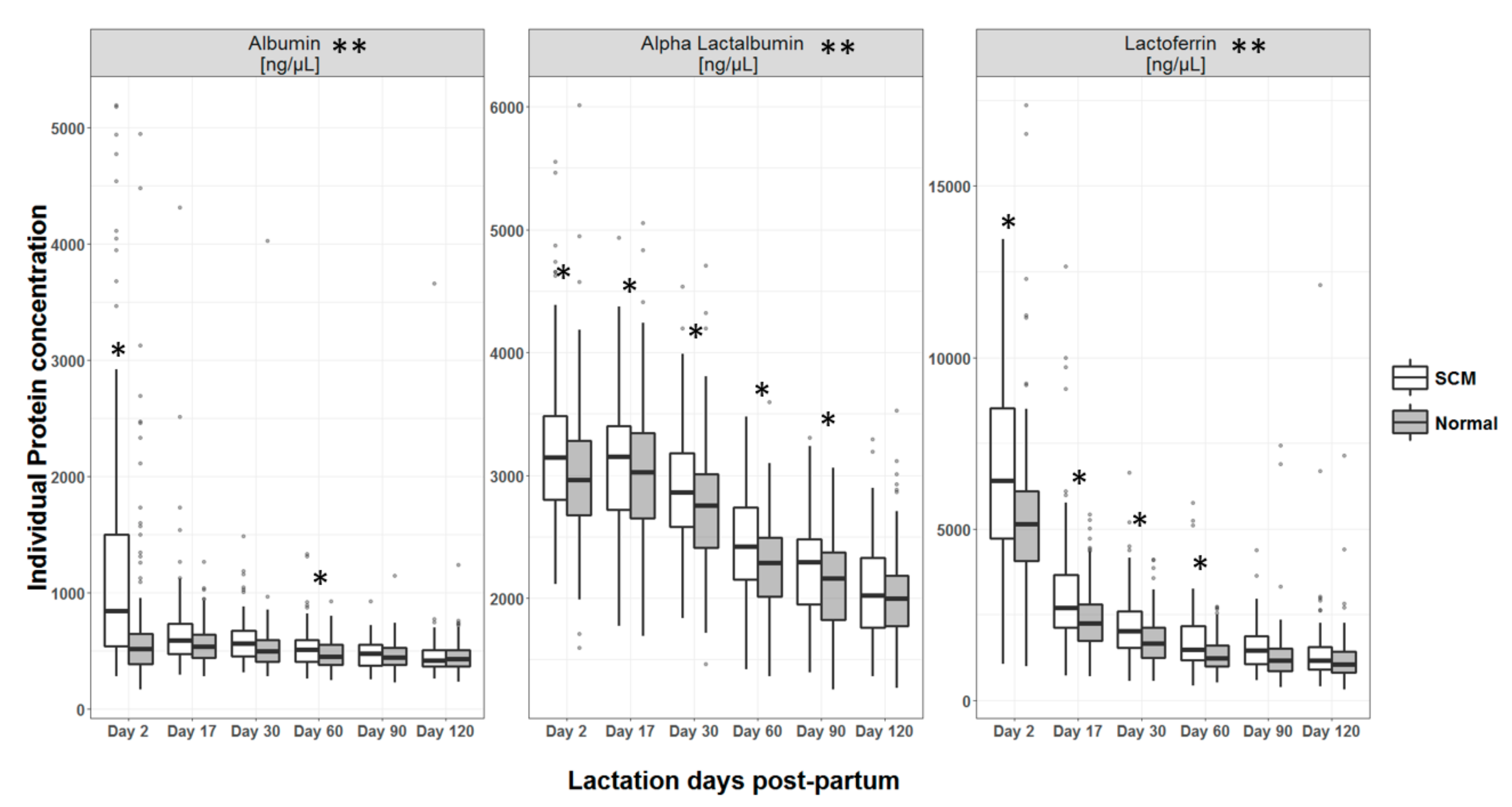
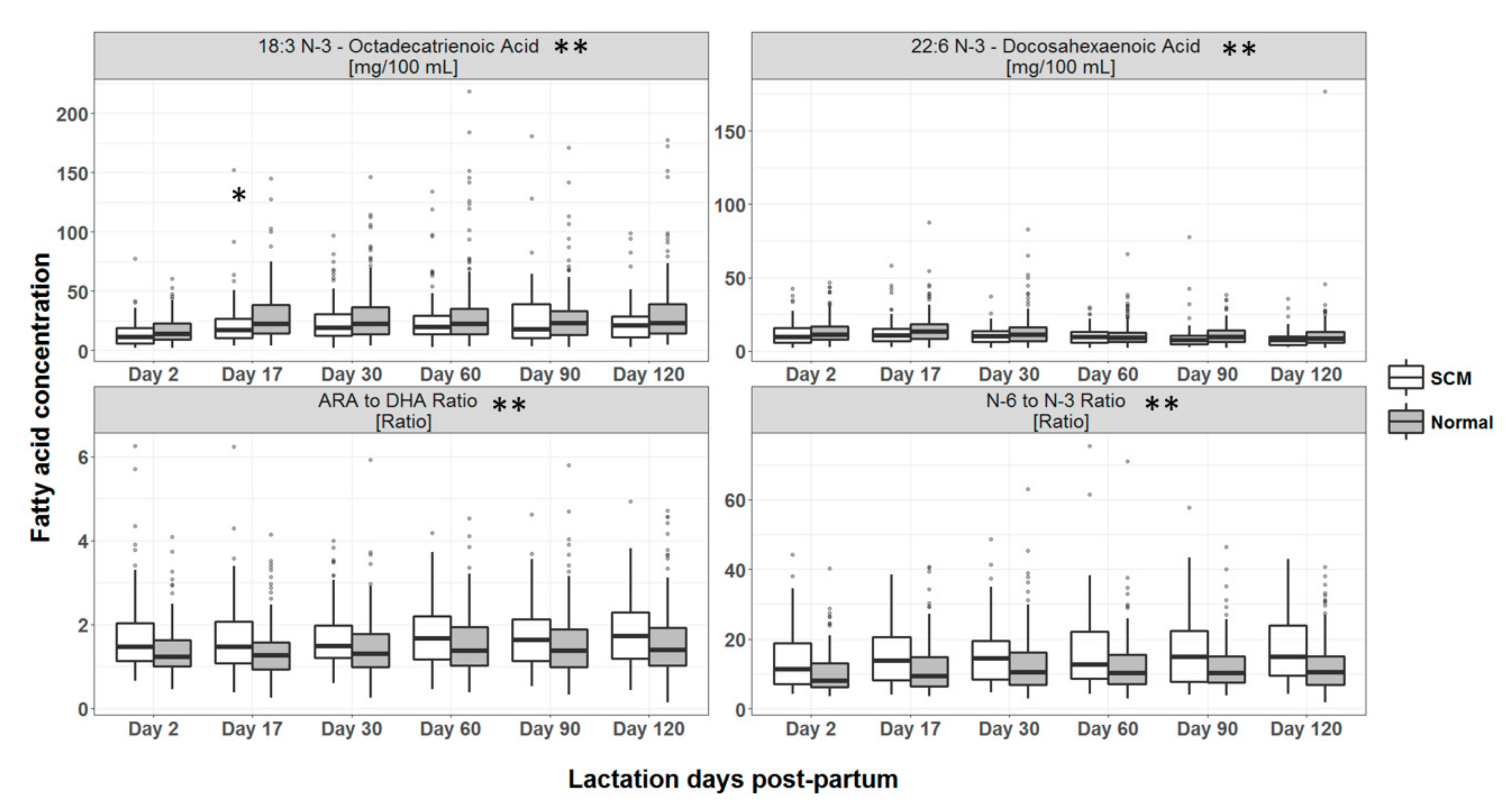
3.4. Macronutrient Composition of HM
3.5. Mineral and Trace Element Concentration in HM
3.6. SCM Status and HM Intake
3.7. Infant Growth Parameters
4. Discussion
4.1. Maternal and Infant Characteristics
4.2. Macronutrient Composition of HM
4.3. Mineral and Trace Element Concentration in HM
4.4. SCM Status and HM Intake
4.5. Infant Growth Parameters
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aryeetey, R.N.O.; Marquis, G.S.; Timms, L.; Lartey, A.; Brakohiapa, L. Subclinical mastitis is common among ghanaian women lactating 3 to 4 months postpartum. J. Hum. Lact. 2008, 24, 263–267. [Google Scholar] [CrossRef]
- Morton, J.A. The Clinical Usefulness of Breast Milk Sodium in the Assessment of Lactogenesis. Pediatrics 1994, 93, 802–806. [Google Scholar] [PubMed]
- Gomo, E.; Filteau, S.M.; Tomkins, A.M.; Ndhlovu, P.; Michaelsen, K.F.; Friis, H. Subclinical mastitis among HIV-infected and uninfected Zimbabwean women participating in a multimicronutrient supplementation trial. Trans. R. Soc. Trop. Med. Hyg. 2003, 97, 212–216. [Google Scholar] [CrossRef]
- Arsenault, J.E.; Aboud, S.; Manji, K.P.; Fawzi, W.W.; Villamor, E. Vitamin Supplementation Increases Risk of Subclinical Mastitis in HIV-Infected Women. J. Nutr. 2010, 140, 1788–1792. [Google Scholar] [CrossRef] [PubMed]
- Nussenblatt, V.; Lema, V.; Kumwenda, N.; Broadhead, R.; Neville, M.C.; Taha, T.E.; Semba, R.D. Epidemiology and microbiology of subclinical mastitis among HIV-infected women in Malawi. Int. J. STD AIDS 2005, 16, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Neville, M.C.; Allen, J.C.; Archer, P.C.; Casey, C.E.; Seacat, J.; Keller, R.P.; Lutes, V.; Rasbach, J.; Neifert, M. Studies in human lactation: Milk volume and nutrient composition during weaning and lactogenesis. Am. J. Clin. Nutr. 1991, 54, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, A.C.; Hansen, K.B.; Moller, B.R. Leukocyte counts and microbiologic cultivation in the diagnosis of puerperal mastitis. Am. J. Obstet. Gynecol. 1983, 146, 938–941. [Google Scholar] [CrossRef]
- Filteau, S.M.; Lietz, G.; Mulokozi, G.; Bilotta, S.; Henry, C.J.K.; Tomkins, A.M. Milk cytokines and subclinical breast inflammation in Tanzanian women: Effects of dietary red palm oil or sunflower oil supplementation. Immunology 1999, 97, 595–600. [Google Scholar] [CrossRef]
- World Health Organization. Mastitis-Causes and Management; World Health Organization: Geneva, Switzerland, 2000; pp. 1–44. [Google Scholar]
- Michie, C.; Lockie, F.; Lynn, W. The Challenge of Addiction. Lancet 1984, 324, 1019–1020. [Google Scholar]
- Fetherston, C. Risk Factors for Lactation Mastitis. J. Hum. Lact. 1998, 14, 101–109. [Google Scholar] [CrossRef]
- Willumsen, J.F.; Filteau, S.M.; Coutsoudis, A.; Uebel, K.E.; Newell, M.-L.; Tomkins, A.M. Subclinical Mastitis as a Risk Factor for Mother-Infant HIV Transmission. Adv. Exp. Med. Biol. 2000, 478, 211–223. [Google Scholar] [PubMed]
- Kantarci, S.; Koulinska, I.N.; Aboud, S.; Fawzi, W.W.; Villamor, E. Subclinical mastitis, cell-associated HIV-1 shedding in breast milk, and breast-feeding transmission of HIV-1. J. Acquir. Immune Defic. Syndr. 2007, 46, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Batavani, R.A.; Asri, S.; Naebzadeh, H. The effect of subclinical mastitis on milk composition in dairy cows. Iran. J. Vet. Res. 2007, 8, 205–211. [Google Scholar]
- Bruckmaier, R.M.; Ontsouka, C.E.; Blum, J.W. Fractionized milk composition in dairy cows with subclinical mastitis. Vet. Med. Czech 2004, 49, 283–290. [Google Scholar] [CrossRef]
- Schukken, Y.H.; Günther, J.; Fitzpatrick, J.; Fontaine, M.C.; Goetze, L.; Holst, O.; Leigh, J.; Petzl, W.; Schuberth, H.; Sipka, A.; et al. Host-response patterns of intramammary infections in dairy cows. Vet. Immunol. Immunopathol. 2011, 144, 270–289. [Google Scholar] [CrossRef]
- Li, C.; Solomons, N.W.; Scott, M.E.; Koski, K.G. Subclinical mastitis (SCM) and proinflammatory cytokines are associated with mineral and trace element concentrations in human breast milk. J. Trace Elem. Med. Biol. 2018, 46, 55–61. [Google Scholar] [CrossRef]
- Leitner, G.; Chaffer, M.; Shamay, A.; Shapiro, F.; Merin, U.; Ezra, E.; Saran, A.; Silanikove, N. Changes in milk composition as affected by subclinical mastitis in sheep. J. Dairy Sci. 2004, 87, 46–52. [Google Scholar] [CrossRef]
- Busato, A.; Trachsel, P.; Schällibaum, M.; Blum, J.W. Udder health and risk factors for subclinical mastitis in organic dairy farms in Switzerland. Prev. Vet. Med. 2000, 44, 205–220. [Google Scholar] [CrossRef]
- Schrick, F.N.; Hockett, M.E.; Saxton, A.M.; Lewis, M.J.; Dowlen, H.H.; Oliver, S.P. Influence of Subclinical Mastitis During Early Lactation on Reproductive Parameters. J. Dairy Sci. 2001, 84, 1407–1412. [Google Scholar] [CrossRef]
- Singh, M.; Yadav, P.; Sharma, A.; Garg, V.K.; Mittal, D. Estimation of Mineral and Trace Element Profile in Bubaline Milk Affected with Subclinical Mastitis. Biol. Trace Elem. Res. 2017, 176, 305–310. [Google Scholar] [CrossRef]
- Silanikove, N.; Merin, U.; Leitner, G. On effects of subclinical mastitis and stage of lactation on milk quality in goats. Small Rumin. Res. 2014, 122, 76–82. [Google Scholar] [CrossRef]
- Tuaillon, E.; Viljoen, J.; Dujols, P.; Cambonie, G.; Rubbo, P.A.; Nagot, N.; Bland, R.M.; Badiou, S.; Newell, M.L.; Van De Perre, P. Subclinical mastitis occurs frequently in association with dramatic changes in inflammatory/anti-inflammatory breast milk components. Pediatr. Res. 2017, 81, 556–564. [Google Scholar] [CrossRef]
- Aryeetey, R.N.O.; Marquis, G.S.; Brakohiapa, L.; Timms, L.; Lartey, A. Subclinical Mastitis May Not Reduce Breastmilk Intake During Established Lactation. Breastfeed. Med. 2009, 4, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Kasonka, L.; Makasa, M.; Marshall, T.; Chisenga, M.; Sinkala, M.; Chintu, C.; Kaseba, C.; Kasolo, F.; Gitau, R.; Tomkins, A.; et al. Risk factors for subclinical mastitis among HIV-infected and uninfected women in Lusaka, Zambia. Paediatr. Perinat. Epidemiol. 2006, 20, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Cruz-hernandez, C.; Thakkar, S.K.; Masserey-elmelegy, I.; Bousi, W.; Fontannaz, P.; Giuffrida, F. Quantification of Fatty Acids in Erythrocytes and Plasma by Fast Gas Chromatography. J. Sep. Sci. 2017, 40, 3289–3300. [Google Scholar] [CrossRef] [PubMed]
- Affolter, M.; Garcia-Rodenas, C.L.; Vinyes-Pares, G.; Jenni, R.; Roggero, I.; Avanti-Nigro, O.; de Castro, C.A.; Zhao, A.; Zhang, Y.; Wang, P.; et al. Temporal changes of protein composition in breast milk of Chinese urban mothers and impact of caesarean section delivery. Nutrients 2016, 8, 504. [Google Scholar] [CrossRef] [PubMed]
- Klein, P.D.; Butte, N.F.; Garza, C.; Patterson, B.W.; Wong, W.W. Human-milk intake measured by administration of deuterium oxide to the mother: A comparison with the test-weighing technique. Am. J. Clin. Nutr. 2018, 47, 815–821. [Google Scholar]
- Macfarlane, A.J.; Blondel, B.; Mohangoo, A.D.; Cuttini, M.; Nijhuis, J.; Novak, Z.; Ólafsdóttir, H.S.; Zeitlin, J. Wide differences in mode of delivery within Europe: Risk-stratified analyses of aggregated routine data from the Euro-Peristat study. BJOG 2016, 123, 559–568. [Google Scholar] [CrossRef]
- Khanal, V.; Scott, J.A.; Lee, A.H.; Binns, C.W. Incidence of Mastitis in the Neonatal Period in a Traditional Breastfeeding Society: Results of a Cohort Study. Breastfeed. Med. 2015, 10, 481–487. [Google Scholar] [CrossRef]
- Say, B.; Dizdar, E.A.; Degirmencioglu, H.; Uras, N.; Sari, F.N.; Oguz, S.; Canpolat, F.E. The effect of lactational mastitis on the macronutrient content of breast milk. Early Hum. Dev. 2016, 98, 7–9. [Google Scholar] [CrossRef]
- Hunt, K.M.; Williams, J.E.; Shafii, B.; Hunt, M.K.; Behre, R.; Ting, R.; McGuire, M.K.; McGuire, M.A. Mastitis Is Associated with Increased Free Fatty Acids, Somatic Cell Count, and Interleukin-8 Concentrations in Human Milk. Breastfeed. Med. 2013, 8, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A.; Prentice, A.M.; Lamb, W.H. Mastitis in rural Gambian mothers and the protection of the breast by milk antimicrobial factors. Trans. R. Soc. Trop. Med. Hyg. 1985, 79, 90–95. [Google Scholar] [CrossRef]
- Fetherston, C.M.; Lai, C.T.; Hartmann, P.E. Relationships Between Symptoms and Changes in Breast Physiology During Lactation Mastitis. Breastfeed. Med. 2006, 1, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.A.; Salah, M.M.; Eid, S.Z. The effect of breast infection on the composition of human milk. Int. J. Biochem. 1972, 3, 543–548. [Google Scholar] [CrossRef]
- Ogola, H.; Shitandi, A.; Nanua, J. Effect of mastitis on raw milk compositional quality. J. Vet. Sci. 2007, 8, 237–242. [Google Scholar] [CrossRef][Green Version]
- Auldist, M.J.; Coats, S.; Rogers, G.L.; McDowell, G.H. Changes in the Composition of Milk From Healthy and Mastitic Dairy-Cows During the Lactation Cycle. Aust. J. Exp. Agric. 1995, 35, 427–436. [Google Scholar] [CrossRef]
- Buescher, E.S.; Hair, P.S. Human milk anti-inflammatory component contents during acute mastitis. Cell. Immunol. 2001, 210, 87–95. [Google Scholar] [CrossRef]
- Layman, D.K.; Lönnerdal, B.; Fernstrom, J.D. Applications for α-lactalbumin in human nutrition. Nutr. Rev. 2018, 76, 444–460. [Google Scholar] [CrossRef]
- Kasuga, K.; Yang, R.; Porter, T.F.; Agrawal, N.; Petasis, N.A.; Irimia, D.; Toner, M.; Serhan, C.N. Rapid Appearance of Resolvin Precursors in Inflammatory Exudates: Novel Mechanisms in Resolution. J. Immunol. 2008, 181, 8677–8687. [Google Scholar] [CrossRef]
- Hogan, J.S.; Weiss, W.P.; Smith, K.L. Role of Vitamin E and Selenium in Host Defense Against Mastitis. J. Dairy Sci. 1993, 76, 2795–2803. [Google Scholar] [CrossRef]
- Smith, K.L.; Harrison, J.H.; Hancock, D.D.; Todhunter, D.A.; Conrad, H.R. Effect of Vitamin E and Selenium Supplementation on Incidence of Clinical Mastitis and Duration of Clinical Symptoms. J. Dairy Sci. 1984, 67, 1293–1300. [Google Scholar] [CrossRef]
- Smith, K.L.; Hogan, J.S.; Weiss, W.P. Dietary vitamin E and selenium affect mastitis and milk quality. J. Anim. Sci. 1997, 75, 1659–1665. [Google Scholar] [CrossRef]
- Duntas, L.H. Selenium and inflammation: Underlying anti-inflammatory mechanisms. Horm. Metab. Res. 2009, 41, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Overton, T.R.; Yasui, T. Practical applications of trace minerals for dairy cattle. J. Anim. Sci. 2014, 92, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Scaletti, R.W.; Trammell, D.S.; Smith, B.A.; Harmon, R.J. Role of Dietary Copper in Enhancing Resistance to Escherichia coli Mastitis. J. Dairy Sci. 2003, 86, 1240–1249. [Google Scholar] [CrossRef]
- Wegner, T.N.; Stull, J.W. Relation between mastitis test score, mineral composition of milk, and blood electrolyte profiles in Holstein cows. J. Dairy Sci. 1978, 61, 1755–1759. [Google Scholar] [CrossRef]
- Martinez, N.; Sinedino, L.D.P.; Bisinotto, R.S.; Ribeiro, E.S.; Gomes, G.C.; Lima, F.S.; Greco, L.F.; Risco, C.A.; Galvão, K.N.; Taylor-Rodriguez, D.; et al. Effect of induced subclinical hypocalcemia on physiological responses and neutrophil function in dairy cows. J. Dairy Sci. 2014, 97, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, K.S.; Alexander, M.P.; Serdula, M.K.; Davis, M.K.; Bowman, B.A. Assessment of Infant Feeding: The Validity of Measuring Milk Intake. Nutr. Rev. 2002, 60, 235–251. [Google Scholar] [CrossRef]
- Savenije, O.E.; Brand, P.L. Accuracy and precision of test weighing to assess milk intake in newborn infants. Arch. Dis. Child. Fetal Neonatal Ed. 2006, 91, 330–332. [Google Scholar] [CrossRef]
- Drewett, R.F.; Woolridge, M.W.; Greasley, V.; Mcleod, C.N.; Hewison, J.; Williams, A.F.; Baum, J.D. Evaluating breast-milk intake by test weighing: A portable electronic balance suitable for community and field studies. Early Hum. Dev. 1984, 10, 123–126. [Google Scholar] [CrossRef]
- Manganaro, R.; Marseglia, L.; Mamì, C.; Palmara, A.; Paolata, A.; Loddo, S.; Gargano, R.; Mondello, M.; Gemelli, M. Breast milk sodium concentration, sodium intake and weight loss in breast-feeding newborn infants. Br. J. Nutr. 2007, 97, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Wren-Atilola, H.M.; Solomons, N.W.; Scott, M.E.; Koski, K.G. Infant growth faltering linked to subclinical mastitis, maternal faecal–oral contamination, and breastfeeding. Matern. Child Nutr. 2019, e12756. [Google Scholar] [CrossRef] [PubMed]
- Filteau, S.M.; Rice, A.L.; Ball, J.J.; Chakraborty, J.; Stoltzfus, R.; De Francisco, A.; Willumsen, J.F. Breast milk immune factors in Bangladeshi women supplemented postpartum with retinol or β-carotene. Am. J. Clin. Nutr. 1999, 69, 953–958. [Google Scholar] [CrossRef]
- Li, C.; Solomons, N.W.; Scott, M.E.; Koski, K.G. Anthropometry before Day 46 and Growth Velocity before 6 Months of Guatemalan Breastfed Infants Are Associated with Subclinical Mastitis and Milk Cytokines, Minerals, and Trace Elements. J. Nutr. 2019, 149, 1651–1659. [Google Scholar] [CrossRef]
| SCM (N = 108), n (%) | Normal (N = 197), n (%) | p Value | |
|---|---|---|---|
| Maternal Characteristics | |||
| Age (y) a | 31.44 ± 4.42 | 31.17 ± 4.09 | 0.596 |
| Country of residence b | |||
| Spain (56% SCM prevalence) | 5 (4.8) | 4 (2.0) | <0.001 |
| France (24% SCM prevalence) | 21 (20.2) | 67 (34.0) | |
| Italy (7% SCM prevalence) | 1 (1.0) | 14 (7.1) | |
| Norway (0% SCM prevalence) | 0 (0.0) | 10 (5.1) | |
| Portugal (37% SCM prevalence) | 36 (33.3) | 62 (31.5) | |
| Romania (67% SCM prevalence) | 28 (25.9) | 14 (7.1) | |
| Sweden (40% SCM prevalence) | 17 (15.7) | 26 (13.2) | |
| Height (cm) a | 164.86 ± 6.06 | 164.89 ± 5.99 | 0.964 |
| Pre-pregnancy weight (kg) a | 61.79 ± 8.18 | 61.63 ± 7.49 | 0.866 |
| Pre-pregnancy BMI (kg/m2) a | 22.73 ± 2.78 | 22.67 ± 2.52 | 0.847 |
| BMI category b | |||
| Normal weight | 85 (78.7) | 158 (80.2) | 0.871 |
| Overweight | 23 (21.3) | 39 (19.8) | |
| Parity b | 0.503 | ||
| Primiparous | 84 (77.8) | 141 (71.6) | |
| Multiparous | 24 (22.2) | 56 (28.4) | |
| Gestational age at delivery (wk) a | 39.07 ± 1.23 | 39.56 ± 1.13 | 0.001 |
| Mode of delivery b | |||
| Caesarian | 41 (38.0) | 35 (17.8) | <0.001 |
| Vaginal | 67 (62.0) | 162 (82.2) | |
| Infant Characteristics | |||
| Infant weight at birth (kg) a | 3.23 (0.51) | 3.42 (0.44) | 0.001 |
| Infant length at birth (cm) a | 49.69 (2.21) | 50.13 (1.97) | 0.086 |
| Infant gender b | |||
| Female | 46 (42.6) | 93 (47.2) | 0.513 |
| Male | 62 (57.4) | 104 (52.8) | |
| Mean ± SD | Day 2 | Day 17 | Day 30 | Day 60 | Day 90 | Day 120 | Overall p Value 1 | |
|---|---|---|---|---|---|---|---|---|
| Energy (kcal/100 mL) | SCM | 60 ± 10 | 69 ± 10 | 71 ± 14 | 72 ± 13 | 70 ± 15 | 70 ± 14 | 0.13 |
| (n = 36) | (n = 91) | (n = 80) | (n = 77) | (n = 70) | (n = 68) | |||
| Normal | 62 ± 9 | 73 ± 12 | 72 ± 13 | 72 ± 15 | 71 ± 16 | 72 ± 17 | ||
| (n = 55) | (n = 172) | (n = 156) | (n = 153) | (n = 150) | (n = 136) | |||
| Lactose (g/100 mL) | SCM | 6.7 ± 0.5 * | 7.2 ± 0.4 * | 7.3 ± 0.4 * | 7.2 ± 0.4 | 7.3 ± 0.3 | 7.3 ± 0.3 | <0.001 |
| (n = 36) | (n = 91) | (n = 80) | (n = 78) | (n = 71) | (n = 68) | |||
| Normal | 7.0 ± 0.3 | 7.3 ± 0.3 | 7.4 ± 0.3 | 7.3 ± 0.3 | 7.3 ± 0.3 | 7.3 ± 0.3 | ||
| (n = 55) | (n = 172) | (n = 157) | (n = 153) | (n = 150) | (n = 139) | |||
| Total protein (g/100 mL) | SCM | 2.7 ± 1.1 ** | 1.7 ± 0.4 | 1.5 ± 0.3 | 1.4 ± 0.3 | 1.3 ± 0.3 | 1.3 ± 0.3 | <0.0001 |
| (n = 104) | (n = 104) | (n = 91) | (n = 80) | (n = 77) | (n = 77) | |||
| Normal | 2.5 ± 1.1 | 1.6 ± 0.3 | 1.4 ± 0.3 | 1.3 ± 0.3 | 1.3 ± 0.3 | 1.3 ± 0.5 | ||
| (n = 150) | (n = 188) | (n = 171) | (n = 162) | (n = 155) | (n = 147) | |||
| Total fat (g/100 mL) | SCM | 2.6 ± 1.0 | 3.7 ± 1.1 | 4.0 ± 1.5 | 4.2 ± 1.5 | 4.0 ± 1.7 | 4.0 ± 1.5 | 0.11 |
| (n = 41) | (n = 92) | (n = 80) | (n = 78) | (n = 71) | (n = 68) | |||
| Normal | 2.9 ± 1.0 | 4.2 ± 1.4 | 4.1 ± 1.4 | 4.1 ± 1.6 | 4.0 ± 1.8 | 4.2 ± 1.8 | ||
| (n = 55) | (n = 173) | (n = 156) | (n = 153) | (n = 150) | (n = 136) |
| Day 2 | Day 17 | Day 30 | Day 60 | Day 90 | Day 120 | Overall p Value 1 | ||
|---|---|---|---|---|---|---|---|---|
| Calcium (mg/L) | SCM | 256 ± 73 * | 281 ± 54 | 296 ± 47 | 299 ± 41 | 288 ± 45 | 271 ± 36 | 0.003 |
| (n = 91) | (n = 97) | (n = 84) | (n = 78) | (n = 74) | (n = 71) | |||
| Normal | 295 ± 72 | 293 ± 55 | 296 ± 51 | 301 ± 45 | 297 ± 48 | 284 ± 42 | ||
| (n = 111) | (n = 192) | (n = 174) | (n = 166) | (n = 156) | (n = 148) | |||
| Phosphorus (mg/L) | SCM | 109 ± 41 * | 150 ± 31 * | 149 ± 29 * | 138 ± 21 | 128 ± 18 | 126 ± 20 | <0.0001 |
| (n = 91) | (n = 97) | (n = 84) | (n = 78) | (n = 74) | (n = 71) | |||
| Normal | 144 ± 32 | 169 ± 28 | 155 ± 24 | 139 ± 21 | 133 ± 20 | 131 ± 22 | ||
| (n = 111) | (n = 192) | (n = 174) | (n = 166) | (n = 156) | (n = 148) | |||
| Iron (μg/L) | SCM | 601 ± 392 * | 461 ± 310* | 346 ± 162 | 292 ± 141 | 264 ± 140 | 216 ± 103 | <0.0001 |
| (n = 91) | (n = 97) | (n = 84) | (n = 78) | (n = 74) | (n = 71) | |||
| Normal | 415 ± 193 | 389 ± 186 | 326 ± 137 | 278 ± 132 | 245 ± 127 | 262 ± 502 | ||
| (n = 111) | (n = 192) | (n = 174) | (n = 166) | (n = 156) | (n = 148) | |||
| Selenium (μg/L) | SCM | 37 ± 19 * | 19 ± 6 | 15 ± 3 | 12 ± 3 | 10 ± 2 | 9 ± 2 | <0.0001 |
| (n = 91) | (n = 97) | (n = 84) | (n = 78) | (n = 74) | (n = 71) | |||
| Normal | 26 ± 12 | 18 ± 3 | 15 ± 3 | 12 ± 2 | 10 ± 2 | 10 ± 6 | ||
| (n = 111) | (n = 192) | (n = 174) | (n = 166) | (n = 156) | (n = 148) | |||
| Manganese (μg/L) | SCM | 6.6 ± 3.9 * | 4.4 ± 1.6 | 3.7 ± 1.1 | 4.5 ± 3.4 | 3.4 ± 0.8 | 3.7 ± 0.9 | <0.0001 |
| (n = 79) | (n = 41) | (n = 26) | (n = 22) | (n = 19) | (n = 13) | |||
| Normal | 5.8 ± 3.1 | 3.9 ± 1.3 | 3.5 ± 1.0 | 3.4 ± 0.9 | 3.3 ± 0.7 | 4.0 ± 2.2 | ||
| (n = 92) | (n = 103) | (n = 75) | (n = 45) | (n = 36) | (n = 37) | |||
| Zinc (μg/L) | SCM | 7975 ± 3093 * | 3234 ± 1274 | 2422 ± 906 | 1616 ± 1208 | 1113 ± 521 | 996 ± 520 | <0.0001 |
| (n = 91) | (n = 97) | (n = 84) | (n = 78) | (n = 74) | (n = 71) | |||
| Normal | 7178 ± 2734 | 3590 ± 1051 | 2686 ± 872 | 1599 ± 692 | 1219 ± 571 | 1031 ± 563 | ||
| (n = 111) | (n = 192) | (n = 174) | (n = 166) | (n = 153) | (n = 148) | |||
| Copper (μg/L) | SCM | 533 ± 267 * | 529 ± 135 | 423 ± 86 | 325 ± 74 | 268 ± 72 | 221 ± 69 | <0.001 |
| (n = 91) | (n = 97) | (n = 84) | (n = 78) | (n = 74) | (n = 71) | |||
| Normal | 472 ± 175 | 522 ± 111 | 422 ± 89 | 304 ± 76 | 256 ± 77 | 228 ± 89 | ||
| (n = 111) | (n = 192) | (n = 174) | (n = 166) | (n = 156) | (n = 148) |
| Days Postpartum | Feeding Frequency (per day) | Intake Per Feed (kg/feed) | Daily intake (kg/day) | ||||
|---|---|---|---|---|---|---|---|
| Median | Average | Median | Average | Median | Average | ||
| (Q1, Q3) | (± SD) | (Q1, Q3) | (± SD) | (Q1, Q3) | (± SD) | ||
| Day 2 | SCM | 9 (8, 10.75) | 9.56 ± 2.25 | 0.02 (0.01, 0.04) | 0.03 ± 0.03 | 0.21 (0.12, 0.36) | 0.29 ± 0.22 |
| Normal | 9 (8, 11) | 9.54 ± 2.55 | 0.03 (0.02, 0.04) | 0.03 ± 0.02 | 0.27 (0.18, 0.35) | 0.28 ± 0.15 | |
| Day 17 | SCM | 8 (6.75, 8) | 7.80 ± 1.51 | 0.07 (0.04, 0.10) | 0.07 ± 0.04 | 0.52 (0.36, 0.74) | 0.55 ± 0.24 |
| Normal | 9 (7, 10) | 8.9 ± 1.95 | 0.07 (0.05, 0.09) | 0.07 ± 0.04 | 0.60 (0.45, 0.70) | 0.61 ± 0.20 | |
| Day 30 | SCM | 7.5 (7, 9) | 7.94 ± 1.95 | 0.09 (0.06, 0.11) | 0.09 ± 0.04 | 0.66 (0.57, 0.73) | 0.67 ± 0.18 |
| Normal | 8 (7, 10) | 8.39 ± 1.88 | 0.09 (0.06, 0.12) | 0.09 ± 0.04 | 0.70 (0.54, 0.87) | 0.73 ± 0.25 | |
| Day 60 | SCM | 7 (6,8) | 7.24 ± 1.64 | 0.10 (0.06, 0.13) | 0.10 ± 0.04 | 0.72 (0.61, 0.82) | 0.69 ± 0.18 |
| Normal | 7 (6, 8) | 7.13 ± 1.78 | 0.11 (0.08, 0.14) | 0.11 ± 0.05 | 0.75 (0.60, 0.90) | 0.77 ± 0.27 | |
| Day 90 | SCM | 7 (6, 8.25) | 7.33 ± 1.78 | 0.12 (0.10, 0.14) | 0.12 ± 0.03 | 0.94 * (0.68, 1.03) | 0.89 * ± 0.25 |
| Normal | 6 (5, 7) | 6.26 ± 1.58 | 0.12 (0.09, 0.16) | 0.13 ± 0.05 | 0.75 (0.62, 0.90) | 0.76 ± 0.21 | |
| Day 120 | SCM | 6 (6, 7.5) | 6.47 ± 1.36 | 0.13 (0.08, 0.15) | 0.12 ± 0.05 | 0.75 (0.66, 0.81) | 0.72 ± 0.25 |
| Normal | 6 (5, 7) | 5.94 ± 1.62 | 0.13 (0.09, 0.17) | 0.14 ± 0.06 | 0.73 (0.66, 0.91) | 0.77 ± 0.23 | |
| V0 (Child Birth) | Overall Effect | |||
|---|---|---|---|---|
| Z Score | Estimate SCM vs. Normal (SE) | p Value | Estimate SCM vs. Normal (SE) 1 | p Value 1 |
| BMI | −0.477 (0.134) | 0.0005 | 0.032 (0.066) | 0.624 |
| Head circumference | −0.581 (0.135) | <0.0001 | −0.022 (0.056) | 0.696 |
| Length | −0.251 (0.130) | 0.059 | −0.073 (0.053) | 0.165 |
| Weight | −0.430 (0.121) | 0.0005 | 0.011 (0.049) | 0.831 |
| Weight for length | −0.421 (0.142) | 0.004 | 0.056 (0.073) | 0.448 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samuel, T.M.; De Castro, C.A.; Dubascoux, S.; Affolter, M.; Giuffrida, F.; Billeaud, C.; Picaud, J.-C.; Agosti, M.; Al-Jashi, I.; Pereira, A.B.; et al. Subclinical Mastitis in a European Multicenter Cohort: Prevalence, Impact on Human Milk (HM) Composition, and Association with Infant HM Intake and Growth. Nutrients 2020, 12, 105. https://doi.org/10.3390/nu12010105
Samuel TM, De Castro CA, Dubascoux S, Affolter M, Giuffrida F, Billeaud C, Picaud J-C, Agosti M, Al-Jashi I, Pereira AB, et al. Subclinical Mastitis in a European Multicenter Cohort: Prevalence, Impact on Human Milk (HM) Composition, and Association with Infant HM Intake and Growth. Nutrients. 2020; 12(1):105. https://doi.org/10.3390/nu12010105
Chicago/Turabian StyleSamuel, Tinu Mary, Carlos Antonio De Castro, Stephane Dubascoux, Michael Affolter, Francesca Giuffrida, Claude Billeaud, Jean-Charles Picaud, Massimo Agosti, Isam Al-Jashi, Almerinda Barroso Pereira, and et al. 2020. "Subclinical Mastitis in a European Multicenter Cohort: Prevalence, Impact on Human Milk (HM) Composition, and Association with Infant HM Intake and Growth" Nutrients 12, no. 1: 105. https://doi.org/10.3390/nu12010105
APA StyleSamuel, T. M., De Castro, C. A., Dubascoux, S., Affolter, M., Giuffrida, F., Billeaud, C., Picaud, J.-C., Agosti, M., Al-Jashi, I., Pereira, A. B., Costeira, M. J., Silva, M. G., Marchini, G., Rakza, T., Haaland, K., Stiris, T., Stoicescu, S.-M., Martínez-Costa, C., Vanpee, M., ... Silva-Zolezzi, I. (2020). Subclinical Mastitis in a European Multicenter Cohort: Prevalence, Impact on Human Milk (HM) Composition, and Association with Infant HM Intake and Growth. Nutrients, 12(1), 105. https://doi.org/10.3390/nu12010105





