Baked Bread Enhances the Immune Response and the Catabolism in the Human Body in Comparison with Steamed Bread
Abstract
1. Introduction
2. Materials and Methods
2.1. Making Steamed Bread and Baked Bread
2.2. Volunteers
2.3. Treatments
2.4. Cytokine Network
2.4.1. Detection of Serum Cytokine Profiles
2.4.2. Construction of Cytokine Network
2.5. Central Metabolic Pathway (CMP) Metabolic Network
2.5.1. Construction of Metabolic Network
2.5.2. Determination of Serum Glucose and Lactate
2.5.3. Determination of Expression Levels of Metabolic Enzymes in CMP
2.6. Statistical Analysis
3. Results
3.1. Serum Cytokines Concentrations
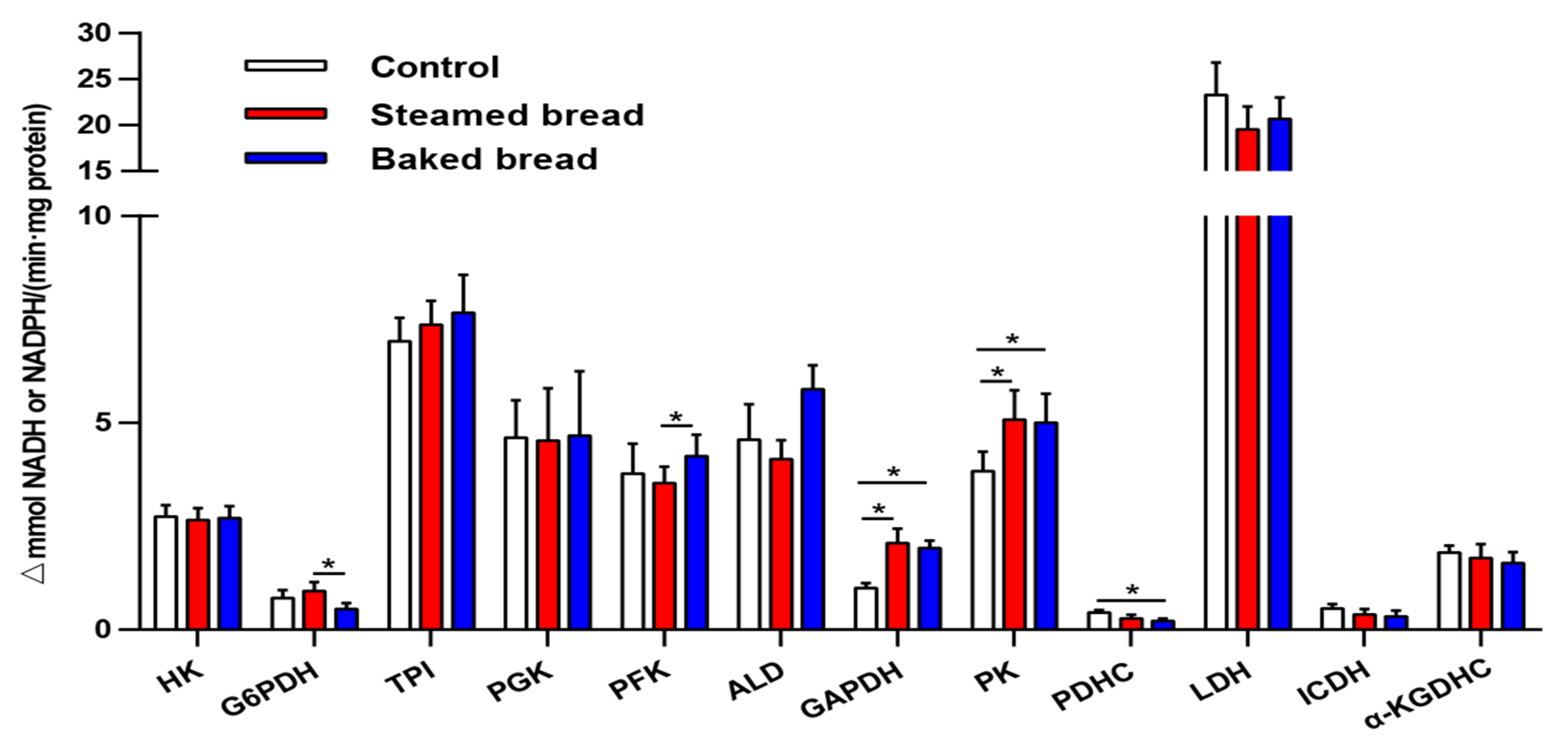
3.2. Serum Metabolic Enzyme Expression Levels
3.3. Cytokine Network and CMP Metabolic Network
3.3.1. Cytokine Network
3.3.2. CMP Metabolic Network
3.3.3. Relationship Between the Cytokine and CMP Metabolic Network
4. Discussion
4.1. Baked Bread Enhanced the Immune Response as Compared with Steamed Bread
4.2. Baked Bread Increased the Catabolism and Decreased the Anabolism as Compared with Steamed Bread
4.3. Steamed Bread and Baked Bread May Affect the Secretion of Cytokines Which Regulate Metabolism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Delgado, A.M.; Khandual, S.; Villanueva, S. Chemical stability of astaxanthin integrated into a food matrix: Effects of food processing and methods for preservation. Food Chem. 2017, 225, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Mosca, A.; Chen, J. Food oral management: Physiology and objective assessment. Curr. Opin. Food Sci. 2016, 9, 11–20. [Google Scholar] [CrossRef]
- Varum, F.; Hatton, G.; Basit, A. Food, physiology and drug delivery. Int. J. Pharm. 2013, 457, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Małgorzata, M. Bioactive phenolics of fresh and freeze-dried sweet and semi-spicy pepper fruits (Capsicum annuum, L.). J. Funct. Foods 2014, 7, 269–277. [Google Scholar]
- Wang, T.; He, F.; Chen, G. Improving bioaccessibility and bioavailability of phenolic compounds in cereal grains through processing technologies: A concise review. J. Funct. Foods 2014, 7, 101–111. [Google Scholar] [CrossRef]
- Mcclements, D.; Xiao, H.; Demokritou, P. Physicochemical and colloidal aspects of food matrix effects on gastrointestinal fate of ingested inorganic nanoparticles. Adv. Colloid. Interface. Sci. 2017, 246, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Narukawa, M. Physiological responses to taste signals of functional food components. Biosci. Biotechnol. Biochem. 2018, 82, 200–206. [Google Scholar] [CrossRef]
- Pang, G.; Xie, J.; Chen, Q.; Hu, Z. How functional foods play critical roles in human health. Food Sci. Human Wellness 2012, 1, 26–60. [Google Scholar] [CrossRef]
- Zachary, T. Gut Hormones and Related Concepts. Diabetes Care 2006, 29, 2319–2324. [Google Scholar]
- Li, H.; Liu, X.; Li, Y.; Hua, Y.; Zhi, D.; Pang, G. Effects of the polysaccharide from Pholiota nameko on human cytokine network in serum. Int. J. Biol. Macromol. 2012, 50, 164–170. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, Y.; Xu, Z.; Yang, W.; Mariga, A.; Pang, G.; Geng, C.; Hu, Q. Immunoregulatory role of Pleurotus eryngii superfine powder through intercellular communication of cytokines. Food Agric. Immunol. 2014, 25, 586–599. [Google Scholar] [CrossRef][Green Version]
- Sun, Y.; Shao, Y.; Zhang, Z.; Wang, L.; Mariga, A.; Pang, G.; Geng, C.; Ho, C.; Hu, Q.; Zhao, L. Regulation of human cytokines by Cordyceps militaris. J. Food Drug Anal. 2014, 22, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Pang, G. Effect of resistant and digestible rice starches on human cytokine and lactate metabolic networks in serum. Cytokine 2017, 93, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, G.; Xie, J.; Pang, G. Influence of JuA in evoking communication changes between the small intestines and brain tissues of rats and the GABA A and GABA B receptor transcription levels of hippocampal neurons. J. Ethnopharmacol. 2015, 159, 215–223. [Google Scholar] [CrossRef]
- Altan-Bonnet, G.; Mukherjee, R. Cytokine-mediated communication: A quantitative appraisal of immune complexity. Nat. Rev. Immunol. 2019, 19, 205–217. [Google Scholar] [CrossRef]
- Kirouac, D.; Madlambayan, G.; Yu, M.; Sykes, E.; Ito, C.; Zandstra, P. Cell-cell interaction networks regulate blood stem and progenitor cell fate. Mol. Syst. Biol. 2009, 5, 293–313. [Google Scholar] [CrossRef]
- Qiao, W.; Wang, W.; Laurenti, E.; Turinsky, A.; Wodak, S.; Bader, G.; Dick, J.; Zandstra, P. Intercellular network structure and regulatory motifs in the human hematopoietic system. Mol. Syst. Biol. 2014, 10, 741. [Google Scholar] [CrossRef]
- Brooks, G. Lactate shuttles in nature. Biochem. Soc. Trans. 2002, 30, 258–264. [Google Scholar] [CrossRef]
- Trabold, O.; Wagner, S.; Wicke, C.; Scheuenstuhl, H.; Hussain, M.; Rosen, N.; Seremetiev, A.; Becker, H.; Hunt, T. Lactate and oxygen constitute a fundamental regulatory mechanism in wound healing. Wound Repair Regen. 2003, 11, 504–509. [Google Scholar] [CrossRef]
- Leite, T.; Coelho, R.; Da Silva, D.; Coelho, W.; Marinho-Carvalho, M.; Sola-Penna, M. Lactate downregulates the glycolytic enzymes hexokinase and phosphofructokinase in diverse tissues from mice. FEBS Lett. 2011, 585, 92–98. [Google Scholar] [CrossRef]
- Imai, S.; Guarente, L. Ten years of NAD-dependent SIR2 family deacetylases: Implications for metabolic diseases. Trends Pharmacol. Sci. 2010, 31, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Limonciel, A.; Aschauer, L.; Wilmes, A.; Prajczer, S.; Leonard, M.; Pfaller, W.; Jennings, P. Lactate is an ideal non-invasive marker for evaluating temporal alterations in cell stress and toxicity in repeat dose testing regimes. Toxicol. In Vitro 2011, 25, 1855–1862. [Google Scholar] [CrossRef] [PubMed]
- Philp, A.; Macdonald, L.; Watt, P. Lactate - A signal coordinating celland systemic function. J. Exp. Biol. 2005, 208, 4561–4575. [Google Scholar] [CrossRef] [PubMed]
- Pang, G.; Xie, J.; Chen, Q.; Hu, Z. Energy intake, metabolic homeostasis, and human health. Food Sci. Human Wellness 2014, 3, 89–103. [Google Scholar] [CrossRef]
- Horecker, B. The pentose phosphate pathway. J. Biol. Chem. 2002, 277, 47965–47971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, B. Fate of deoxynivalenol and deoxynivalenol-3-glucoside during wheat milling and Chinese steamed bread processing. Food Control 2014, 44, 86–91. [Google Scholar] [CrossRef]
- Wronkowska, M.; Haros, M.; Soral-Śmietana, M. Effect of starch substitution by buckwheat flour on gluten-free bread quality. Food Bioprocess Tech. 2013, 6, 1820–1827. [Google Scholar] [CrossRef]
- Gui, M. Evaluation of a functional food in improving human memory. Chin. Prev. Med. 2010, 11, 31–34. [Google Scholar]
- Xie, J.; Guo, L.; Pang, G.; Wu, X.; Zhang, M. Modulation effect of Semen Ziziphi Spinosae extractson IL-1β, IL-4, IL-6, IL-10, TNF-α and IFN-γ in mouse serum. Nat. Prod. Res. 2011, 25, 464–467. [Google Scholar] [CrossRef]
- Giavedoni, L. Simultaneous detection of multiple cytokines and chemokines from nonhuman primates using luminex technology. J. Immunol. Methods 2005, 301, 89–101. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, T.; Pang, G. Intercellular wireless communication network between mother and fetus in rat pregnancy-a study on directed and weighted network. Reprod. Biol. Endocrinol. 2019, 17, 40. [Google Scholar] [CrossRef] [PubMed]
- Frankenstein, Z.; Alon, U.; Cohen, I. The immune-body cytokine network defines a social architecture of cell interactions. Biol. Direct. 2006, 1, 32. [Google Scholar] [CrossRef] [PubMed]
- American Association for the Advancement of Science. SITE VISIT: Sorting Out Cytokines. Science 2000, 288, 1131b. [Google Scholar] [CrossRef]
- Hsieh, M.; Sze, S. Finding Alignments of Conserved Graphlets in Protein Interaction Networks. J. Comput. Biol. 2014, 21, 234–246. [Google Scholar] [CrossRef]
- Stephanopoulos, G.; Aristidou, A.; Nielsen, N. Metabolic Engineering - Principles and Methodologies; Academic Press: Pittsburgh, PA, USA, 1998; pp. 1–694. [Google Scholar]
- Almaas, E.; Kovács, B.; Vicsek, T.; Oltvai, Z.; Barabási, A.L. Global organization of metabolic fluxes in the bacterium Escherichia coli. Nature 2004, 427, 839–843. [Google Scholar] [CrossRef]
- Teusink, B.; Passarge, J.; Reijenga, C.; Esgalhado, E.; van der Weijden, C.; Schepper, M.; Walsh, M.; Bakker, B.; van Dam, K.; Westerhoff, H.; et al. Can yeast glycolysis be understood in terms of in vitro kinetics of the constituent enzymes? Testing biochemistry. Eur. J. Biochem. 2000, 267, 5313–5329. [Google Scholar] [CrossRef]
- Pierce, V.; Crawford, D. Rapid enzyme assays investigating the variation in the glycolytic pathway in field-caught populations of Fundulus heteroclitus. Biochem. Genet. 1994, 32, 315–330. [Google Scholar] [CrossRef]
- Cai, X.; Wang, H.; Pang, G. Flux control analysis of a lactate and sucrose metabolic network at different storage temperatures for Hami melon (Cucumis melo var. saccharinus). Sci. Hortic. 2005, 181, 4–12. [Google Scholar] [CrossRef]
- Mathers, J.; Movahedi, M.; Macrae, F.; Mecklin, J.; Moeslein, G.; Olschwang, S.; Eccles, D.; Evans, G.; Maher, E.; Bertario, L.; et al. Long-term effect of resistant starch on cancer risk in carriers of hereditary colorectal cancer: An analysis from the CAPP2 randomised controlled trial. Lancet Oncol. 2012, 13, 1242–1249. [Google Scholar] [CrossRef]
- Proost, P.; Struyf, S.; Van, D.; Fiten, P.; Ugarte-Berzal, E.; Opdenakker, G. Chemokine isoforms and processing in inflammation and immunity. J. Autoimmun. 2017, 85, 45–57. [Google Scholar] [CrossRef]
- Romagnani, S. Th1/Th2 cells. Inflamm. Bowel Dis. 1999, 5, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Mottram, D.; Wedzicha, B.; Dodson, A. Acrylamide is formed in the Maillard reaction. Nature 2000, 419, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Van der Fels-Klerx, H.; Peters, R.; Van Boekel, M. Acrylamide and 5-hydroxymethylfurfural formation during baking of biscuits: Part I: Effects of sugar type. Food Chem. 2016, 192, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Hasawi, N.; Khandari, M.; Luqmani, Y. Phosphofructokinase: A mediator of glycolytic flux in cancer progression. Crit. Rev. Oncol. Hematol. 2014, 92, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Mathis, D.; Shoelson, S. Immunometabolism: An emerging frontier. Nat. Rev. Immunol. 2011, 11, 81–83. [Google Scholar] [CrossRef]
- Cheng, S.; Quintin, J.; Cramer, R.; Shepardson, K.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.; Martens, J.; Rao, N.; Aghajanirefah, A.; et al. mTOR- and HIF-1α–mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef]
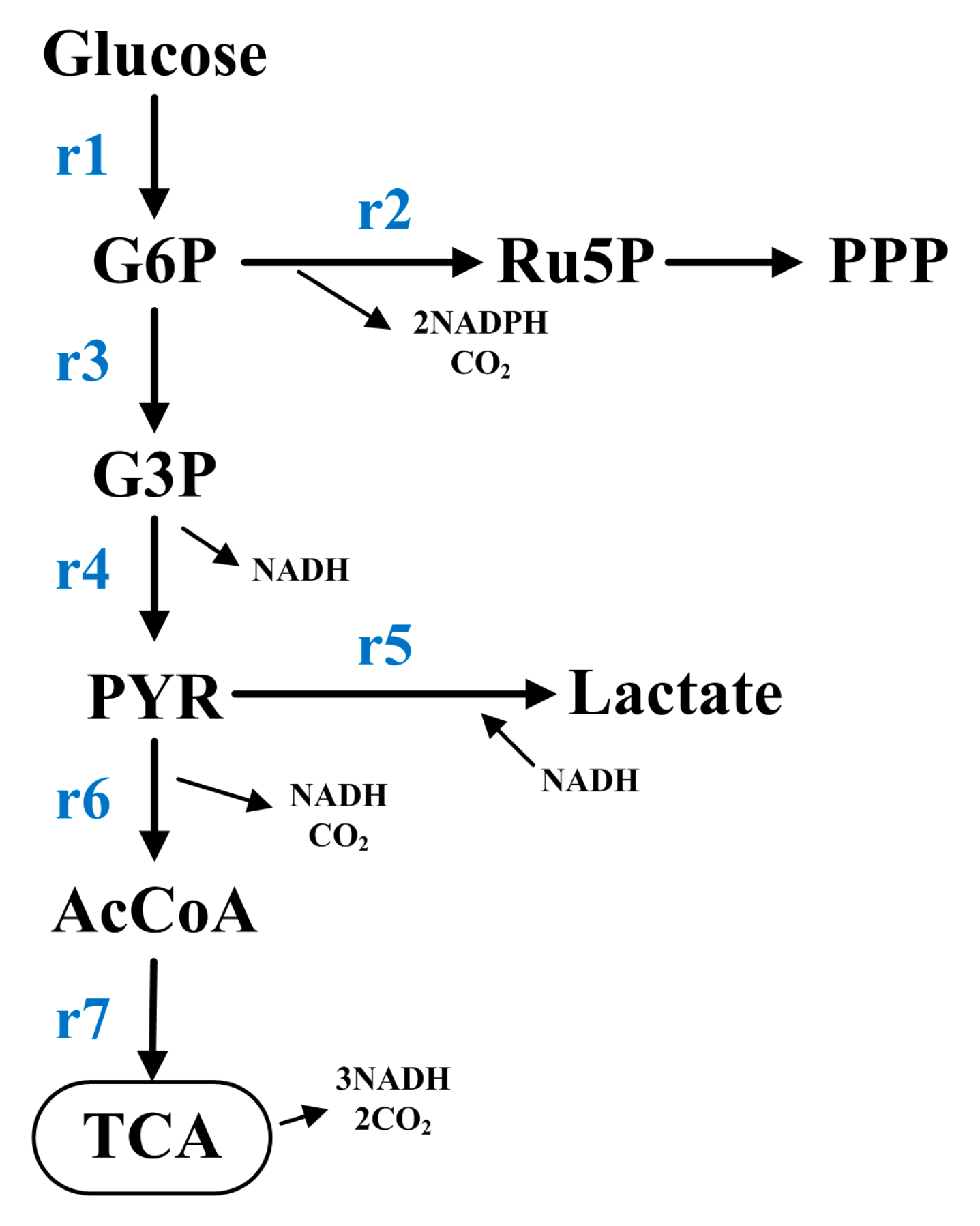


| Intermediate Metabolites | Mass Balance Equations |
|---|---|
| Glucose-6-phosphate | X1 = r1 − r2 − r3 |
| Glyceraldehyde-3-phosphate | X2 = r3 − r4 |
| Pyruvate | X3 = r4 − r5 − r6 |
| Acetyl Coenzyme A | X4 = r6 − r7 |
| Immune Cells | Output/Input | Nonimmune Cells | Output/Input | Total Network Strength (St) | |||
|---|---|---|---|---|---|---|---|
| Nodes Strength 2 | Nodes Number | Nodes Strength 2 | Nodes Number | ||||
| Steamed Bread | Th1 | 2.18/−2.15 | 15/23 | Fibroblast | −3.99/11.89 | 21/20 | 36.39 |
| Th2 | 3.72/−6.65 | 15/22 | Hematopoietic stem cell | 0/11.83 | 0/15 | ||
| Monocyte | 0.24/4.65 | 19/22 | Endothelial cell | −1.27/−7.20 | 18/15 | ||
| Macrophage | 0.24/4.65 | 19/22 | Smooth muscle cell | 0.52/−5.15 | 19/8 | ||
| Natural killer cell | −3.90/−2.11 | 16/19 | Osteoblast | 2.63/4.38 | 10/14 | ||
| B cell | 0.24/−3.34 | 19/17 | Astrocyte | −5.12/−2.18 | 16/4 | ||
| Neutrophil | −3.24/15.87 | 9/15 | Stromal cell | 2.70/0 | 14/0 | ||
| Eosinophil | −0.12/4.27 | 12/23 | Thyroid cell | 0/−2.18 | 0/4 | ||
| Basophil | 4.14/−6.86 | 14/18 | Epithelial cell | −0.09/−2.18 | 15/4 | ||
| Dendritic cell | 0.33/10.10 | 18/13 | Synovial cell | 2.63/0 | 10/0 | ||
| Mast cell | 5.36/−0.80 | 14/22 | Chondrocyte | 2.63/0 | 10/0 | ||
| Cytotoxic T lymphocyte | 0/−11.21 | 0/13 | Adipocyte | 2.63/0 | 10/0 | ||
| Microglia | 2.63/2.56 | 10/18 | |||||
| Thymocyte | 3.09/0 | 12/0 | |||||
| Baked Bread | Th1 | 6.31/2.60 | 14/10 | Fibroblast | 0/3.16 | 10/6 | 98.54 |
| Th2 | 6.31/−4.5 | 12/6 | Hematopoietic stem cell | 0/3.16 | 0/6 | ||
| Monocyte | 2.10/7.50 | 11/12 | Endothelial cell | 6.31/−0.07 | 12/12 | ||
| Macrophage | 10.11/8.91 | 17/10 | Smooth muscle cell | 1.70/−4.5 | 4/6 | ||
| Natural killer cell | 0/1.26 | 10/3 | Osteoblast | 0/3.16 | 0/6 | ||
| B-cell | 2.10/−1.34 | 11/10 | Astrocyte | 0/1.28 | 0/3 | ||
| Neutrophil | 0/8.91 | 0/10 | Stromal cell | 1.70/0 | 4/0 | ||
| Eosinophil | 0/3.16 | 0/6 | Myoblast | 0/1.28 | 0/3 | ||
| Basophil | 0/7.66 | 0/10 | |||||
| Mast cell | 6.31/0 | 12/0 | |||||
| Dendritic cell | 6.31/7.66 | 12/10 | |||||
| Items | Control | Steamed Bread | Baked Bread | |
|---|---|---|---|---|
| Serum collected at 14:30 | Glucose (mg/dL) | 89.14 ± 2.99 | 88.94 ± 2.46 | 87.67 ± 2.88 |
| Lactate (mg/dL) | 22.76 ± 0.88 | 21.00 ± 0.85 | 24.63 ± 1.01 | |
| NADH (mg/L) | 38.70 ± 7.29 | 69.26 ± 12.54 | 33.31 ± 6.23 | |
| Serum collected at 15:30 | Glucose (mg/dL) | 91.78 ± 1.65 | 79.15 ± 1.61 | 84.29 ± 2.76 |
| Lactate (mg/dL) | 19.57 ± 0.91 | 17.35 ± 0.82 | 17.73 ± 0.95 | |
| NADH (mg/L) | 62.29 ± 9.40 | 53.73 ± 9.65 | 61.51 ± 8.55 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Pang, G. Baked Bread Enhances the Immune Response and the Catabolism in the Human Body in Comparison with Steamed Bread. Nutrients 2020, 12, 1. https://doi.org/10.3390/nu12010001
Wang H, Pang G. Baked Bread Enhances the Immune Response and the Catabolism in the Human Body in Comparison with Steamed Bread. Nutrients. 2020; 12(1):1. https://doi.org/10.3390/nu12010001
Chicago/Turabian StyleWang, Huisong, and Guangchang Pang. 2020. "Baked Bread Enhances the Immune Response and the Catabolism in the Human Body in Comparison with Steamed Bread" Nutrients 12, no. 1: 1. https://doi.org/10.3390/nu12010001
APA StyleWang, H., & Pang, G. (2020). Baked Bread Enhances the Immune Response and the Catabolism in the Human Body in Comparison with Steamed Bread. Nutrients, 12(1), 1. https://doi.org/10.3390/nu12010001





