Worm-Based Alternate Assessment of Probiotic Intervention against Gut Barrier Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. C. elegans Strains and Culture Conditions
2.2. RNA Preparation, Reverse Transcription, and Real-Time Quantitative PCR (qPCR)
2.3. Antibacterial Activities of EcN in the Intestine of C. elegans
2.4. Staining with Nile Red and Oil Red O
2.5. Intestinal Barrier Function Assay (Smurf Assay)
2.6. Intestinal Injury Models in Mice
2.7. Fluorometric Assay of FITC-Conjugated Dextran In Vivo
2.8. Immunohistochemistry
2.9. Alcian Blue (AB) Staining of Murine Tissues
2.10. Statistical Analysis
3. Results
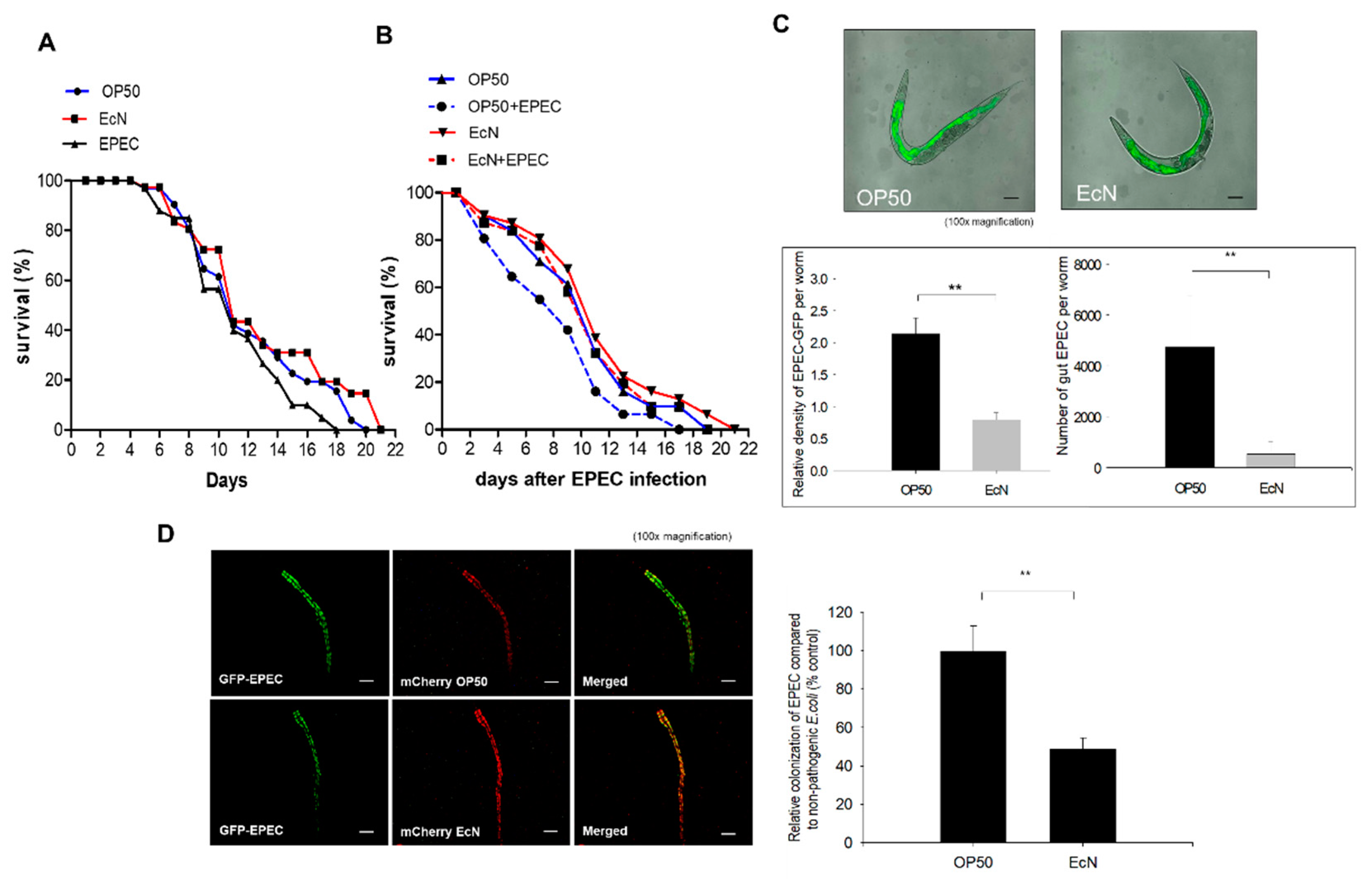
3.1. Antagonistic Actions of EcN against the Gut Colonization of EPEC in the Worms
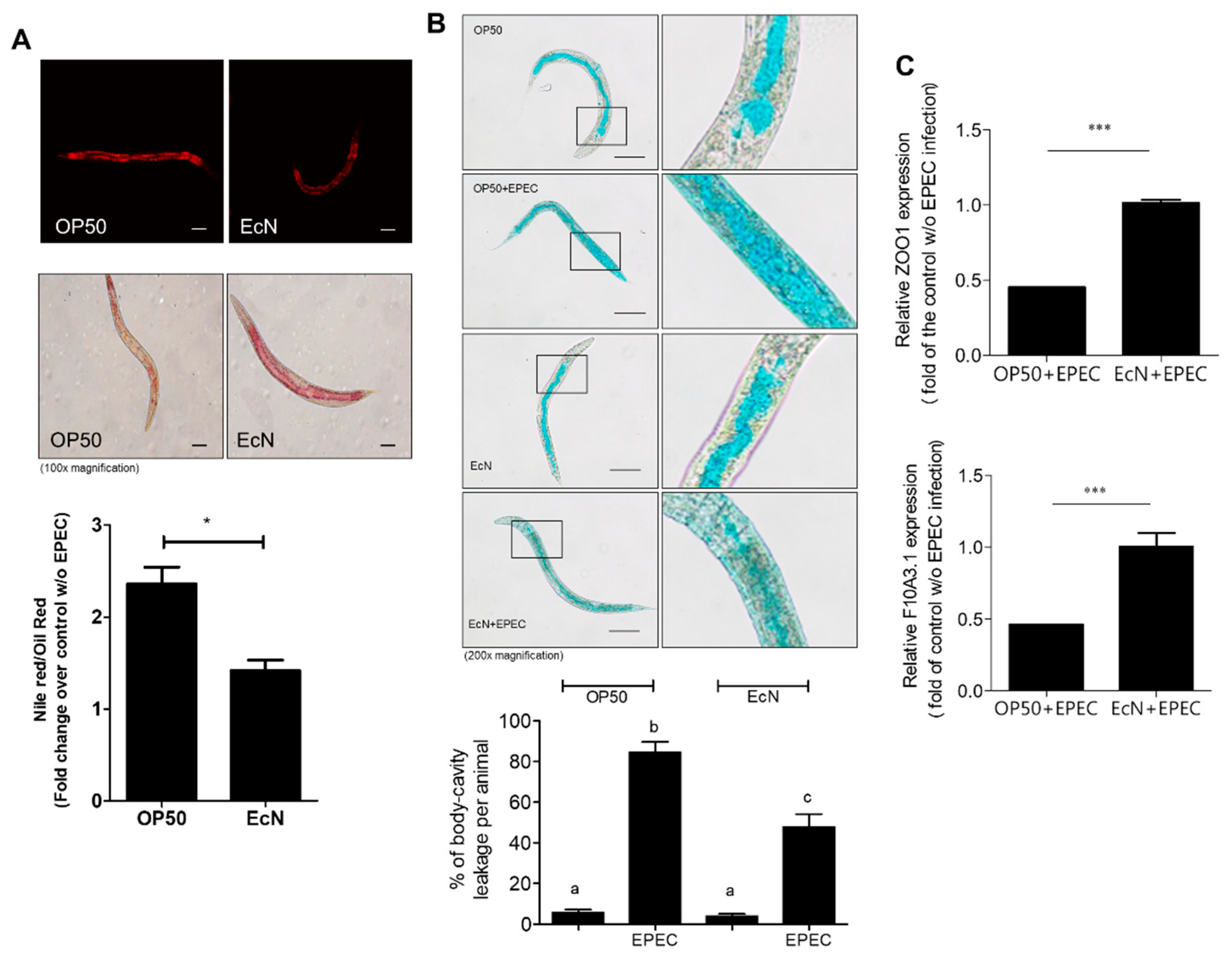
3.2. Improved Gut Permeability by EcN Treatment in Response to EPEC Infection
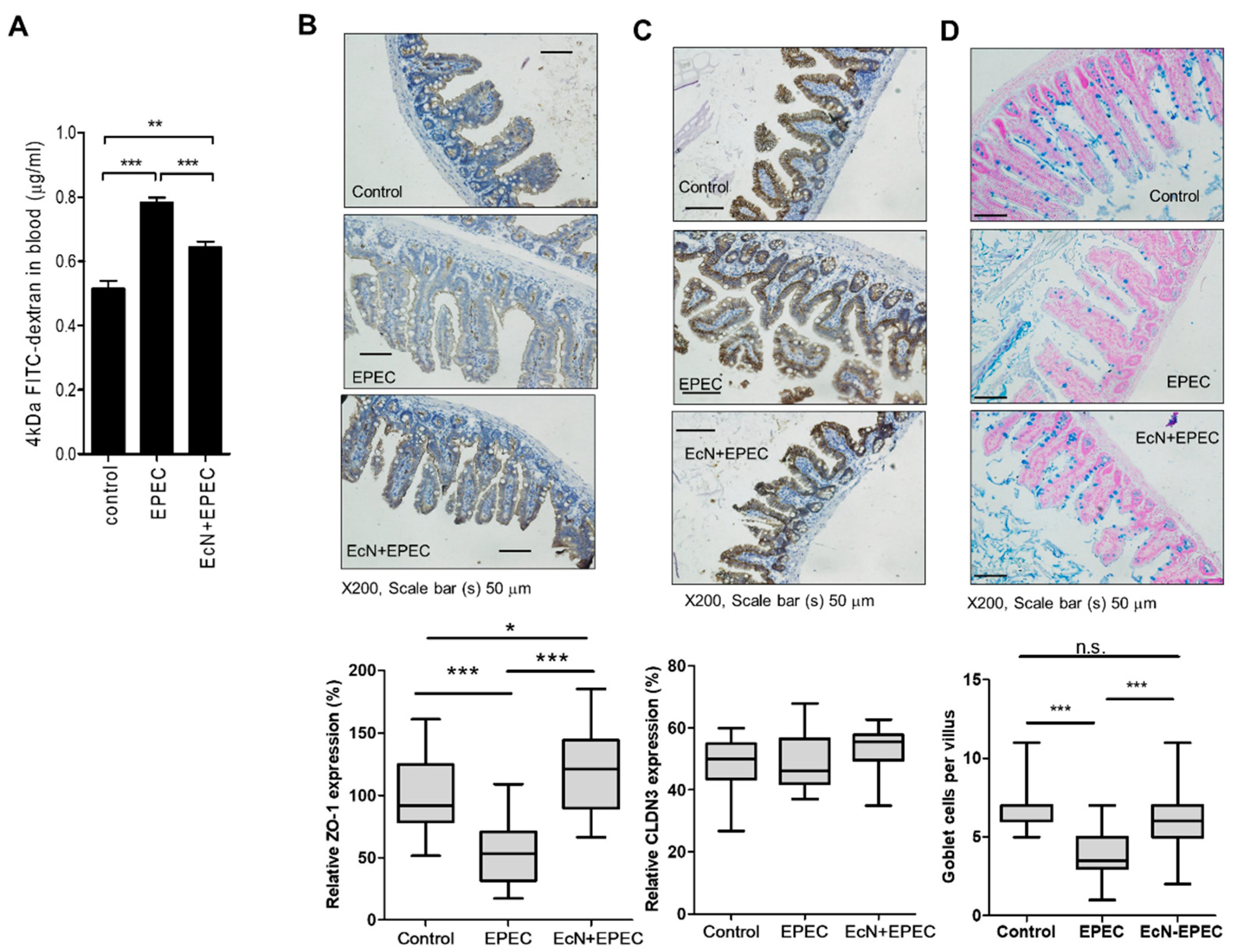
3.3. EcN-Improved Gut Integrity-Related Features in the Mammalian Infection Model
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- D’Souza, A.L.; Rajkumar, C.; Cooke, J.; Bulpitt, C.J. Probiotics in prevention of antibiotic associated diarrhoea: Meta-analysis. BMJ 2002, 324, 1361. [Google Scholar] [CrossRef] [PubMed]
- Duncker, S.C.; Lorentz, A.; Schroeder, B.; Breves, G.; Bischoff, S.C. Effect of orally administered probiotic E. coli strain Nissle 1917 on intestinal mucosal immune cells of healthy young pigs. Vet. Immunol. Immunopathol. 2006, 111, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Kruis, W.; Schutz, E.; Fric, P.; Fixa, B.; Judmaier, G.; Stolte, M. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment. Pharmacol. Ther. 1997, 11, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Hancock, V.; Dahl, M.; Klemm, P. Probiotic Escherichia coli strain Nissle 1917 outcompetes intestinal pathogens during biofilm formation. J. Med. Microbiol. 2010, 59, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Abdulkadir, S.A.; Qu, Z.; Garabedian, E.; Song, S.K.; Peters, T.J.; Svaren, J.; Carbone, J.M.; Naughton, C.K.; Catalona, W.J.; Ackerman, J.J.; et al. Impaired prostate tumorigenesis in Egr1-deficient mice. Nat. Med. 2001, 7, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Henker, J.; Muller, S.; Laass, M.W.; Schreiner, A.; Schulze, J. Probiotic Escherichia coli Nissle 1917 (EcN) for successful remission maintenance of ulcerative colitis in children and adolescents: An open-label pilot study. Z. Gastroenterol. 2008, 46, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Matthes, H.; Krummenerl, T.; Giensch, M.; Wolff, C.; Schulze, J. Clinical trial: Probiotic treatment of acute distal ulcerative colitis with rectally administered Escherichia coli Nissle 1917 (EcN). BMC Complement. Altern. Med. 2010, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Plassmann, D.; Schulte-Witte, H. Treatment of irritable bowel syndrome with Escherichia coli strain Nissle 1917 (EcN): A retrospective survey. Med. Klin. 2007, 102, 888–892. [Google Scholar]
- Gronbach, K.; Eberle, U.; Muller, M.; Olschlager, T.A.; Dobrindt, U.; Leithauser, F.; Niess, J.H.; Doring, G.; Reimann, J.; Autenrieth, I.B.; et al. Safety of probiotic Escherichia coli strain Nissle 1917 depends on intestinal microbiota and adaptive immunity of the host. Infect. Immun. 2010, 78, 3036–3046. [Google Scholar] [CrossRef]
- Blum, G.; Marre, R.; Hacker, J. Properties of Escherichia coli strains of serotype O6. Infection 1995, 23, 234–236. [Google Scholar] [CrossRef]
- Mäkelä, P.H.; Sütonen, A. Oral administration of a certain strain of live Escherichia coli for intestinal disorders? Infection 1995, 23, 184–188. [Google Scholar] [CrossRef]
- Schulze, U.S.J. The non-pathogenic Escherichia coli strain Nissle 1917—Features of a versatile probiotic. Microb. Ecol. Health Dis. 2009, 21, 122–158. [Google Scholar]
- Hoytema van Konijnenburg, D.P.; Reis, B.S.; Pedicord, V.A.; Farache, J.; Victora, G.D.; Mucida, D. Intestinal epithelial and intraepithelial T cell crosstalk mediates a dynamic response to infection. Cell 2017, 171, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Kallinowski, F.; Wassmer, A.; Hofmann, M.A.; Harmsen, D.; Heesemann, J.; Karch, H.; Herfarth, C.; Buhr, H.J. Prevalence of enteropathogenic bacteria in surgically treated chronic inflammatory bowel disease. Hepatogastroenterology 1998, 45, 1552–1558. [Google Scholar] [PubMed]
- La Ferla, K.; Seegert, D.; Schreiber, S. Activation of NF-kappaB in intestinal epithelial cells by E. coli strains isolated from the colonic mucosa of IBD patients. Int. J. Colorectal Dis. 2004, 19, 334–342. [Google Scholar]
- Maddocks, O.D.; Short, A.J.; Donnenberg, M.S.; Bader, S.; Harrison, D.J. Attaching and effacing Escherichia coli downregulate DNA mismatch repair protein in vitro and are associated with colorectal adenocarcinomas in humans. PLoS ONE 2009, 4, e5517. [Google Scholar] [CrossRef] [PubMed]
- Weber, P.; Koch, M.; Heizmann, W.R.; Scheurlen, M.; Jenss, H.; Hartmann, F. Microbic superinfection in relapse of inflammatory bowel disease. J. Clin. Gastroenterol. 1992, 14, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L.A.; Bajaj-Elliott, M.; Klein, N.J.; Murch, S.H.; Phillips, A.D. Bacterial-epithelial contact is a key determinant of host innate immune responses to enteropathogenic and enteroaggregative Escherichia coli. PLoS ONE 2011, 6, e27030. [Google Scholar] [CrossRef]
- Ruchaud-Sparagano, M.H.; Muhlen, S.; Dean, P.; Kenny, B. The enteropathogenic E. coli (EPEC) tir effector inhibits NF-kappab activity by targeting TNFalpha receptor-associated factors. PLoS Pathog. 2011, 7, e1002414. [Google Scholar] [CrossRef]
- Sham, H.P.; Shames, S.R.; Croxen, M.A.; Ma, C.; Chan, J.M.; Khan, M.A.; Wickham, M.E.; Deng, W.; Finlay, B.B.; Vallance, B.A. Attaching and effacing bacterial effector NleC suppresses epithelial inflammatory responses by inhibiting NF-kappaB and p38 mitogen-activated protein kinase activation. Infect. Immun. 2011, 79, 3552–3562. [Google Scholar] [CrossRef]
- Pukkila-Worley, R.; Ausubel, F.M. Immune defense mechanisms in the Caenorhabditis elegans intestinal epithelium. Curr. Opin. Immunol. 2012, 24, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Zugasti, O.; Bose, N.; Squiban, B.; Belougne, J.; Kurz, C.L.; Schroeder, F.C.; Pujol, N.; Ewbank, J.J. Activation of a G protein-coupled receptor by its endogenous ligand triggers the innate immune response of Caenorhabditis elegans. Nat. Immunol. 2014, 15, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.; Lynn, G.; Ngo, V.; Wong, D.; Moseley, S.L.; Ewbank, J.J.; Goncharov, A.; Wu, Y.C.; Pujol, N.; Chisholm, A.D. Negative regulation of Caenorhabditis elegans epidermal damage responses by death-associated protein kinase. Proc. Natl. Acad. Sci. USA 2009, 106, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yin, L.; Li, X.; Tang, M.; Zhang, T.; Wang, D. Contributions of altered permeability of intestinal barrier and defecation behavior to toxicity formation from graphene oxide in nematode Caenorhabditis elegans. Nanoscale 2013, 5, 9934–9943. [Google Scholar] [CrossRef]
- Tsukita, S.; Furuse, M. Claudin-Based barrier in simple and stratified cellular sheets. Cur. Opin. Cell Biol. 2002, 14, 531–536. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar]
- Daniells, C.; Duce, I.; Thomas, D.; Sewell, P.; Tattersall, J.; de Pomerai, D. Transgenic nematodes as biomonitors of microwave-induced stress. Mutat. Res. 1998, 399, 55–64. [Google Scholar] [CrossRef]
- Anyanful, A.; Dolan-Livengood, J.M.; Lewis, T.; Sheth, S.; Dezalia, M.N.; Sherman, M.A.; Kalman, L.V.; Benian, G.M.; Kalman, D. Paralysis and killing of Caenorhabditis elegans by enteropathogenic Escherichia coli requires the bacterial tryptophanase gene. Mol. Microbiol. 2005, 57, 988–1007. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, E.J.; Soukas, A.A.; Carr, C.E.; Ruvkun, G.C. elegans major fats are stored in vesicles distinct from lysosome-related organelles. Cell Metab. 2009, 10, 430–435. [Google Scholar] [CrossRef]
- Wells, J.M.; Brummer, R.J.; Derrien, M.; MacDonald, T.T.; Troost, F.; Cani, P.D.; Theodorou, V.; Dekker, J.; Meheust, A.; de Vos, W.M.; et al. Homeostasis of the gut barrier and potential biomarkers. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G171–G193. [Google Scholar] [CrossRef]
- De Santis, S.; Cavalcanti, E.; Mastronardi, M.; Jirillo, E.; Chieppa, M. Nutritional keys for intestinal barrier modulation. Front. Immunol. 2015, 6, 612. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.Y.; Osaka, T.; Moriyama, E.; Date, Y.; Kikuchi, J.; Tsuneda, S. Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum. Physiol. Rep. 2015, 3, e12327. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.B.; Tebbutt, N.C.; Buchert, M.; Putoczki, T.L.; Doggett, K.; Bao, S.; Johnstone, C.N.; Masson, F.; Hollande, F.; Burgess, A.W.; et al. Glycoprotein A33 deficiency: A new mouse model of impaired intestinal epithelial barrier function and inflammatory disease. Dis. Model. Mech. 2015, 8, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xiao, L.; Rao, J.N.; Zou, T.; Liu, L.; Bellavance, E.; Gorospe, M.; Wang, J.Y. JunD represses transcription and translation of the tight junction protein zona occludens-1 modulating intestinal epithelial barrier function. Mol. Biol. Cell 2008, 19, 3701–3712. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Pazin, M.J.; D’Souza, T.; Ji, H.; Morin, P.J. Regulation of the CLDN3 gene in ovarian cancer cells. Cancer Biol. Ther. 2007, 6, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, G.H. Roles of claudin-2, ZO-1 and occludin in leaky HK-2 cells. PLoS ONE 2017, 12, e0189221. [Google Scholar] [CrossRef] [PubMed]
- Samak, G.; Chaudhry, K.K.; Gangwar, R.; Narayanan, D.; Jaggar, J.H.; Rao, R. Calcium/Ask1/MKK7/JNK2/c-Src signalling cascade mediates disruption of intestinal epithelial tight junctions by dextran sulfate sodium. Biochem. J. 2015, 465, 503–515. [Google Scholar] [CrossRef]
- Breese, E.J.; Michie, C.A.; Nicholls, S.W.; Murch, S.H.; Williams, C.B.; Domizio, P.; Walker-Smith, J.A.; MacDonald, T.T. Tumor necrosis factor alpha-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology 1994, 106, 1455–1466. [Google Scholar] [CrossRef]
- Reinecker, H.C.; Steffen, M.; Witthoeft, T.; Pflueger, I.; Schreiber, S.; MacDermott, R.P.; Raedler, A. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn’s disease. Clin. Exp. Immunol. 1993, 94, 174–181. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Guo, S.; Ye, D.; Dokladny, K.; Alhmoud, T.; Ereifej, L.; Said, H.M.; Ma, T.Y. Mechanism of IL-1beta modulation of intestinal epithelial barrier involves p38 kinase and activating transcription factor-2 activation. J. Immunol. 2013, 190, 6596–6606. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Guo, S.; Ye, D.; Ma, T.Y. TNF-Alpha modulation of intestinal epithelial tight junction barrier is regulated by ERK1/2 activation of Elk-1. Am. J. Pathol. 2013, 183, 1871–1884. [Google Scholar] [CrossRef] [PubMed]
- Savkovic, S.D.; Villanueva, J.; Turner, J.R.; Matkowskyj, K.A.; Hecht, G. Mouse model of enteropathogenic Escherichia coli infection. Infect. Immun. 2005, 73, 1161–1170. [Google Scholar] [CrossRef]
- Shifflett, D.E.; Clayburgh, D.R.; Koutsouris, A.; Turner, J.R.; Hecht, G.A. Enteropathogenic E. coli disrupts tight junction barrier function and structure in vivo. Lab. Invest. 2005, 85, 1308–1324. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Ben-Zeev Brik, R.; Federici, S.; et al. Post-Antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell 2018, 174, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Nigro, G.; Hanson, M.; Fevre, C.; Lecuit, M.; Sansonetti, P.J. Intestinal organoids as a novel tool to study microbes-epithelium interactions. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2016; pp. 1–12. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Wu, S.; Xia, Y.; Sun, J. Salmonella-infected crypt-derived intestinal organoid culture system for host-bacterial interactions. Physiol. Rep. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Moon, Y. Worm-Based Alternate Assessment of Probiotic Intervention against Gut Barrier Infection. Nutrients 2019, 11, 2146. https://doi.org/10.3390/nu11092146
Kim J, Moon Y. Worm-Based Alternate Assessment of Probiotic Intervention against Gut Barrier Infection. Nutrients. 2019; 11(9):2146. https://doi.org/10.3390/nu11092146
Chicago/Turabian StyleKim, Juil, and Yuseok Moon. 2019. "Worm-Based Alternate Assessment of Probiotic Intervention against Gut Barrier Infection" Nutrients 11, no. 9: 2146. https://doi.org/10.3390/nu11092146
APA StyleKim, J., & Moon, Y. (2019). Worm-Based Alternate Assessment of Probiotic Intervention against Gut Barrier Infection. Nutrients, 11(9), 2146. https://doi.org/10.3390/nu11092146




