Low Serum Vitamin D Concentrations Are Associated with Insulin Resistance in Mexican Children and Adolescents
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Demographic and Lifestyle Measures
2.3. Biologic and Anthropometric Measures
2.4. Insulin Resistance and Vitamin D
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Flores, M.; Sánchez-Romero, L.; Macías, N.; Lozada, A.; Diaz, E.; Barquera, S. Concentraciones Séricas de Vitamina D en Niños, Adolescentes y Adultos Mexicanos Resultados de la ENSANUT 2006; Instituto Nacional de Salud Pública: Cuernavaca, Mexico, 2011. [Google Scholar]
- Van Schoor, N.; Lips, P. Worldwide vitamin D status. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 671–680. [Google Scholar] [CrossRef]
- Alemzadeh, R.; Kichler, J.; Babar, G.; Calhoun, M. Hypovitaminosis D in obese children and adolescents: Relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism 2008, 57, 183–191. [Google Scholar] [CrossRef]
- Alcubierre, N.; Castelblanco, E.; Martínez-Alonso, M.; Granado-Casas, M.; Esquerda, A.; Traveset, A.; Martinez-Gonzalez, D.; Franch-Nadal, J.; Mauricio, D. Vitamin D deficiency is associated with poorer satisfaction with diabetes-related treatment and quality of life in patients with type 2 diabetes: A cross-sectional study. Health Qual. Life Outcomes 2018, 16, 44. [Google Scholar] [CrossRef]
- Catena, C.; Colussi, G.; Novello, M.; Bertin, N.; Brosolo, G.; Palomba, A.; Duratti, A.; Sechi, L. Vitamin D deficiency and glucose metabolism in non-diabetic essential hypertensive patients. J. Hypertens. 2018, 36, e26. [Google Scholar] [CrossRef]
- Wimalawansa, S.J.; Wimalawansa, P.S. Associations of vitamin D with insulin resistance, obesity, type 2 diabetes, and metabolic syndrome. J. Steroid Biochem. Mol. Boil. 2018, 175, 177–189. [Google Scholar] [CrossRef]
- Svoren, B.M.; Volkening, L.K.; Wood, J.R.; Laffel, L.M. Significant vitamin D deficiency in youth with type 1 diabetes mellitus. J. Pediatr. 2009, 154, 132–134. [Google Scholar] [CrossRef][Green Version]
- Chiu, K.C.; Chu, A.; Go, V.L.W.; Saad, M.F. Hypovitaminosis D is associated with insulin resistance and β cell dysfunction. Am. J. Clin. Nutr. 2004, 79, 820–825. [Google Scholar] [CrossRef]
- Thorand, B.; Zierer, A.; Huth, C.; Linseisen, J.; Meisinger, C.; Roden, M.; Peters, A.; Koenig, W.; Herder, C. Effect of Serum 25-Hydroxyvitamin D on Risk for Type 2 Diabetes May Be Partially Mediated by Subclinical Inflammation: Results from the MONICA/KORA Augsburg study. Diabetes Care 2011, 34, 2320–2322. [Google Scholar] [CrossRef]
- Hernández-Cordero, S.; Cuevas-Nasu, L.; Morán-Ruán, M.C.; Humaran, I.M.G.; Ávila-Arcos, M.A.; Rivera-Dommarco, J.A. Overweight and obesity in Mexican children and adolescents during the last 25 years. Nutr. Diabetes 2017, 7, e247. [Google Scholar] [CrossRef]
- Llewellyn, A.; Simmonds, M.; Owen, C.G.; Woolacott, N. Childhood obesity as a predictor of morbidity in adulthood: A systematic review and meta-analysis. Obes. Rev. 2016, 17, 56–67. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Denova-Gutiérrez, E.; Ramírez-Silva, I.; Rodríguez-Ramírez, S.; Jiménez-Aguilar, A.; Shamah-Levy, T.; Rivera-Dommarco, J.A. Validity of a food frequency questionnaire to assess food intake in Mexican adolescent and adult population. Salud Pública Mex. 2016, 58, 617. [Google Scholar] [CrossRef]
- Hernández-Ávila, J.E.; González-Avilés, L.; Rosales-Mendoza, E. Manual de Usuario. SNUT Sistema de Evaluación de Hábitos Nutricionales y Consumo de Nutrimentos; Instituto Nacional de Salud Pública: Cuernavaca, Mexico, 2003. (In Spanish) [Google Scholar]
- De Onis, M.; Lobstein, T. Defining obesity risk status in the general childhood population: Which cut-offs should we use? Pediatr. Obes. 2010, 5, 458–460. [Google Scholar] [CrossRef]
- Keskin, M.; Kurtoglu, S.; Kendirci, M.; Atabek, M.E.; Yazici, C. Homeostasis Model Assessment Is More Reliable Than the Fasting Glucose/Insulin Ratio and Quantitative Insulin Sensitivity Check Index for Assessing Insulin Resistance Among Obese Children and Adolescents. Pediatrics 2005, 115, e500–e503. [Google Scholar] [CrossRef]
- Shamah-Levy, T.; Cuevas-Nasu, L.; Gaona-Pineda, E.B.; Gómez-Acosta, L.M.; Morales-Ruán, M.D.; Hernández-Ávila, M.; Rivera-Dommarco, J.A. Sobrepeso y obesidad en niños y adolescentes en México, actualización de la Encuesta Nacional de Salud y Nutrición de Medio Camino 2016. Salud Pública Mex. 2018, 60, 244–253. [Google Scholar] [CrossRef]
- Flores, M.; Macias, N.; Lozada, A.; Sánchez, L.M.; Díaz, E.; Barquera, S. Serum 25-hydroxyvitamin D levels among Mexican children ages 2 y to 12 y: A national survey. Nutrition 2013, 29, 802–804. [Google Scholar] [CrossRef]
- Clark, P.; Vivanco-Muñoz, N.; Piña, J.T.; Rivas-Ruiz, R.; Huitrón, G.; Chico-Barba, G.; Reza-Albarran, A.A. High prevalence of hypovitaminosis D in Mexicans aged 14 years and older and its correlation with parathyroid hormone. Arch. Osteoporos. 2015, 10, 225. [Google Scholar] [CrossRef]
- López-González, D.; Méndez-Sánchez, L.; Guagnelli, M.A.; Clark, P. Deficiencia de vitamina D en la edad pediátrica. Una oportunidad de prevención. Mex. Child. Hosp. Med Bull. 2015, 72, 225–234. [Google Scholar]
- Contreras-Manzano, A.; Villalpando, S.; Robledo-Pérez, R. Estado de la vitamina D por factores sociodemográficos e índice de masa corporal en mujeres mexicanas en edad reproductiva. Salud Publica Mex. 2017, 59, 518–525. [Google Scholar] [CrossRef]
- Cediel, G.; Corvalán, C.; Aguirre, C.; de Romaña, D.; Uauy, R. Serum 25-Hydroxyvitamin D associated with indicators of body fat and insulin resistance in prepubertal Chilean children. Int. J. Obes. 2015, 40, 147–152. [Google Scholar] [CrossRef]
- Chung, S.J.; Lee, Y.A.; Hong, H.; Kang, M.J.; Kwon, H.J.; Shin, C.H.; Yang, S.W. Inverse relationship between vitamin D status and insulin resistance and the risk of impaired fasting glucose in Korean children and adolescents: The Korean National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Public Health Nutr. 2013, 17, 795–802. [Google Scholar] [CrossRef]
- Ersfeld, D.L.; Rao, D.S.; Body, J.J.; Sackrison, J.L., Jr.; Miller, A.B.; Parikh, N.; Eskridge, T.L.; Polinske, A.; Olson, G.T.; MacFarlane, G.D. Analytical and clinical validation of the 25 OH vitamin D assay for the LIAISON automated analyzer. Clin. Biochem. 2004, 37, 867–874. [Google Scholar] [CrossRef]
- Flores, M.; Macías, N.; Rivera, M.; Barquera, S.; Hernández, L.; García-Guerra, A.; Rivera, J.A. Energy and nutrient intake among Mexican school-aged children, Mexican National Health and Nutrition Survey 2006. Salud Pública Mex. 2009, 51, S540–S550. [Google Scholar] [CrossRef]
| Variable | Girls (n = 244) | Boys (n = 289) | Total (n = 533) |
|---|---|---|---|
| Age 1, years | 11.7 ± 4.1 | 11.5 ± 3.5 | 11.6 ± 3.9 |
| Weight, kg | 42.4 ± 17.4 | 44.4 ± 17.2 | 43.5 ± 17.3 |
| Height, cm | 142.2 ± 16.6 | 147.5 ± 19.4 *** | 145.1 ± 18.4 |
| BMI Φ, kg/m2 | |||
| Normal, % | 170 (69.6) | 198 (68.4) | 367 (68.9) |
| Overweight, % | 57 (23.4) | 60 (20.8) | 118 (22.1) |
| Obesity, % | 17 (7.0) | 31 (10.8) | 48 (9.0) |
| Body fat percentage, | 33.9 ± 6.9 | 28.5 ± 8.6 *** | 31.2 |
| Triglycerides, mg/dL | 92.7 ± 10.7 | 89.2 ± 6.8 | 90.9 ± 8.8 |
| HDL-c ϕ, mg/dL | 55.2 ± 13.2 | 55.6 ± 13.7 | 55.4 ± 13.6 |
| Glucose, mg/dL | 80.4 ± 7.7 | 82.3 ± 7.1 *** | 81.4 ± 7.5 |
| Insulin, mU/L | 9.9 ± 6.6 | 8.4 ± 5.2 ** | 9.1 ± 5.9 |
| HOMA index δ | 1.9 ± 1.4 | 1.7 ± 1.1 *** | 1.8 ± 1.3 |
| Insulin resistance Ω, % | 25 (10.3) | 28 (9.7) * | 53 (9.9) |
| Tanner, % | |||
| I | 93 (37.9) | 129 (44.6) | 221 (41.5) |
| II | 32 (13.3) | 36 (12.3) | 68 (12.8) |
| III | 28 (11.7) | 36 (12.6) | 65 (12.2) |
| IV | 47 (19.2) | 56 (19.3) | 103 (19.2) |
| V | 44 (17.9) | 32 (11.2) | 76 (14.3) |
| 25(OH)D, ng/mL | 20.4 ± 6.3 | 22.8 ± 6.4 *** | 21.7 ± 6.5 |
| ≥30, % | 20 (8.2) | 38 (13.2) *** | 58 (10.9) |
| ≥20 and <30, % | 99 (40.6) | 147 (50.9) * | 246 (46.2) |
| <20, % | 125 (51.2) | 104 (35.9) *** | 229 (42.9) |
| Energy intake, kcal/day | 2390.0 ± 1069.4 | 2611.9 ± 1056.1 * | 2496.8 ± 1068.2 |
| Physical activity, min/day | 39.2 ± 38.7 | 58.0 ± 57.6 ** | 48.2 ± 49.6 |
| Variable | High n = 173 | Medium n = 175 | Low n = 183 |
|---|---|---|---|
| 25(OH)D (ng/mL) | 29.2 ± 3.9 | 21.3 ± 1.6 | 15.1 ± 2.6 *** |
| Age (years) | 10.7 ± 3.9 | 11.7 ± 3.7 | 12.4 ± 3.7 |
| Women | 59 (34.1) | 81 (46.3) | 103 (56.3) *** |
| Weight (kg) | 38.2 ± 16.4 | 45.1 ± 17.8 | 46.9 ± 16.5 ** |
| Height (cm) | 140.8 ± 20.1 | 146.2 ± 18.5 | 148.1 ± 15.8 *** |
| BMI Φ (kg/m2) | |||
| Overweight | 28 (16.2) | 40 (22.9) | 49 (26.8) ** |
| Obesity | 10 (5.8) | 21 (12.0) | 18 (10.0) ** |
| Body fat | 28.9 ± 7.7 | 31.1 ± 8.3 | 33.0 ± 8.2 ** |
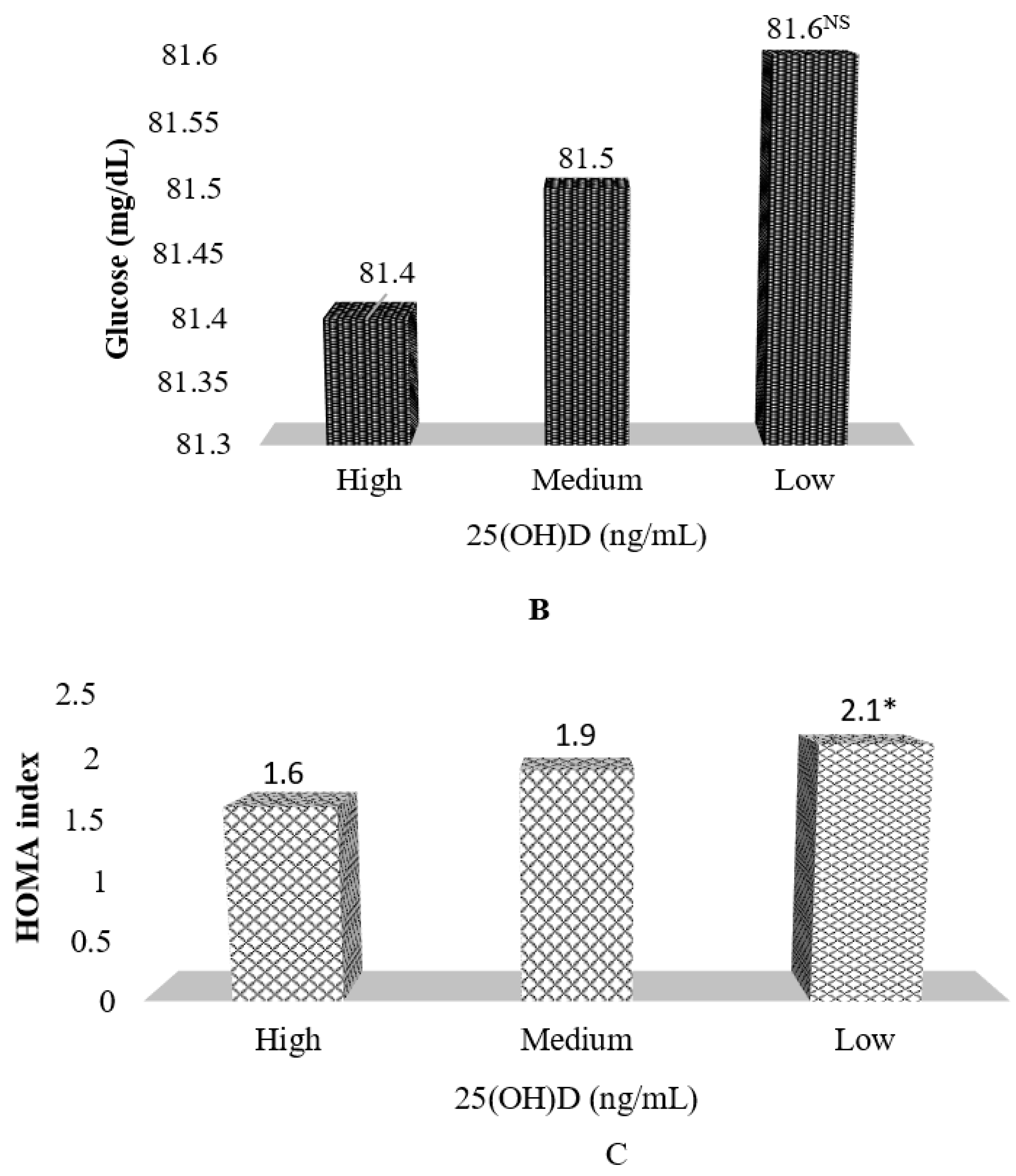
| Glucose (mg/dL) | 81.4 ± 7.5 | 81.8 ± 7.3 | 81.5 ± 7.5 |
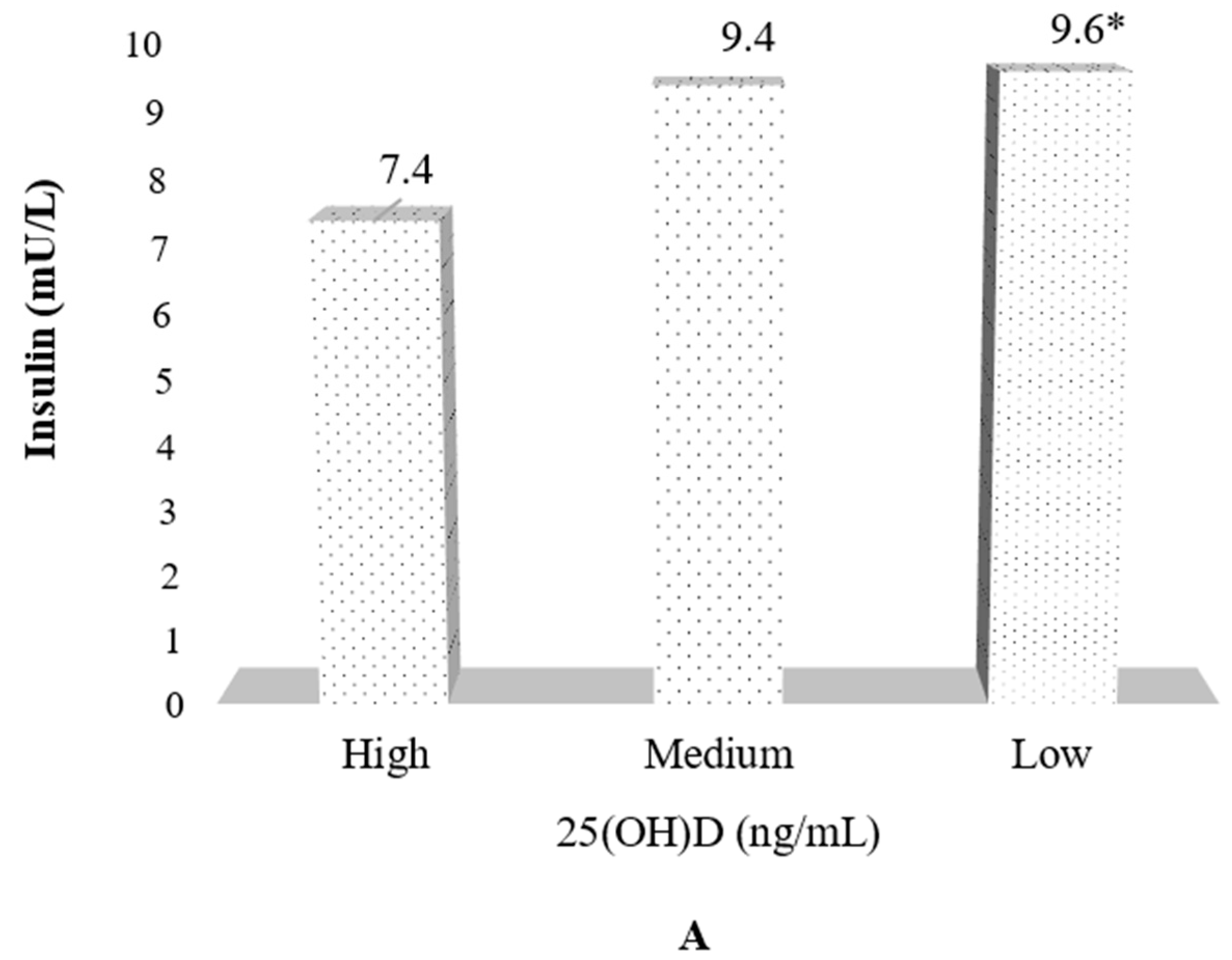
| Insulin (mU/L) | 7.0 ± 3.7 | 9.5 ± 5.8 | 10.7 ± 7.1 ** |
| HOMA index δ | 1.4 ± 0.8 | 1.9 ± 1.2 | 2.2 ± 1.6 *** |
| Insulin resistance, (%) Ω | 4.0 | 11.4 | 13.8 *** |
| Tanner, (%) | |||
| I | 90 (52.3) | 66 (37.6) | 64 (34.8) ** |
| II | 18 (10.5) | 26 (15.0) | 23 (12.4) |
| III | 13 (7.5) | 26 (15.0) | 26 (14.0) |
| IV | 27 (15.7) | 29.4 (16.8) | 46 (25.3) |
| V | 24 (14.0) | 27 (15.6) | 25 (13.5) |
| Variable | Crude | Adjusted ^ | ||||
|---|---|---|---|---|---|---|
| β | SE | p-Value | β | SE | p-Value | |
| Glucose (mg/dL) | −0.11 | −0.210 | 0.409 | −0.02 | 0.051 | 0.72 |
| Insulin (mU/L) | −0.24 | 0.030 | <0.001 | −0.14 | 0.033 | <0.001 |
| HOMA index δ | −0.05 | 0.008 | <0.001 | −0.03 | 0.007 | <0.001 |
| Variable | Crude | Adjusted ^ | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Trend | OR | 95% CI | p Trend | |
| Vitamin D tertile | ||||||
| Medium | 3.0 | 1.3, 7.4 | 0.003 | 2.0 | 0.8, 5.2 | 0.030 |
| Low | 3.8 | 1.6, 8.9 | 2.9 | 1.1, 7.2 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denova-Gutiérrez, E.; Muñoz-Aguirre, P.; López, D.; Flores, M.; Medeiros, M.; Tamborrel, N.; Clark, P. Low Serum Vitamin D Concentrations Are Associated with Insulin Resistance in Mexican Children and Adolescents. Nutrients 2019, 11, 2109. https://doi.org/10.3390/nu11092109
Denova-Gutiérrez E, Muñoz-Aguirre P, López D, Flores M, Medeiros M, Tamborrel N, Clark P. Low Serum Vitamin D Concentrations Are Associated with Insulin Resistance in Mexican Children and Adolescents. Nutrients. 2019; 11(9):2109. https://doi.org/10.3390/nu11092109
Chicago/Turabian StyleDenova-Gutiérrez, Edgar, Paloma Muñoz-Aguirre, Desiree López, Mario Flores, Mara Medeiros, Natalia Tamborrel, and Patricia Clark. 2019. "Low Serum Vitamin D Concentrations Are Associated with Insulin Resistance in Mexican Children and Adolescents" Nutrients 11, no. 9: 2109. https://doi.org/10.3390/nu11092109
APA StyleDenova-Gutiérrez, E., Muñoz-Aguirre, P., López, D., Flores, M., Medeiros, M., Tamborrel, N., & Clark, P. (2019). Low Serum Vitamin D Concentrations Are Associated with Insulin Resistance in Mexican Children and Adolescents. Nutrients, 11(9), 2109. https://doi.org/10.3390/nu11092109





