Native Whey Induces Similar Adaptation to Strength Training as Milk, despite Higher Levels of Leucine, in Elderly Individuals
Abstract
1. Introduction
2. Materials and Methods
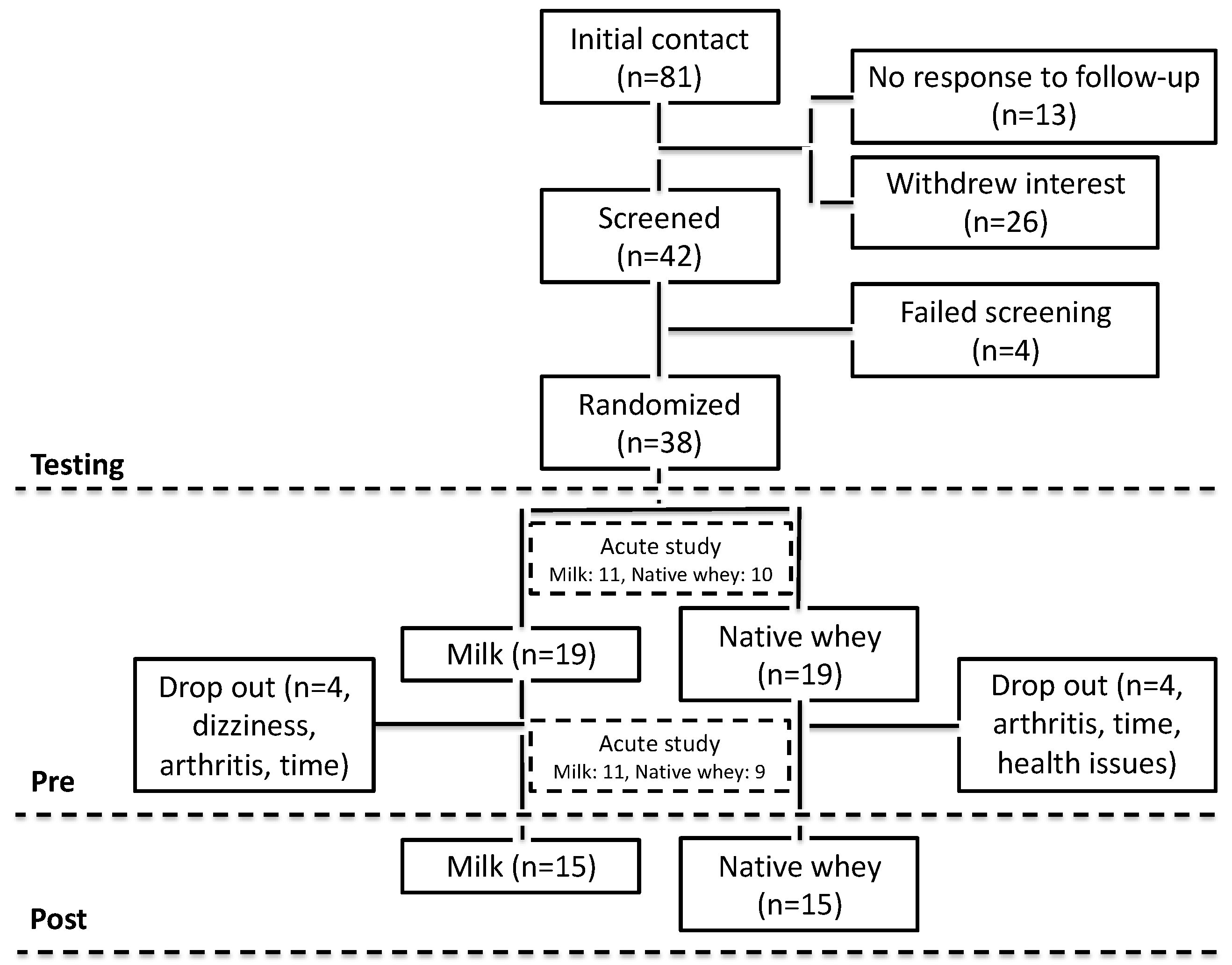
2.1. Participants and Ethical Approval
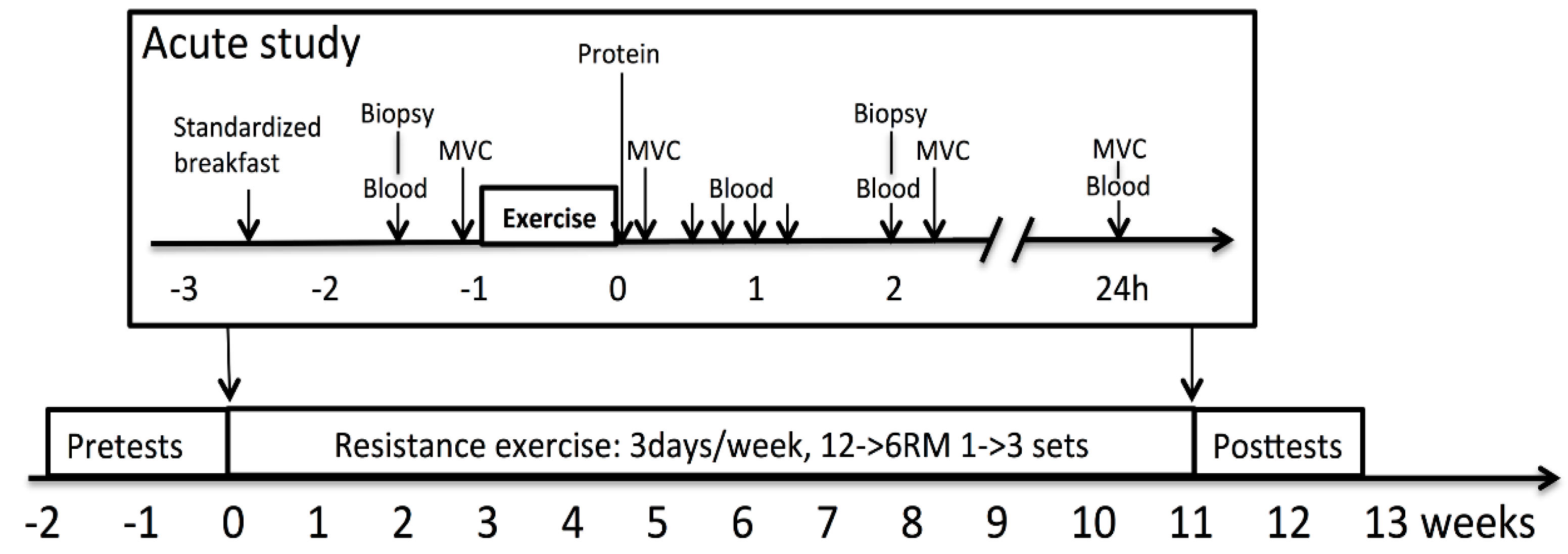
2.2. Study Design
2.3. Supplements
2.4. Daily Food Intake
2.5. Training Program
2.6. Dual-Energy X-ray Absorptiometry
2.7. Ultrasonography
2.8. Maximal Strength
2.9. Functional Tests
2.10. Blood Analyses
2.11. Biopsy Collection and Pre-Analytical Processing
2.12. Western Blot
2.13. Immunohistochemistry
2.14. Acute Strength Training Experiment
2.15. Statistics
3. Results
3.1. Participant Characteristics and Compliance
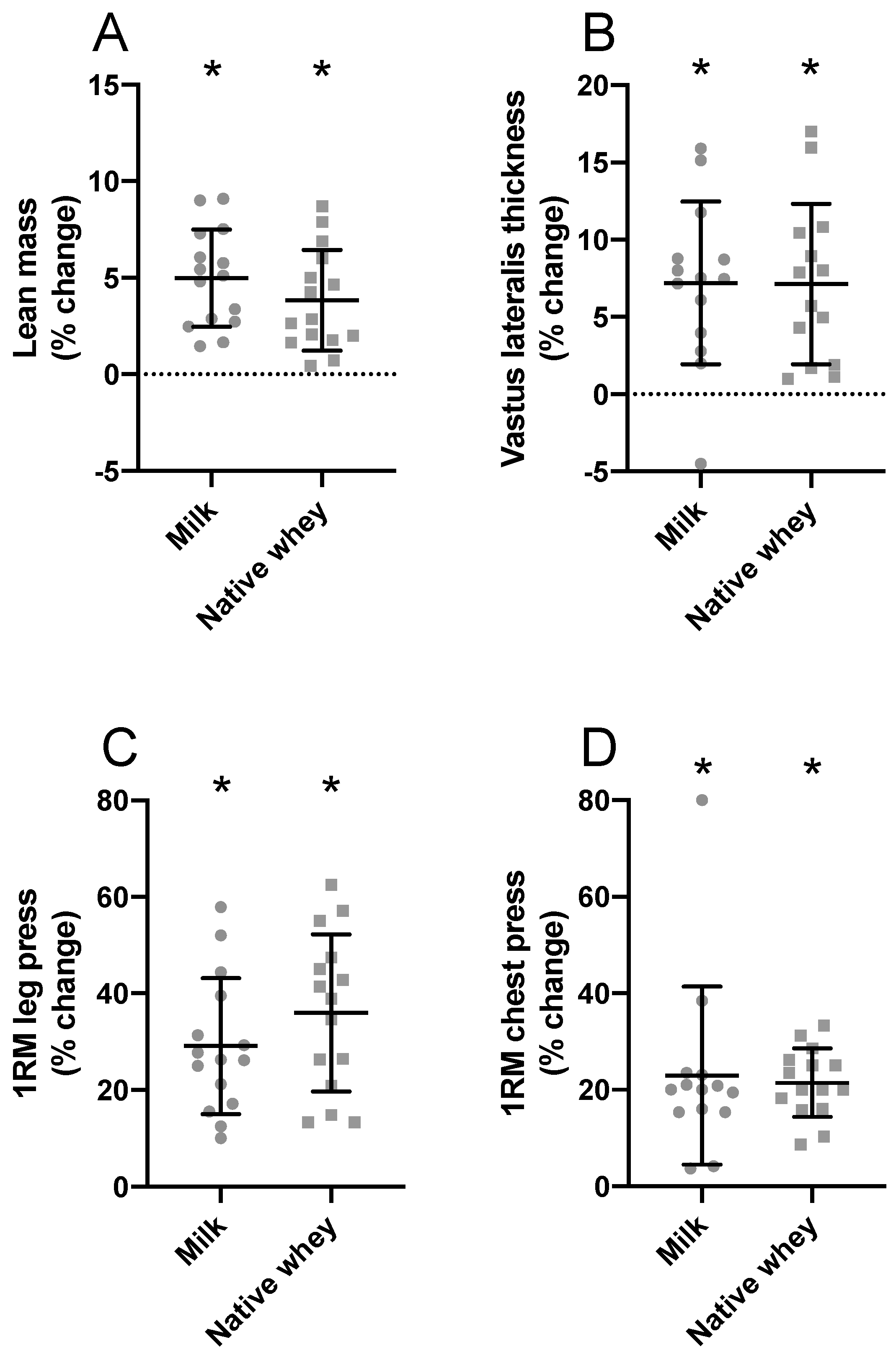
3.2. Muscle Mass and Muscle Fiber Cross-Sectional Area
3.3. Muscle Strength and Performance
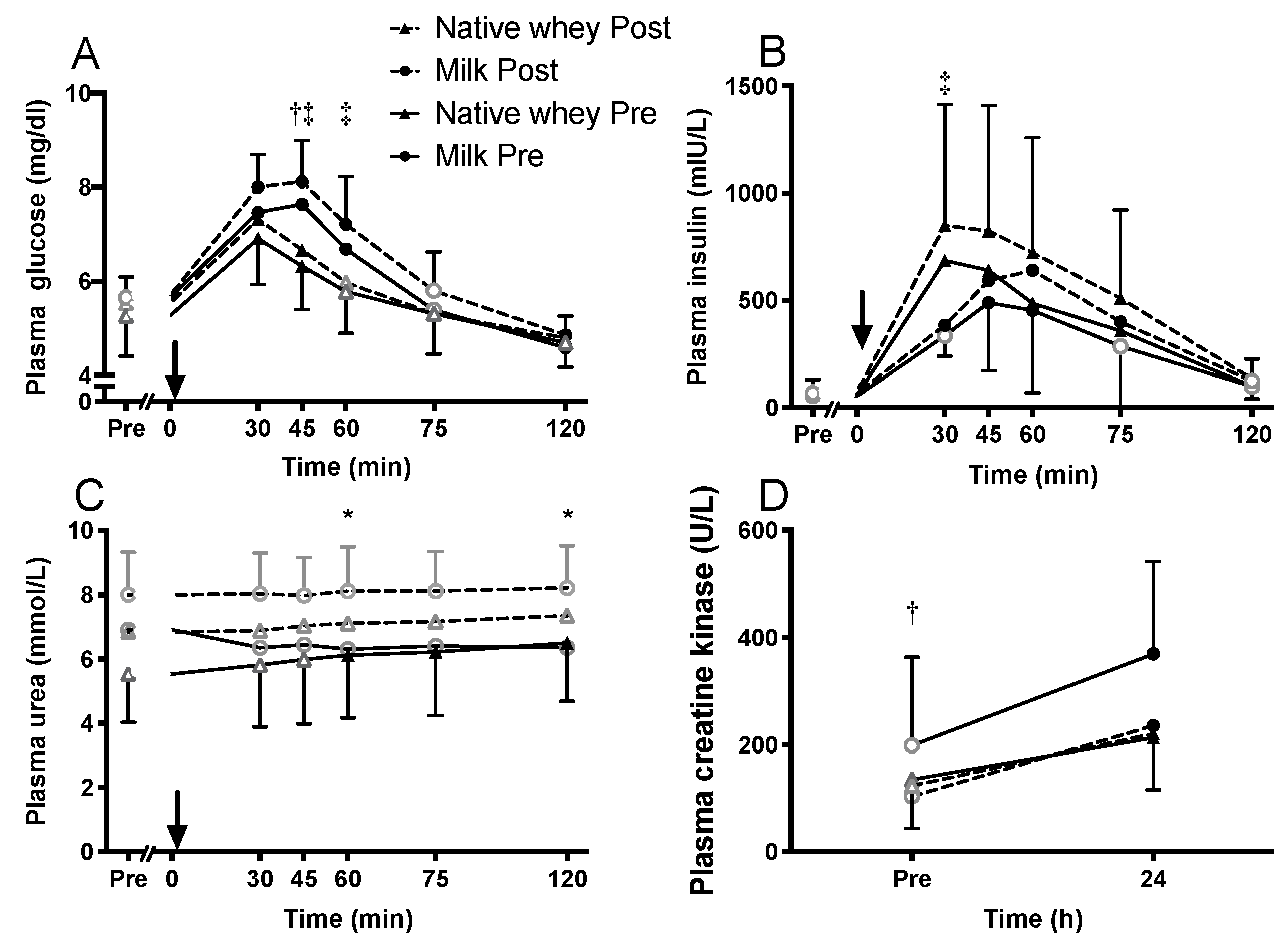
3.4. Acute Experiment
3.5. Blood Measures
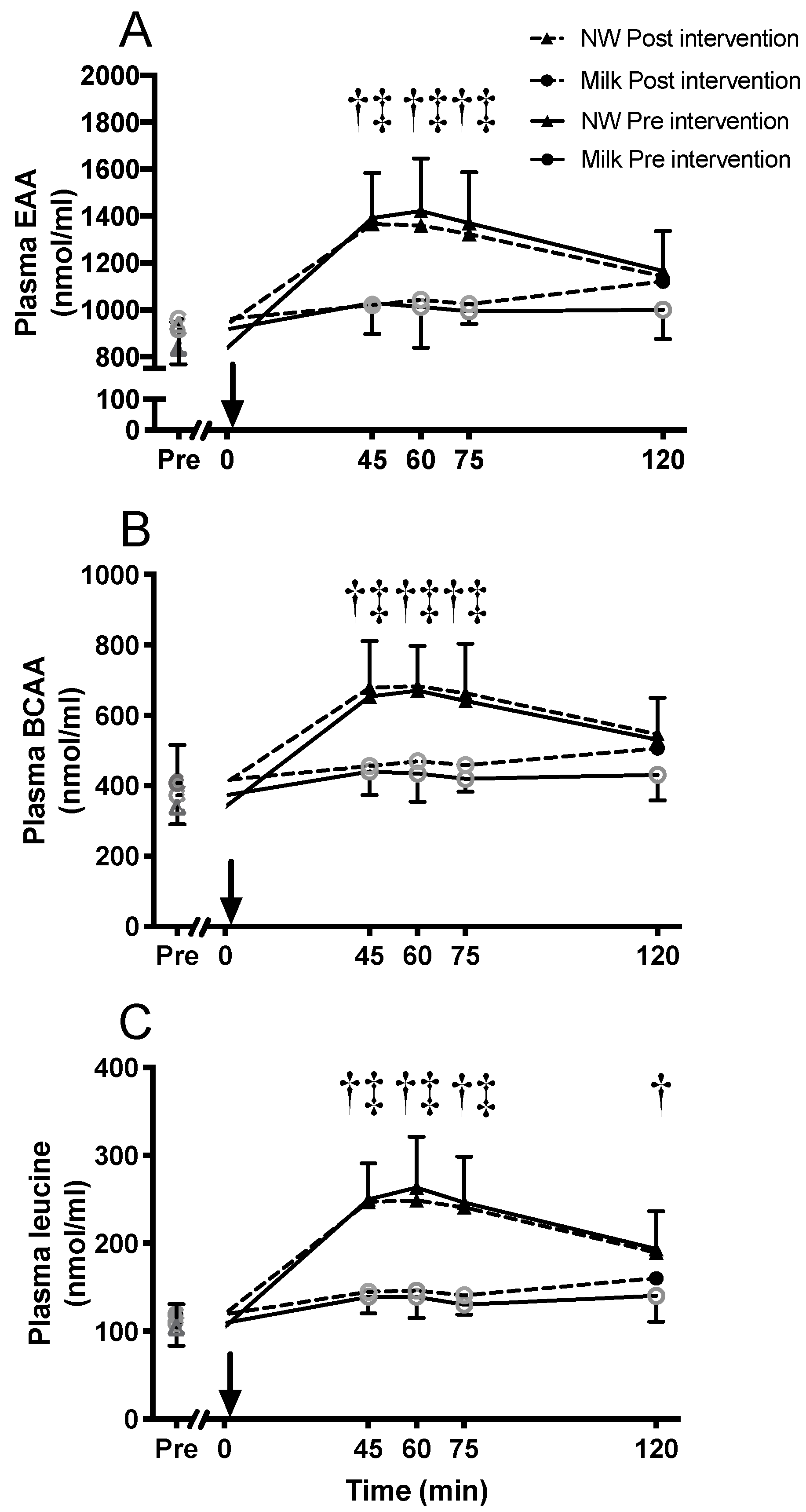
3.6. Amino Acid Concentrations in Blood
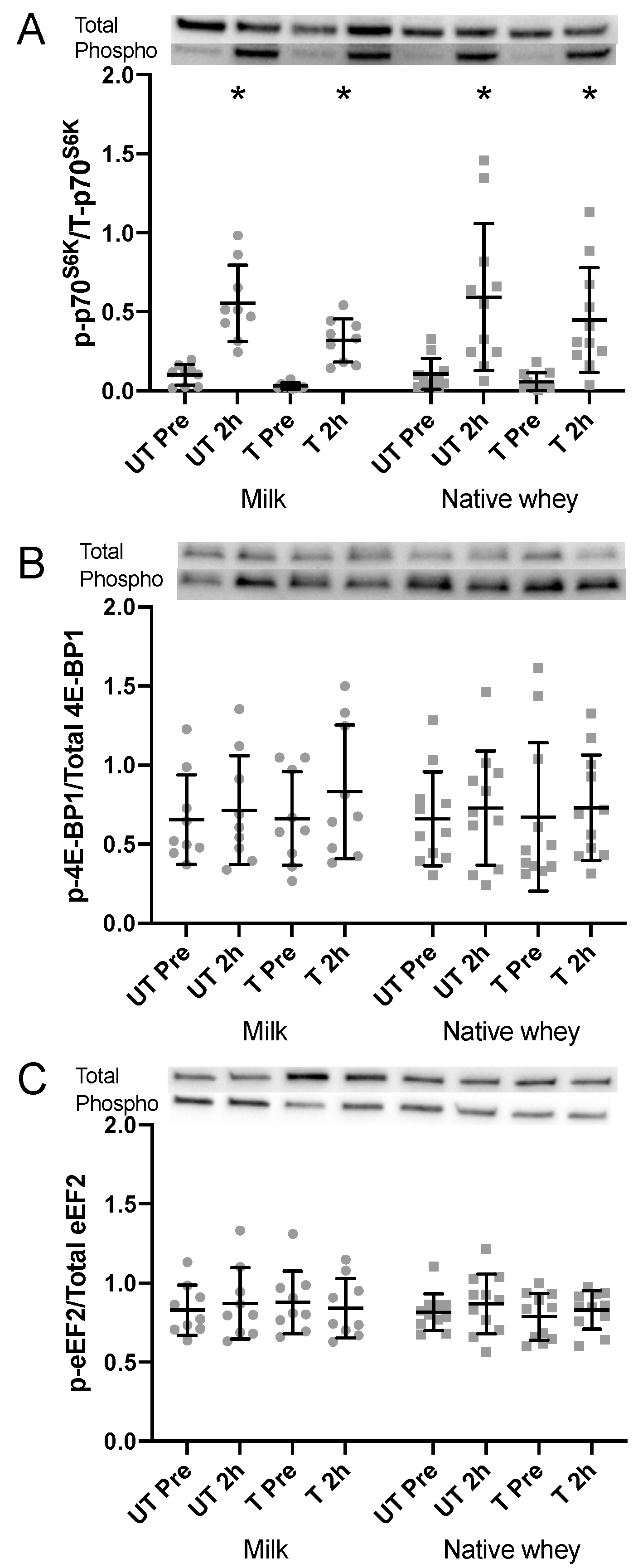
3.7. Protein Signaling
3.8. Correlations
4. Discussion
4.1. Effect of Protein Type on Muscle Mass and Strength
4.2. Amino Acid Concentrations in Blood
4.3. Intracellular Signaling
4.4. Recovery of Force-Generating Capacity
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Atkins, J.L.; Whincup, P.H.; Morris, R.W.; Lennon, L.T.; Papacosta, O.; Wannamethee, S.G. Sarcopenic Obesity and Risk of Cardiovascular Disease and Mortality: A Population-Based Cohort Study of Older Men. J. Am. Geriatr. Soc. 2014, 62, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Barbagallo, M. The cardiometabolic syndrome and sarcopenic obesity in older persons. J. Cardiometab. Syndr. 2007, 2, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Naseeb, M.A.; Volpe, S.L. Protein and exercise in the prevention of sarcopenia and aging. Nutr. Res. 2017, 40, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Rennie, M.J. Anabolic resistance: The effects of aging, sexual dimorphism, and immobilization on human muscle protein turnover. Appl. Physiol. Nutr. Metab. 2009, 34, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Booth, F.W.; Tseng, B.S.; Flück, M.; Carson, J.A. Molecular and cellular adaptation of muscle in response to physical training. Acta Physiol. Scand. 1998, 162, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Coffey, V.G.; Hawley, J.A. The molecular bases of training adaptation. Sports Med. 2007, 37, 737–763. [Google Scholar] [CrossRef]
- Pennings, B.; Boirie, Y.; Senden, J.M.G.; Gijsen, A.P.; Kuipers, H.; van Loon, L.J.C. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011, 93, 997–1005. [Google Scholar] [CrossRef]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef]
- West, D.W.; Burd, N.A.; Coffey, V.G.; Baker, S.K.; Burke, L.M.; Hawley, J.A.; Moore, D.R.; Stellingwerff, T.; Phillips, S.M. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. Am. J. Clin. Nutr. 2011, 94, 795–803. [Google Scholar] [CrossRef]
- Volpi, E.; Kobayashi, H.; Sheffield-Moore, M.; Mittendorfer, B.; Wolfe, R.R. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am. J. Clin. Nutr. 2003, 78, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Atherton, P.J.; Smith, K.; Etheridge, T.; Rankin, D.; Rennie, M.J. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids 2010, 38, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Atherton, P.J.; Kumar, V.; Selby, A.; Rankin, D.; Hildebrandt, W.; Phillips, B.; Williams, J.; Hiscock, N.; Smith, K. Enriching a protein drink with leucine augments muscle protein synthesis after resistance exercise in young and older men. Clin. Nutr. 2017, 36, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.S.; Phillips, B.E.; Wilkinson, D.J.; Limb, M.C.; Rankin, D.; Mitchell, W.K.; Koboyashi, H.; Greenhaff, P.L.; Smith, K.; Atherton, P.J. Intake of low-dose leucine-rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women, at rest and after exercise. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E1057–E1065. [Google Scholar] [CrossRef] [PubMed]
- Wall, B.T.; Hamer, H.M.; de Lange, A.; Kiskini, A.; Groen, B.B.L.; Senden, J.M.G.; Gijsen, A.P.; Verdijk, L.B.; van Loon, L.J.C. Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men. Clin. Nutr. 2013, 32, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Hamarsland, H.; Aas, S.N.; Nordengen, A.L.; Holte, K.; Garthe, I.; Paulsen, G.; Cotter, M.; Borsheim, E.; Benestad, H.B.; Raastad, T. Native Whey Induces Similar Post Exercise Muscle Anabolic Responses as Regular Whey, Despite Greater Leucinemia, in Elderly Individuals. J. Nutr. Health Aging 2019, 23, 42–50. [Google Scholar] [CrossRef]
- Cermak, N.M.N.; Res, P.T.P.; de Groot, L.C.L.; Saris, W.H.W.; van Loon, L.J.L. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: A meta-analysis. Am. J. Clin. Nutr. 2012, 96, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; Tieland, M.; Dirks, M.L.; van der Zwaluw, N.; van der Zwaluw, N.; Verdijk, L.B.; van de Rest, O.; van de Rest, O.; de Groot, L.C.P.G.M.; de Groot, L.C.P.G.M.; et al. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2012, 13, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Chale, A.; Cloutier, G.J.; Hau, C.; Phillips, E.M.; Dallal, G.E.; Fielding, R.A. Efficacy of Whey Protein Supplementation on Resistance Exercise–Induced Changes in Lean Mass, Muscle Strength, and Physical Function in Mobility-Limited Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 68, 682–690. [Google Scholar] [CrossRef]
- Verdijk, L.B.; Jonkers, R.A.; Gleeson, B.G.; Beelen, M.; Meijer, K.; Savelberg, H.H.; Wodzig, W.K.; Dendale, P.; van Loon, L.J. Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am. J. Clin. Nutr. 2009, 89, 608–616. [Google Scholar] [CrossRef]
- Hamarsland, H.; Laahne, J.A.L.; Paulsen, G.; Cotter, M.; Borsheim, E.; Raastad, T. Native whey induces higher and faster leucinemia than other whey protein supplements and milk: A randomized controlled trial. BMC Nutr. 2017, 3, 10. [Google Scholar] [CrossRef]
- Leenders, M.; Verdijk, L.B.; Van der Hoeven, L.; Van Kranenburg, J.; Nilwik, R.; Wodzig, W.K.W.H.; Senden, J.M.G.; Keizer, H.A.; van Loon, L.J.C. Protein supplementation during resistance-type exercise training in the elderly. Med. Sci. Sport Exerc. 2013, 45, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Volk, B.M.; Gómez, A.L.; Kunces, L.J.; Kupchak, B.R.; Freidenreich, D.J.; Aristizabal, J.C.; Saenz, C.; Dunn-Lewis, C.; Ballard, K.D.; et al. Whey protein supplementation during resistance training augments lean body mass. J. Am. Coll. Nutr. 2013, 32, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.W.; Tang, J.E.; Wilkinson, S.B.; Tarnopolsky, M.A.; Lawrence, R.L.; Fullerton, A.V.; Phillips, S.M. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am. J. Clin. Nutr. 2007, 86, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, G.; Hamarsland, H.; Cumming, K.T.; Johansen, R.E.; Hulmi, J.J.; Borsheim, E.; Wiig, H.; Garthe, I.; Raastad, T. Vitamin C and E supplementation alters protein signalling after a strength training session, but not muscle growth during 10 weeks of training. J. Physiol. 2014, 592, 5391–5408. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein Ingestion to Stimulate Myofibrillar Protein Synthesis Requires Greater Relative Protein Intakes in Healthy Older Versus Younger Men. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 70, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Breen, L.; Burd, N.A.; Hector, A.J.; Hector, A.J.; Churchward-Venne, T.A.; Josse, A.R.; Josse, A.R.; Tarnopolsky, M.A.; Phillips, S.M. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br. J. Nutr. 2012, 108, 1780–1788. [Google Scholar] [CrossRef] [PubMed]
- Churchward-Venne, T.A.; Breen, L.; Di Donato, D.M.; Hector, A.J.; Mitchell, C.J.; Moore, D.R.; Stellingwerff, T.; Breuille, D.; Offord, E.A.; Baker, S.K.; et al. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: A double-blind, randomized trial. Am. J. Clin. Nutr. 2014, 99, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.A.; Yang, Y.; Moore, D.R.; Tang, J.E.; Tarnopolsky, M.A.; Phillips, S.M. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br. J. Nutr. 2012, 108, 958–962. [Google Scholar] [CrossRef]
- Dideriksen, K.J.; Reitelseder, S.; Petersen, S.G.; Hjort, M.; Helmark, I.C.; Kjaer, M.; Holm, L. Stimulation of muscle protein synthesis by whey and caseinate ingestion after resistance exercise in elderly individuals. Scand. J. Med. Sci. Sports 2011, 21, E372–E383. [Google Scholar] [CrossRef]
- Shad, B.J.; Thompson, J.L.; Breen, L. Does the muscle protein synthetic response to exercise and amino acid-based nutrition diminish with advancing age? A systematic review. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E803–E817. [Google Scholar] [CrossRef] [PubMed]
- Arnarson, A.; Geirsdottir, O.G.; Ramel, A.; Briem, K.; Jonsson, P.V.; Thorsdottir, I. Effects of whey proteins and carbohydrates on the efficacy of resistance training in elderly people: Double blind, randomised controlled trial. Eur. J. Clin. Nutr. 2013, 67, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Bemben, M.G.; Witten, M.S.; Carter, J.M.; Eliot, K.A.; Knehans, A.W.; Bemben, D.A. The Effects of Supplementation with Creatine and Protein on Muscle Strength Following a Traditional Resistance Training Program in Middle-Aged and Older Men. J. Nutr. Health Aging 2010, 14, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Kukuljan, S.; Kukuljan, S.; Nowson, C.A.; Nowson, C.A.; Sanders, K.; Sanders, K.; Daly, R.M.; Daly, R.M. Effects of resistance exercise and fortified milk on skeletal muscle mass, muscle size, and functional performance in middle-aged and older men: An 18-mo randomized controlled trial. J. Appl. Physiol. 2009, 107, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, K.; Chen, G.C.; Wang, Y.; Zhang, Z.; Dai, X.; Szeto, I.M.Y.; Qin, L.-Q. Effects of milk proteins supplementation in older adults undergoing resistance training: A meta-analysis of randomized control trials. J. Nutr. Health Aging 2018, 22, 237–245. [Google Scholar] [CrossRef]
- Dirks, M.L.; Tieland, M.; Verdijk, L.B.; Losen, M.; Nilwik, R.; Mensink, M.; de Groot, L.C.P.G.M.; van Loon, L.J.C. Protein Supplementation Augments Muscle Fiber Hypertrophy but Does Not Modulate Satellite Cell Content During Prolonged Resistance-Type Exercise Training in Frail Elderly. J. Am. Med. Dir. Assoc. 2017, 18, 608–615. [Google Scholar] [CrossRef]
- Holm, L.; Olesen, J.L.; Matsumoto, K.; Doi, T.; Mizuno, M.; Alsted, T.J.; Mackey, A.L.; Schwarz, P.; Kjaer, M. Protein-containing nutrient supplementation following strength training enhances the effect on muscle mass, strength, and bone formation in postmenopausal women. J. Appl. Physiol. 2008, 105, 274–281. [Google Scholar] [CrossRef]
- Hamarsland, H.; Handegard, V.; Kåshagen, M.; Benestad, H.B.; Raastad, T. No Difference between Spray Dried Milk and Native Whey Supplementation with Strength Training. Med. Sci. Sport Exerc. 2019, 51, 75–83. [Google Scholar] [CrossRef]
- Hamarsland, H.; Nordengen, A.L.; Nyvik Aas, S.; Holte, K.; Garthe, I.; Paulsen, G.; Cotter, M.; Borsheim, E.; Benestad, H.B.; Raastad, T. Native whey protein with high levels of leucine results in similar post-exercise muscular anabolic responses as regular whey protein: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2017, 14, 43. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Snijders, T.; Linkens, A.M.A.; Hamer, H.M.; Van Kranenburg, J.; van Loon, L.J.C. Ingestion of Casein in a Milk Matrix Modulates Dietary Protein Digestion and Absorption Kinetics but Does Not Modulate Postprandial Muscle Protein Synthesis in Older Men. J. Nutr. 2015, 145, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Dangin, M.; Boirie, Y.; Garcia-Rodenas, C.; Gachon, P.; Fauquant, J.; Callier, P.; Ballèvre, O.; Beaufrère, B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E340–E348. [Google Scholar] [CrossRef] [PubMed]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.P.; Maubois, J.L.; Beaufrère, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef] [PubMed]
- Farnfield, M.M.; Breen, L.; Carey, K.A.; Garnham, A.; Cameron-Smith, D. Activation of mTOR signalling in young and old human skeletal muscle in response to combined resistance exercise and whey protein ingestion. Appl. Physiol. Nutr. Metab. 2012, 37, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Baar, E.L.; Carbajal, K.A.; Ong, I.M.; Lamming, D.W. Sex- and tissue-specific changes in mTOR signaling with age in C57BL/6J mice. Aging Cell 2016, 15, 155–166. [Google Scholar] [CrossRef]
- Markofski, M.M.; Dickinson, J.M.; Drummond, M.J.; Fry, C.S.; Fujita, S.; Gundermann, D.M.; Glynn, E.L.; Jennings, K.; Paddon-Jones, D.; Reidy, P.T.; et al. Effect of age on basal muscle protein synthesis and mTORC1 signaling in a large cohort of young and older men and women. Exp. Gerontol. 2015, 65, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Inoki, K.; Brooks, S.V.; Okazawa, H.; Lee, M.; Wang, J.; Kim, M.; Kennedy, C.L.; Macpherson, P.C.D.; Ji, X.; et al. mTORC1 underlies age-related muscle fiber damage and loss by inducing oxidative stress and catabolism. Aging Cell 2019, 18, e12943. [Google Scholar] [CrossRef]
- Joseph, G.A.; Wang, S.; Zhou, W.; Kimble, G.; Tse, H.; Eash, J.; Shavlakadze, T.; Glass, D.J. Inhibition of mTORC1 signaling in aged rats counteracts the decline in muscle mass and reverses multiple parameters of muscle signaling associated with sarcopenia. bioRxiv 2019, 73, 591891. [Google Scholar]
- Paulsen, G.; Mikkelsen, U.R.; Raastad, T.; Peake, J.M. Leucocytes, cytokines and satellite cells: What role do they play in muscle damage and regeneration following eccentric exercise? Exerc. Immunol. Rev. 2012, 18, 42–97. [Google Scholar]
| Amino Acids (Per Serving) | Native Whey | Milk |
|---|---|---|
| Alanine | 0.6 | 1.0 |
| Arginine | 0.6 | 0.6 |
| Aspartic acid | 1.5 | 2.2 |
| Cysteine | 0.2 | 0.5 |
| Phenylalanine | 0.9 | 0.9 |
| Glutamic acid | 4.1 | 3.9 |
| Glycine | 0.4 | 0.4 |
| Histidine | 0.5 | 0.5 |
| Isoleucine | 1.0 | 1.1 |
| Leucine | 1.9 | 2.5 |
| Lysine | 1.6 | 2.1 |
| Methionine | 0.5 | 0.5 |
| Proline | 1.9 | 1.3 |
| Serine | 1.1 | 1.0 |
| Threonine | 0.8 | 1.0 |
| Tyrosine | 0.8 | 0.7 |
| Valine | 1.2 | 1.2 |
| Tryptophan | 0.2 | 0.4 |
| Total protein | 19.7 | 21.8 |
| Fat | 19.1 | 20.0 |
| Carbohydrate | 6.9 | 7.5 |
| Milk | Native Whey | p Values for Group Differences | |
|---|---|---|---|
| N (♂/♀) | 15 (9/6) | 15 (9/6) | |
| Age (years) | 74.3 ± 3.6 | 72.9 ± 1.8 | 0.18 |
| Body mass (kg) | 74.6 ± 14.0 | 78.3 ± 16.2 | 0.81 |
| Fat mass (kg) | 22.1 ± 7.0 | 23.9 ± 8.9 | 0.55 |
| Lean body mass (kg) | 49.8 ± 9.2 | 49.5 ± 10.9 | 0.95 |
| Body fat (%) | 30.5 ± 5.5 | 32.0 ± 9.1 | 0.60 |
| VL thickness (cm) | 2.1 ± 0.4 | 2.3 ± 0.5 | 0.20 |
| Leg press 1 RM (kg) | 176 ± 55 | 158 ± 50 | 0.36 |
| Bench press 1 RM (kg) | 46.8 ± 20 | 42.8 ± 17 | 0.56 |
| Milk | Native Whey | |||
|---|---|---|---|---|
| Baseline | Intervention | Baseline | Intervention | |
| Energy (KJ) | 7800 ± 2100 | 9400 ± 1500 * | 7400 ± 1900 | 9800 ± 1500 * |
| Protein g·kg body mass−1 | 1.0 ± 0.3 | 1.3 ± 0.2 * | 1.1 ± 0.3 | 1.3 ± 0.4 * |
| Protein (E%) | 17 ± 4 | 17 ± 3 | 19 ± 3 | 17 ± 2 |
| Carbohydrate (E%) | 39 ± 5 | 46 ± 3 * | 39 ± 6 | 43 ± 5 * |
| Fat (E%) | 44 ± 8 | 37 ± 4 * | 42 ± 7 | 40 ± 6 |
| Milk | Native Whey | p Values for Group Difference (% Change) | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | % Change | Pre | Post | % Change | ||
| Body mass (kg) | 74.6 ± 14.0 | 77.2 ± 14.0 | 3.6 ± 2.2 * | 75.9 ± 16.1 | 78.3 ± 16.2 | 3.3 ± 1.6 * | 0.618 |
| Fat mass (kg) | 22.1 ± 7.0 | 22.4 ± 6.8 | 1.7 ±5.3 | 23.9 ± 8.9 | 24.4 ± 8.9 | 2.4 ± 4.9 | 0.721 |
| Leg lean mass (kg) | 17.0 ± 3.8 | 18.0 ± 3.8 | 6.3 ± 3.6 * | 17.3 ± 4.7 | 18.0 ± 4.5 | 4.6 ± 3.4 * | 0.199 |
| Arm lean mass (kg) | 5.6 ± 1.5 | 6.0 ± 1.6 | 6.4 ± 3.6 * | 5.3 ± 1.5 | 5.6 ± 1.5 | 5.2 ± 3.9 * | 0.377 |
| Trunk lean mass (kg) | 24.0 ± 3.9 | 24.8 ± 3.7 * | 3.4 ± 3.6 * | 23.7 ± 4.8 | 24.4 ± 5.0 * | 3.0 ± 3.1 * | 0.791 |
| VL thickness (cm) | 2.06 ± 0.36 | 2.21 ± 0.39 * | 7.2 ± 5.3 * | 2.28 ± 0.51 | 2.43 ± 0.50 * | 7.1 ± 5.2 * | 0.971 |
| Stair climb | |||||||
| BW (s) | 7.48 ± 1.0 | 7.18 ± 0.97 | −3.8 ± 5.4 * | 7.54 ± 0.94 | 7.03 ± 0.93 | −6.56 ± 7.1 * | 0.257 |
| 10 kg (s) | 7.35 ± 1.0 | 7.21 ± 1.07 | −3.3 ± 5.0 * | 7.62 ± 1.34 | 7.09 ± 1.07 | −6.29 ± 8.1 * | 0.267 |
| 20 kg (s) | 7.91 ± 1.53 | 7.58 ± 1.50 | −3.8 ± 8.4 * | 8.07 ± 1.73 | 7.41 ± 1.26 | −7.26 ± 8.57 * | 0.293 |
| Sit to stand (s) | 7.0 ± 2.1 | 6.29 ± 1.3 | −8.1 ± 13.9 * | 6.70 ± 1.20 | 5.92 ± 0.97 | −11.0 ± 9.2 * | 0.504 |
| MFA type I (μm2) | 4519 ± 1030 | 4667 ± 1187 | 4.7 ± 22.0 | 4897 ± 919 | 4933 ± 785 | 2.8 ± 19.7 | 0.823 |
| MFA type II (μm2) | 3862 ± 1984 | 4502 ±1397 | 34.2 ± 56.7 * | 3740 ± 1608 | 4692 ± 1441 * | 33.8 ± 27.4 * | 0.935 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamarsland, H.; Johansen, M.K.; Seeberg, F.; Brochmann, M.; Garthe, I.; Benestad, H.B.; Raastad, T. Native Whey Induces Similar Adaptation to Strength Training as Milk, despite Higher Levels of Leucine, in Elderly Individuals. Nutrients 2019, 11, 2094. https://doi.org/10.3390/nu11092094
Hamarsland H, Johansen MK, Seeberg F, Brochmann M, Garthe I, Benestad HB, Raastad T. Native Whey Induces Similar Adaptation to Strength Training as Milk, despite Higher Levels of Leucine, in Elderly Individuals. Nutrients. 2019; 11(9):2094. https://doi.org/10.3390/nu11092094
Chicago/Turabian StyleHamarsland, Håvard, Mathias K. Johansen, Fridtjof Seeberg, Marie Brochmann, Ina Garthe, Haakon B. Benestad, and Truls Raastad. 2019. "Native Whey Induces Similar Adaptation to Strength Training as Milk, despite Higher Levels of Leucine, in Elderly Individuals" Nutrients 11, no. 9: 2094. https://doi.org/10.3390/nu11092094
APA StyleHamarsland, H., Johansen, M. K., Seeberg, F., Brochmann, M., Garthe, I., Benestad, H. B., & Raastad, T. (2019). Native Whey Induces Similar Adaptation to Strength Training as Milk, despite Higher Levels of Leucine, in Elderly Individuals. Nutrients, 11(9), 2094. https://doi.org/10.3390/nu11092094





